Abstract
Spatiotemporal wall temperature (Twall) distributions resulting from flame-wall interactions of lean H2-air and CH4-air flames are measured using phosphor thermometry. Such measurements are important to understand transient heat transfer and wall heat flux associated with various flame features. This is particularly true for hydrogen, which can exhibit a range of unique flame features associated with combustion instabilities. Experiments are performed within a two-wall passage, in an optically accessible chamber. The phosphor ScVO4:Bi3+ is used to measure Twall in a 22 × 22 mm2 region with 180 µm/pixel resolution and repetition rate of 1 kHz. Chemiluminescence imaging is combined with phosphor thermometry to correlate the spatiotemporal dynamics of the flame with the heat signatures imposed on the wall. Measurements are performed for lean H2-air flames with equivalence ratio Φ = 0.56 and compared to CH4-air flames with Φ = 1. Twall signatures for H2-air Φ = 0.56 exhibit alternating high and low-temperature vertical streaks associated with finger-like flame structures, while CH4-air flames exhibit larger scale wrinkling with identifiable crest/cusp regions that exhibit higher/lower wall temperatures, respectively. The underlying differences in flame morphology and Twall distributions observed between the CH4-air and lean H2-air mixtures are attributed to the differences in their Lewis number (CH4-air Φ = 1: Le = 0.94; H2-air Φ = 0.56: Le = 0.39). Findings are presented at two different passage spacings to study the increased wall heat loss with larger surface-area-to-volume ratios. Additional experiments are conducted for H2-air mixtures with Φ = 0.45, where flame propagation was slower and was more suitable to resolve the wall heat signatures associated with thermodiffusive instabilities. These unstable flame features impose similar wall heat fluxes as flames with 2–3 times greater flame power. In this study, these flame instabilities occur within a small space/time domain, but demonstrate the capability to impose appreciable heat fluxes on surfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Flame-wall interaction (FWI) is an important topic within gas turbine and internal combustion (IC) engines, where a flame is bounded by solid surfaces. As a flame interacts with the wall, it imposes a significant heat flux on the wall, which subsequently weakens the flame. This mutual interaction defines the flame quenching distance from the wall, the near-wall emission formation, and the thermal fatigue imposed on the chamber walls (Dreizler and Böhm 2015). Modern engines are designed to be smaller and operate with higher power densities, as these conditions are conducive for reducing fuel consumption and CO2 emissions (Szybist et al. 2021; Leach et al. 2020). However, in these downsized combustors, the surface-area to volume ratio increases such that the flame is more exposed to walls, and higher pressures are such that more chemical reactions occur near surfaces (Johe et al. 2022). These trends are making FWI an increasingly important topic within combustion research.
Another prevailing topic in combustion research is the transition to low-carbon and zero-carbon fuels (Leach et al. 2020; Masri 2021; Onorati et al. 2022). In this area, hydrogen (H2) has received significant attention as a potential fuel that can help decarbonize transportation and power generation sectors (Onorati et al. 2022; Taamallah et al. 2015; Verhelst and Wallner 2009). Substantial research efforts have demonstrated the successful implementation of baseline IC engines converted to operate with hydrogen fuel (Leite et al. 2023a; 2023b) and the use of hydrogen enrichment with traditional jet fuels in model gas turbine combustors (Agostinelli et al. 2022; Aniello et al. 2022; Chterev and Boxx 2021; Miniero et al. 2023). Such research has yielded promising results in terms of hydrogen feasibility, while achieving low carbon, soot, and nitric oxide emissions.
