Abstract
Background & Aims: Intestinal adaptation in short bowel syndrome (SBS) consists of increased epithelial cell (EC) proliferation as well as apoptosis. Previous microarray analyses of intraepithelial lymphocytes (IEL) gene expression after SBS showed an increased expression of angiotensin converting enzyme (ACE). Because ACE has been shown to promote alveolar EC apoptosis, we examined if IEL-derived ACE plays a role in intestinal EC apoptosis.
Methods: Mice underwent either a 70% mid-intestinal resection (SBS group) or a transection (Sham group) and were studied at 7 days. ACE expression was measured, and ACE inhibition (ACE-I, enalaprilat) was used to assess ACE function.
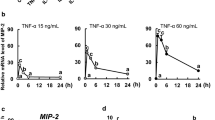
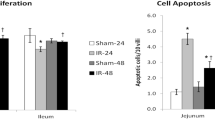
Results: IEL-derived ACE was significantly elevated in SBS mice. The addition of an ACE-I to SBS mice resulted in a significant decline in EC apoptosis. To address a possible mechanism, tumor necrosis factor alpha (TNF-α) mRNA expression was measured. TNF-α was significantly increased in SBS mice, and decreased with ACE-I. Interestingly, ACE-I was not able to decrease EC apoptosis in TNF-α knockout mice.
Conclusions: This study shows a previously undescribed expression of ACE by IEL. SBS was associated with an increase in IEL-derived ACE. ACE appears to be associated with an up-regulation of intestinal EC apoptosis. ACE-I significantly decreased EC apoptosis.
Similar content being viewed by others
References
Ray EC, Avissar NE, Sax HC. Growth factor regulation of enterocyte nutrient transport during intestinal adaptation. Am J Surg 2002; 183: 361–371.
Westergaard H. Short bowel syndrome. Seminars in Gastrointestinal Disease 2002; 13: 210–220.
Vanderhoof JA, Langnas AN, Pinch LW, Thompson JS, Kaufman SS. Short bowel syndrome. J Pediatr Gastroenterol Nutr 1992; 14: 359–370.
Petersen Y, Burrin D, Sangild P. GLP-2 has differential effects on small intestine growth and function in fetal and neonatal pigs. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1986–R1993.
Stern LE, Erwin CR, O'Brien DP, Huang F, Warner BW. Epidermal growth factor is critical for intestinal adaptation following small bowel resection. Microsc Res Tech 2000; 51: 138–148.
Thiesen A, Wild G, Tappenden K, et al. The locally acting glucocorticosteroid budesonide enhances intestinal sugar uptake following intestinal resection in rats. Gut 2003; 52: 252–259.
Sukhotnik I, Lerner A, Sabo E, et al. Effects of enteral arginine supplementation on the structural intestinal adaptation in a rat model of short bowel syndrome. Dig Dis Sci 2003; 48: 1346–1351.
O'Brien DP, Nelson LA, Kemp CJ, et al. Intestinal permeability and bacterial translocation are uncoupled after small bowel resection. J Pediatr Surg 2002; 37: 390–394.
Welters CF, Dejong CH, Deutz NE, Heineman E. Intestinal adaptation in short bowel syndrome. ANZ J Surg 2002; 72: 229–236.
Falcone RA, Jr, Shin CE, Erwin CR, Warner B. The adaptive intestinal response to massive enterectomy is preserved in c-SRC-deficient mice. J Pediatr Surg 1999; 34: 800–804.
Klein RM, McKenzie JC. The role of cell renewal in the ontogeny of the intestine. II. Regulation of cell proliferation in adult, fetal, and neonatal intestine. J Pediatr Gastroenterol Nutr 1983; 2: 204–228.
Sham J, Martin G, Meddings JB, Sigalet DL. Epidermal growth factor improves nutritional outcome in a rat model of short bowel syndrome. J Pediatr Surg 2002; 37: 765–769.
Gillingham MB, Dahly EM, Carey HV, Clark MD, Kritsch KR, Ney DM. Differential jejunal and colonic adaptation due to resection and IGF-I in parenterally fed rats. Am J Physiol—Gastrointest Liver Physiol 2000; 278: G700–G709.
