Abstract
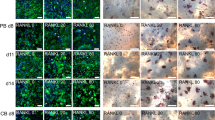
Osteoclasts (OCs) are specialized bone-resorbing cells. For “in vitro” analysis, they may be obtained from the precursors present in peripheral blood (PB) or umbilical cord blood (UCB), but there has been no detailed analysis of how the kind of source and cell culture conditions may affect the behavior of these cells. Here we analyzed the behavior of OCs after transfection with specific transcription factor decoy molecules founding that the OCs from PB undergo apoptosis when nuclear factor kappa B (NF-kB) or NFATc1 were removed, or when ERα expression was increased. Conversely, OCs from UCB showed a strong resistance to apoptotic stimuli. We found that survival signals including Bcl-2, Bcl-XL, and Survivin are present in the OCs/UCB, but not in OCs/PB. The resistance to apoptosis seems to be not correlated with NF-kB, NFATc1, or ERα expression level, or with the activation of ERK and Akt proteins. One of the mechanisms responsible for bone remodeling is apoptosis, and being susceptible of therapeutic manipulation, the OCs are extensively employed to investigate cell response to therapies for the treatment of bone loss associated with several diseases, including periodontitis, osteoporosis, and metastatic osteolysis. Therefore, our evidences are to be taken in consideration when both the effects of biological modifiers are tested and OCs apoptosis molecular mechanisms are investigated.





Similar content being viewed by others
References
Zaidi M (2007) Skeletal remodeling in health and disease. Nat Med 13:791–801
Tanaka S (2007) Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol 27:466–478
Tanaka SN, Takahashi, Udagawa N et al (1993) Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest 91:257–263
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12:17–25
Quinn JM, Elliott J, Gillespie MT, Martin TJ (1998) A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology 139:4424–4427
Atkins GJ, Kostakis P, Vincent C et al (2006) RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res 21:1339–1349
Erices A, Conget P, Minguell JJ (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109:235–242
Sun B, Jeong YH, Jung JW, Seo K, Lee YS, Kang KS (2007) Regulation of human umbilical cord blood-derived multi-potent stem cells by autogenic osteoclast-based niche-like structure. Biochem Biophys Res Commun 357:92–98
Mann MJ, Dzau VJ (2000) Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest 106:1071–1075
Gao H, Xiao J, Sun Q et al (2006) A single decoy oligodeoxynucleotides targeting multiple oncoproteins produces strong anticancer effects. Mol Pharmacol 70:1621–1629
Penolazzi L, Borgatti M, Lambertini E et al (2004) Peptide nucleic acid-DNA decoy chimeras targeting NF-kappaB transcription factors: induction of apoptosis in human primary osteoclasts. Int J Mol Med 14:145–152
Day CJ, Kim MS, Lopez CM, Nicholson GC, Morrison NA (2005) NFAT expression in human osteoclasts. J Cell Biochem 95:17–23
Nakamura T, Imai Y, Matsumoto T et al (2007) Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130:811–823
Piva R, Penolazzi L, Lambertini E, Giordano S, Gambari R (2005) Induction of apoptosis of human primary osteoclasts treated with a transcription factor decoy mimicking a promoter region of estrogen receptor alpha. Apoptosis 10:1079–1094
Matsuzaki K, Katayama K, Takahashi Y et al (1999) Human osteoclast-like cells are formed from peripheral blood mononuclear cells in a coculture with SaOS-2 cells transfected with the parathyroid hormone (PTH)/PTH-related protein receptor gene. Endocrinology 140:925–932
Piva R, Penolazzi L, Zennaro M et al (2006) Induction of apoptosis of osteoclasts by targeting transcription factors with decoy molecules. Ann NY Acad Sci 1091:509–516
Penolazzi L, Zennaro M, Lambertini E et al (2007) Induction of estrogen receptor alpha expression with decoy oligonucleotide targeted to NFATc1 binding sites in osteoblasts. Mol Pharmacol 71:1457–1462
Evans CE, Mylchreest S, Andrew JG (2006) Age of donor alters the effect of cyclic hydrostatic pressure on production by human macrophages and osteoblasts of sRANKL, OPG and RANK. BMC Musculoskelet Disord 7:21
Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. Bone 40:251–264
Bruzzaniti A, Baron R (2006) Molecular regulation of osteoclast activity. Rev Endocr Metab Disord 7:123–139
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245
Akiyama T, Bouillet P, Miyazaki T et al (2003) Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J 22:6653–6664
Sevilla L, Zaldumbide A, Carlotti F, Dayem MA, Pognonec P, Boulukos KE (2001) Bcl-XL expression correlates with primary macrophage differentiation, activation of functional competence, and survival and results from synergistic transcriptional activation by Ets2 and PU.1. J Biol Chem 276:17800–17807
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58:5315–5320
Gingery A, Bradley E, Shaw A, Oursler MJ (2003) Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem 89:165–179
Stern PH (2007) Antiresorptive agents and osteoclast apoptosis. J Cell Bioch 101:1087–1096
Vaux DL, Cory S, Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335:440–442
Brandwood CP, Hoyland JA, Hillarby MC et al (2003) Apoptotic gene expression in Paget’s disease: a possible role for Bcl-2. J Pathol 201:504–512
Hentunen TA, Reddy SV, Boyce BF et al (1998) Immortalization of osteoclast precursors by targeting Bcl-XL and Simian virus 40 large T antigen to the osteoclast lineage in transgenic mice. J Clin Invest 102:88–97
Zhang Q, Badell IR, Schwarz EM et al (2005) Tumor necrosis factor prevents alendronate-induced osteoclast apoptosis in vivo by stimulating Bcl-xL expression through Ets-2. Arthritis Rheum 52:2708–2718
Shin S, Sung BJ, Cho YS et al (2001) An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40:1117–1123
Ahn KS, Sethi G, Chao TH et al (2007) Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products. Blood 110:2286–2295
Bharti AC, Aggarwal BB (2004) Ranking the role of RANK ligand in apoptosis. Apoptosis 9:677–690
Hodge MJ, Kirkland MA, Nicholson G (2007) Multiple roles of M-CSF in human osteoclastogenesis. J Cell Biochem 102:759–768
Felix R, Hofstetter W, Wetterwald A, Cecchini MG, Fleisch H (1994) Role of colony-stimulating factor-1 in bone metabolism. J Cel Bioch 55:340–349
Bharti AC, Takada Y, Shishodia S, Aggarwal BB (2004) Evidence that receptor activator of nuclear factor (NF)-kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB independent and TRAF6-dependent mechanism. J Biol Chem 279:6065–6076
Del Fattore A, Teti A, Rucci N (2008) Osteoclast receptors and signaling. Arch Biochem Biophys. doi:10.1016/j.abb.2008.01.011 (In press)
Lamoureux F, Richard P, Wittrant Y et al (2007) Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res 67:7308–7318
Hettinger K, Vikhanskaya F, Poh MK et al (2007) c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ 14:218–229
Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA (2003) M-CSF, TNFa and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 10:1165–1177
Acknowledgments
This research was supported by grants from MIUR FIRB-2001, MIUR COFIN-2005, STAMINA project, Fondazione Cassa di Risparmio di Padova e Rovigo. E.L. is a recipient of a fellowship from Fondazione Cassa di Risparmio di Cento.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penolazzi, L., Pocaterra, B., Tavanti, E. et al. Human osteoclasts differentiated from umbilical cord blood precursors are less prone to apoptotic stimuli than osteoclasts from peripheral blood. Apoptosis 13, 553–561 (2008). https://doi.org/10.1007/s10495-008-0188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0188-7