Although hydrogen has emerged as a promising fuel for reducing carbon emissions, several aspects need to be addressed regarding its implementation. Among these aspects are FWI and heat transfer to surfaces. Hydrogen exhibits high heating values, high adiabatic flame temperatures, and has one of the highest flame speeds among the available fuels (Konnov et al. 2018). Flame quenching distance is inversely proportional to flame temperature and flame speed (Ferguson and Keck 1977; Lavoie 1978), such that wall heat fluxes are often greater with hydrogen. Lean mixtures of hydrogen can reduce flame temperature and flame speed, but lean H2 flames are subject to thermodiffusive instabilities. These combustion instabilities occur when the Lewis number (Le) is low such that for a wrinkled lean hydrogen flame the local mass flux of H2 into the reaction zone is greater than the heat flux out of the reaction zone. This preferential diffusion promotes local H2 enrichment along a corrugated flame surface (Berger et al. 2019), which increases the local flame speed and the local flame temperatures (Frouzakis et al. 2015, Berger et al. 2022; Howarth and Aspden 2022; Howarth et al. 2023). These higher temperature flame regions can cause concerns of high wall heat fluxes and thermal fatigue of surfaces.
Wall temperature is a key component to understand FWI and wall heat fluxes. Thermocouples, heat flux gauges, and phosphor thermometry are common experimental techniques used to measure wall temperature during FWI (e.g., Padhiary et al. 2023; Boust et al. 2007; Aldén et al. 2011). In terms of hydrogen FWI, experimental measurements of wall temperature are sparse, with only a few exceptions reported in the literature (Guiberti et al. 2015; Feuk et al. 2022; Nau et al. 2023). Direct numerical simulations (DNS) have played an instrumental role in understanding FWI for H2 flames (e.g., Owston et al. 2007; Gruber et al. 2010; Mari et al. 2016; Lai et al. 2022; Zhao et al. 2022; Zhu et al. 2024). The majority of DNS studies employ isothermal or adiabatic wall boundary conditions. While these simplified conditions provide the means to perform DNS in a reasonable manner, these conditions can deviate from true FWI conditions. One-dimensional simulations employing conjugate heat transfer (CHT) have revealed significant differences in FWI characteristics compared to isothermal and adiabatic conditions (Mari et al. 2016). To understand the effects of wall heat transfer, Agostinelli et al. (2022) employed CHT in Large Eddy Simulations (LES) of a swirl burner to resolve the increased bluff-body surface temperature induced by shorter flame lengths from H2 enrichment. The higher wall temperature led to increased heat transfer to the gaseous fluid, which modified the flame/flow dynamics and was argued to play an underlying role in the observed thermoacoustic instabilities in experiments. These findings reveal the important effects of wall heat transfer associated with H2 flames.
The wide-spread use of hydrogen in gas turbines and IC engines is still in development, and with this, hydrogen combustion heat transfer models are also still under development (Demuynck et al. 2011; Michl et al. 2016). The lack of experimental data on wall temperature and boundary conditions impedes the development of such models. Consequently, most engine combustion simulations are not fully predictive with respect to wall heat transfer. Most engine-related wall models utilize empirical constants that are adjusted to match experimental conditions, such as gas pressure or heat release (Rakopoulos et al. 2010). Recent studies employing hydrogen in IC engines revealed that wall model empirical constants are appreciably different for hydrogen than hydrocarbon fuels (Leite et al. 2023a, b). Detailed measurements of wall temperature during hydrogen FWI are needed to improve the predictive capability of wall models.
Within gas turbine and IC engines, flames impinge onto individual chamber surfaces, as well as within two-wall engine passages (e.g., annular slots, flame holders, crevices, etc.). Heat transfer and flame quenching are often most severe in two-wall passages where the flame is more exposed to walls. The heat transfer in these passages can affect acoustic instabilities in gas turbine combustors (Ketelheun et al. 2013; Mejia et al. 2015; Agostinelli et al. 2022) and the unburned fuel emissions and temperature stratification in IC engines (Alkidas 1999; Dronniou and Dec 2012; Peterson et al. 2013, 2014). Measuring wall temperature and FWI in two-wall passages is often not performed due to limited measurement access. Numerical simulations have highlighted the need for detailed wall temperature measurements in various narrow passage configurations to validate simulations and better understand the underlying FWI physics (Janas et al. 2015; Agostinelli et al. 2022). Such statements are further emphasized for hydrogen flames, which can penetrate further into narrow passages owing to their high flame speed and smaller flame thicknesses.