Yang H, Wildhaber BE, Teitelbaum DH. 2003 Harry M. Vars Research Award. Keratinocyte growth factor improves epithelial function after massive small bowel resection. JPEN J Parenter Enteral Nutr 2003; 27: 198–206; discussion 206–207.
Tappenden K, Albin D, Bartholome A, Mangian H. Glucagon-like peptide-2 and short-chain fatty acids: A new twist to an old story. J Nutr 2003; 133: 3717–7320.
Chen Y, Chou K, Fuchs E, Havran W, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Aacd Sci USA 2002; 99: 14338–14343.
Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 2004; 172: 4151–4158.
Wildhaber B, Yang H, Coran A, Teitelbaum D. Gene alteration of intestinal intraepithelial lymphocytes in response to massive small bowel resection. Ped Surg Int 2003; 19: 310–315.
Wang R, Zagariya A, Ibarra-Sunga O, et al. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 1999; 276: L885–L889.
Siragy HM. AT(1) and AT(2) receptors in the kidney: Role in disease and treatment. Am J Kidney Dis 2000; 36: S4–S9.
Schiffrin EL. Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med 2002; 113: 409–418.
Ward PE, Sheridan MA, Hammon KJ, Erdos EG. Angiotensin I converting enzyme (kininase II) of the brush border of human and swine intestine. Biochem Pharmacol 1980; 29: 1525–1529.
Defendini R, Zimmerman EA, Weare JA, Alhenc-Gelas F, Erdos EG. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology 1983; 37: 32–40.
Erickson RH, Suzuki Y, Sedlmayer A, Song IS, Kim YS. Rat intestinal angiotensin-converting enzyme: purification, properties, expression, and function. Am J Physiol 1992; 263: G466–G473.
Danilov SM, Faerman AI, Printseva O, Martynov AV, Sakharov I, Trakht IN. Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry 1987; 87: 487–490.
Tamura K, Yokoyama N, Sumida Y, et al. Tissue-specific changes of type 1 angiotensin II receptor and angiotensin-converting enzyme mRNA in angiotensinogen gene-knockout mice. J Endocrinol 1999; 160: 401–408.
Skidgel RA, Erdos EG. Angiotensin I converting enzyme. Adv Exp Med Biol 1989; 247A: 25–28.
Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 1996; 183: 441–449.
Wilkins HR, Ohneda K, Keku TO, et al. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol 2002; 283: G457–G464.
Marshman E, Ottewell P, Potten C, Watson A. Caspase activation during spontaneous and radiationinduced apoptosis in the murine intestine. J Pathol 2001; 195: 285–292.
Kiristioglu I, Teitelbaum DH. Alteration of the intestinal intraepithelial lymphocytes during total parenteral nutrition. J Surg Res 1998; 79: 91–96.
Yang H, Fan Y, Finaly R, Teitelbaum DH. Alteration of intestinal intraepithelial lymphocytes after massive small bowel resection. J Surg Res 2003; 110: 276–286.
Yang H, Wildhaber B, Tazuke Y, Teitelbaum DH. 2002 Harry M. Vars Research Award. Keratinocyte growth factor stimulates the recovery of epithelial structure and function in a mouse model of total parenteral nutrition. JPEN J Parenter Enteral Nutr 2002; 26: 333–340; discussion 340–341.
Akbar AN. Life (and death) in the Fas lane [comment]. Gut 1998; 43: 5–6.
Odaka C, Mizuochi T. Angiotensin-converting enzyme inhibitor captopril prevents activation-induced apoptosis by interfering with T cell activation signals. Clin Exp Immunol 2000; 121: 515–522.
Costerousse O, Allegrini J, Lopez M, Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: Genetic polymorphism of expression in T-lymphocytes. Biochem J 1993; 290: 33–40.
Wang R, Ramos C, Joshi I, Zagariya A, Pardo A, Selman M, Uhal BD. Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol 1999; 277: L1158–L1164.
Yu G, Liang X, Xie X, Su M, Zhao S. Diverse effects of chronic treatment with losartan, fosinopril, and amlodipine on apoptosis, angiotensin II in the left ventricle of hypertensive rats. Int J Cardiol 2001; 81: 123–129; discussion 129–130.