In this work, we experimentally measure wall temperatures (Twall) associated with FWI of hydrogen within a two-wall narrow passage. Phosphor thermometry is used to obtain spatially resolved 2D Twall measurements during FWI. The thermographic phosphor used is bismuth-doped scandium vanadate (ScVO4:Bi3+), which offers high temperature sensitivity and measurement precision (Abram et al. 2020; Ojo et al. 2022). Experiments are performed in a fixed-volume chamber (FVC) designed to emulate a simplified piston engine geometry and is equipped with an optically accessible two-wall passage. Phosphor thermometry is performed simultaneously with chemiluminescence imaging to understand the spatiotemporal features of Twall and the flame front. Experiments are performed with lean H2-air mixtures (equivalence ratio, Ф = 0.56), as well as stoichiometric CH4-air mixtures. Findings from CH4 flames are presented to highlight the unique spatiotemporal Twall signatures associated with the intrinsically different flame structure between H2 and CH4 flames. Findings are presented for two passage spacings to study the wall heat transfer as the surface-are to volume ratio increases. This work concludes by presenting additional experiments with leaner H2-air mixtures (Φ = 0.45) to investigate wall heat flux signatures associated with thermodiffusive instabilities. These unstable flame features are shown to impose similar magnitudes of wall heat flux as flames with 2–3 times greater flame power.
2 Experimental
2.1 Fixed Volume Chamber (FVC)
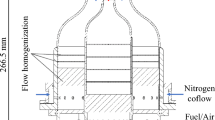
Experiments were performed in an optically accessible fixed volume chamber (FVC) dedicated for FWI and heat transfer studies. A schematic of the FVC is shown in Fig. 1. The FVC features a test section (150 cm3) and a back pressure section (6 cm3). These sections are separated by a 6 mm thick orifice plate with 81 equidistant holes each with 0.5 mm diameter. The test section emulates a simplified IC engine geometry at top-dead center. At the far end of the test section, a two-walled crevice region exists, which is designed to emulate an enlarged piston crevice. Fused silica windows are used to provide optical access into the test section and crevice region. All metal components of the FVC are comprised of 304 stainless-steel.
The chamber operation has been described by Escofet-Martin et al. (2021) and Ojo et al. (2021) and is briefly described here. The contents inside the FVC are initially evacuated to 20 mbar. A homogenous fuel–air mixture is then introduced into the chamber until an initial pressure of 2 bar is reached. The fuel–air mixture is ignited by the spark plug. Heat release initiates an exponential pressure rise as the flame propagates towards the opposite end of the chamber before entering the crevice region. At a preselected chamber pressure, a dump-valve with 8 ms response time is activated to evacuate the gas inside the chamber. The exhaust flow exiting the FVC is choked by the orifice plate to create an exponential pressure decay that closely mirrors the exponential pressure rise. The chamber operation is intended to simulate a pressure time history similar to that of an IC engine albeit at lower pressures.
Similar to our previous works (Ojo et al. 2022, 2023), this work focuses on Twall measurements and flame front imaging within the crevice passage of the FVC. The crevice region is 70 mm deep (\(\Delta y\)) and 158 mm wide (\(\Delta z\)). The crevice is characterized by two walls: one is the front fused silica window surface, and the other is the 304 stainless steel wall (see Fig. 1a). These walls are not externally cooled or heated. The distance between the walls is referred to as the crevice spacing (CS). The placement of the front window can be adjusted to select a CS between 0.5–5.0 mm (Ojo et al. 2022). The front window with size of 60 \(\times\) 110 mm2 (\(\Delta y\times \Delta z\)) provides optical access to the majority of the crevice region, as well as 2 mm (\(\Delta y\)) above the crevice within the test section.
In this work, three different homogeneous fuel–air mixtures were investigated: (1) a H2-air mixture with equivalence ratio of Ф = 0.56, (2) a CH4-air mixture with Ф = 1.0, and (3) a H2-air mixture with Ф = 0.45. The first two mixtures were chosen in an attempt to provide flames with a comparable flame power. Flame power (\({Q}_{\Sigma }\)) represents a laminar heat release reference, and is often used to normalize the wall heat flux in FWI studies (Poinsot and Veynante 2005; Dreizler and Böhm 2015). \({Q}_{\Sigma }\) is defined as:
where \({\rho }_{u}\) is the unburned gas density, \({S}_{L}\) is the unstretched laminar flame speed, \({C}_{p}\) is the heat capacity of the unburned mixture, \({T}_{ad}\) is the adiabatic flame temperature, and \({T}_{u}\) is the unburned gas temperature.
Table 1 reports the operating conditions explored in this work and their corresponding \({Q}_{\Sigma }\), \({S}_{L}\), \({T}_{ad}\), and Lewis number (Le). These properties were calculated from Cantera (1D flame simulation), which utilized the GRI 3.0 Mechanism (Smith et al. 2011). Properties are reported for time-averaged pressure/temperature conditions when the flame is within the measurement region of interest (ROI). The fast heat release of hydrogen made it challenging to control the chamber pressure during the time of FWI at the measurement location. The pressure affects the value of \({S}_{L}\), which consequently affects the value of \({Q}_{\Sigma }\). A H2-air mixture with Ф = 0.56 provided repeatable chamber operation, but yielded a flame power 45% higher than the flame power for CH4-air Ф = 1.0. Leaner H2-air mixtures produced larger variability in the flame arrival time at the measurement ROI. The variability in the flame arrival produced a variability of the chamber pressure, which made it difficult to control variables such as \({S}_{L}\) and \({Q}_{\Sigma }\). It was decided that a repeatable operating condition was preferred over ensuring an iso-condition between the two mixtures. As such, the H2-air Ф = 0.56 operation was chosen for qualitative comparison to the CH4-air Ф = 1.0 operation. Given that the mixtures do not have an iso-condition (i.e., similar value of \({Q}_{\Sigma }\), \({S}_{L}\), or \({T}_{ad}\)), comparison of the findings between these mixtures is performed with caution.
The H2-air Ф = 0.56 mixture has a low Lewis number of 0.39, for which the flame can undergo thermodiffusive instabilities (Berger et al. 2022). However, under the H2-air Ф = 0.56 condition, the flame propagation is fast and it is difficult to capture a series of consecutive images of the flame passing through the ROI. Therefore, the leaner H2-air mixture of Ф = 0.45, shown in Table 1, was chosen to slow the H2 flame propagation to resolve the spatiotemporal Twall distribution for a series of consecutive images as the flame propagates in the ROI. Under these conditions, it was more conducive to capture the temporal sequence of thermodiffusive instabilities associated with lean H2-air flames.
As shown in Table 1, experiments were performed with two different crevice spacings of 2 mm and 1.2 mm. The CS prescribes the surface-area to volume ratio (SA/V) in the crevice, which largely governs gas/wall heat exchange (Ojo et al. 2022). The corresponding SA/V values for the 2 mm and 1.2 mm spacings are 1.02 mm−1 and 1.69 mm−1, respectively. SA/V values for real piston engines (including optical engines) can range from 4 to 10 mm−1 (Heywood 1988; Cheng et al. 1993; Baum et al. 2014; Janas et al. 2015). The SA/V values in this work are smaller than real engines due to a larger crevice spacing, which gives a larger volume. Although SA/V values are not matched to that of a piston engine, this work provides fundamental measurements to demonstrate the effect of SA/V on the wall heat loss.