Coopersmith CM, D OD, Gordon JI. Bcl-2 inhibits ischemia-reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol 1999; 276: G677–G686.
Jarboe M, Juno R, Bernal N, Knott A, Zhang Y, Erwin C, Warner B. Bax deficiency rescues resection-induced enterocyte apoptosis in mice with perturbed EGF receptor function. Surgery 2004; 136: 121–126.
Schindler R, Dinarello C, Koch K. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine 1995; 7: 526–533.
Fukuzawa M, Satoh J, Sagara M, et al. Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-alpha in vitro and in vivo. Immunopharmacology 1997; 36: 49–55.
Ortiz LA, Champion HC, Lasky JA, et al. Enalapril protects mice from pulmonary hypertension by inhibiting TNF-mediated activation of NF-kappaB and AP-1. Am J Physiol Lung Cell Mol Physiol 2002; 282: L1209–L1221.
Wang R, Ibarra-Sunga O, Verlinski L, Pick R, Uhal B. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 2000; 279: L143–L151.
Horiuchi M, Akishita M, Dzau V. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 1999; 33: 613–621.
Suzuki J, Iwai M, Nakagami H, et al. Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation 2002; 106: 847–852.
Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol 2003; 163: 2523–2530.
Sabri A, Levy B, Poitevin P, et al. Differential roles of AT1 and AT2 receptor subtypes in vascular trophic and phenotypic changes in response to stimulation with angiotensin II. Arterioscler Thromb Vasc Biol 1997; 17: 257–264.
Schiffrin E. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension 1992; 19: 1–9.
Naim HY. Human small intestinal angiotensin-converting enzyme: intracellular transport, secretion and glycosylation. Biochem J 1993; 296(Pt 3): 607–615.
Abreu-Martin MT, Palladino AA, Faris M, Carramanzana NM, Nel AE, Targan SR. Fas activates the JNK pathway in human colonic epithelial cells: Lack of a direct role in apoptosis. Am J Physiol 1999; 276: G599–G605.
Knott AW, O'Brien DP, Juno RJ, et al. Enterocyte apoptosis after enterectomy in mice is activated independent of the extrinsic death receptor pathway. Am J Physiol—Gastrointest Liver Physiol 2003; 285: G404–G413.
Tang Y, Swartz-Basile D, Swietlicki E, Yi L, Rubin D, Levin M. Bax is required for resection-induced changes in apoptosis, proliferation, and members of the extrinsic cell death pathways. Gastroenterology 2004; 126: 220–225.
Stern LE, Falcone RA, Jr, Huang F, Kemp CJ, Erwin CR, Warner BW. Epidermal growth factor alters the bax:bcl-w ratio following massive small bowel resection. J Surg Res 2000; 91: 38–42.
Stern LE, Huang F, Kemp CJ, Falcone RA, Jr, Erwin CR, Warner BW. Bax is required for increased enterocyte apoptosis after massive small bowel resection. Surgery 2000; 128: 165–170.
Stern LE, Falcone RA, Jr, Kemp CJ, Stuart LA, Erwin CR, Warner BW. Effect of massive small bowel resection on the Bax/Bcl-w ratio and enterocyte apoptosis. J Gastrointest Surg 2000; 4: 93–100.
Leri A, Claudio P, Li Q, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 1998; 101: 1326–1331.
Abbate A, Biondi-Zoccai G, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol 2002; 193: 145–151.
Kobara M, Tatsumi T, Kambayashi D, et al. Effects of ACE inhibition on myocardial apoptosis in an ischemia-reperfusion rat heart model. J Cardiovasc Pharmacol 2003; 41: 880–885.
Pang J, Xu R, Xu X, et al. Hexarelin protects rat cardiomyocytes from angiotensin II-induced apoptosis in vitro. Am J Physiol Heart Circ Physiol 2004; 286: H1063–H1067.
Kumar D, Zimpelmann J, Robertson S, Burns K. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol 2004; 96: e77–e79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wildhaber, B.E., Yang, H., Haxhija, E.Q. et al. Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis 10, 1305–1315 (2005). https://doi.org/10.1007/s10495-005-2138-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-2138-y