Figure 2 shows the pressure curves from individual experiments at each operating condition. The chamber pressures were measured using a piezo-electric pressure transducer installed in the FVC. The pressure at which the dump-valve was activated for each operating condition is shown in Fig. 2. The dump valve activation initiates the evacuation of the chamber gases, which in part influences the peak pressures and symmetry of the pressure curves. For each operation condition, the variation of the pressure curve was less than 5% from those shown in Fig. 2. The higher heat release of H2 is obvious from the pressure trace; the pressure rise for H2-air Ф = 0.56 conditions is ~ 1.37 bar/ms, while the pressure rise is ~ 0.105 bar/ms for the CH4-air Ф = 1.0 conditions. The peak pressures are also 2–3 bar higher for the H2-air Ф = 0.56 operation. The leaner H2-air mixture of Ф = 0.45 reaches peak pressures similar to the CH4-air mixture. The lower peak pressure is attributed to the leaner mixture and the timing of the dump valve relative to the flame progression.
The pressure curves at different CS reveal the influence of heat transfer on the available heat release. The pressure differences between a CS of 2.0 mm and 1.2 mm are noticeable for both the H2 and CH4 mixtures. Higher peak pressures are recorded for the 2.0 mm spacing, indicating higher heat release due to a larger volume available for gases to burn within the crevice region. The longer duration of FWI for the CH4-air condition yields more time for heat loss, which is largely attributed to more noticeable differences in the pressure curves at different CS.
2.2 Phosphor Thermometry
The phosphor used for surface thermometry was bismuth-doped (1%) scandium vanadate (ScVO4:Bi3+). ScVO4:Bi3+ features a fast phosphorescence decay with a lifetime < 3 µs at room temperature, which makes it ideal for kHz-rate measurements (Ojo et al. 2022). ScVO4:Bi3+ has a broadband emission centred around 640 nm with emission extending from ~ 400 nm to the infrared above 780 nm. Figure 3a shows an example of the phosphorescence decay signal of ScVO4:Bi3+ at room temperature. ScVO4:Bi3+ also exhibits a high temperature sensitivity, which can yield single-shot temperature precision less than 0.5 K in the temperature range of 295–335 K (Abram et al. 2020; Ojo et al. 2022, 2023). The phosphor used in this work was synthesized by Phosphor Technology Ltd, and has been used previously in FWI environments (Ojo et al. 2022). Further details of the phosphor’s luminescence properties can be found in Escofet-Martin (2022).
ScVO4:Bi3+ powder was mixed with a temperature-resistant HPC binder (ZYP coatings) and was applied using an airbrush to produce a 30 mm × 36 mm (\(\Delta y\times \Delta z\)) coating on the metal wall in the crevice region (see Fig. 1). The coating thickness was measured at various spatial locations using a coating thickness gauge (PCE Instruments; PCE-CT-65). The average coating thickness was \(\Delta x\) = 6 ± 1 µm.
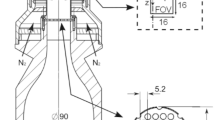
Two different experimental setups were used for phosphor thermometry. The first setup measured Twall at a single location (0D) at 10 kHz repetition rate (see Fig. 1b), while the second setup measured spatially-resolved 2D Twall distributions at 1 kHz repetition rate (see Fig. 1c). The former was used to better resolve the temporal evolution of Twall, which was not well resolved at 1 kHz for H2-air Ф = 0.56 conditions.
For the 0D measurements, the phosphor coating was excited by a frequency-quadrupled Nd:YAG laser (Edgewave) emitting light at 266 nm. Using a pinhole, the laser beam covered an area of about 7 mm2 on the phosphor coating and was placed at the central \(\Delta y\) location on the phosphor coating. The laser operated at 10 kHz and had a laser energy of 0.004 mJ/pulse. A photomultiplier tube (PMT; R955HA, Hamamatsu) and a 50 mm lens were used to collect the phosphorescence decay. A 607 ± 18 nm filter was mounted to the lens to detect the peak of the ScVO4:Bi3+ emission. An oscilloscope (Tektronix MDO3054) was used to acquire the detected signals. Luminescence decays were processed on a single-shot basis to evaluate individual luminescence lifetimes. Calibration of the lifetime with temperature was performed by measuring the temperature of a phosphor-coated aluminium-block, which was heated on a hotplate up to 373 K and then allowed to cool slowly to ambient. The block was equipped with a thermocouple attached to its surface, providing a measure of the surface temperature.
For the 2D measurements, the phosphor coating was excited by a 400 nm laser beam generated from a Ti:Sapphire laser (Astrella, Coherent) emitting light at 800 nm with 35 fs pulse width. A BBO crystal was used to convert the laser to the 400 nm wavelength. It should be noted that while nanosecond excitation of thermographic phosphors is conventional for laser-based phosphor thermometry applications, femtosecond excitation of ScVO4:Bi3+ does not impede the exploitation of its emission or the lifetime for thermometry (Ojo et al. 2023, Escofet-Martin et al. 2022). A − 75 mm lens was used to expand the 400 nm laser beam to a diameter of ~ 35 mm onto the phosphor coating. The laser operated at 1 kHz and had a laser energy of 0.15 mJ/pulse at 400 nm. A high-speed camera (VEO 710L, Phantom) was used to image the phosphorescence from the laser excitation. A 50 mm Nikon lens (f/1.2) fitted with a 607 ± 18 nm bandpass filter was mounted onto the camera. The camera imaged a 22 \(\times\) 22 mm2 region of interest on the phosphor coating with a projected pixel size of 180 µm/pixel.
The lifetime-based intensity ratio method (Ojo et al. 2023; Burnford et al. 2024) was used to measure surface temperature from the phosphorescence signal. The high-speed camera operated at a frame rate of 60 kHz, with an interframe time of 400 ns and camera exposure of 16.35 µs. For each 1 kHz laser pulse, three camera frames were acquired. The first frame provided a background image (BG). The second frame (F1) imaged the first 0.7 µs of the phosphorescence decay, while the third frame (F2) imaged the remaining phosphorescence signal within the 16.35 µs exposure. Figure 3a shows these frames in relation to the phosphor decay. For each decay signal, the lifetime-based intensity ratio (IR) image was evaluated as:
A flat-field correction was applied to correct for spatial variation of the laser beam. A time-averaged IR image recorded at room temperature was used to provide a flat-field correction ratio image (R):
Calibration of R as a function of temperature was performed in a similar manner as the 0D measurements. The same phosphor-coated aluminium-block was placed on a hotplate and then removed to cool to ambient temperature. The block was equipped with a thermocouple attached to its surface. While recording the temperature from the thermocouple, phosphor images were recorded and the ratio image R was determined at select temperatures to provide a calibration curve. Figure 3d shows the calibration curve depicting the temperature dependence of the spatially averaged ratio \({\text{R}}_{\text{mean}}\). This calibration curve was used to convert \(\text{R}\) to surface temperature on a pixel-by-pixel basis. Figure 3d also shows the temperature dependence of the phosphor lifetime used for 0D measurements. It is shown that \({\text{R}}_{\text{mean}}\) exhibits a strong dependence on temperature as the phosphorescence lifetime decreases with temperature due to thermal quenching. Further details and various applications of the lifetime-based intensity ratio method are described in Ojo et al. (2023), Burnford et al. (2024), Padhiary et al. (2024), and Morrisset et al. (2024).
The uncertainty of the 2D phosphor thermometry measurements is reported as the spatial standard deviation evaluated from a 37 mm2 region in the calibration experiments. For the range of temperatures presented in the findings in Sect. 3 (290–320 K), the uncertainty is reported as 0.6–0.7 K. This uncertainty is slightly higher than reported in our previous work which reported an uncertainty of 0.3 -0.4 K for the same temperature range (Ojo et. al. 2023). The lower uncertainty in Ojo et al. (2023) was achieved by applying a 2 × 2 binning to the phosphor images, which was not performed in the current work.
2.3 Flame Front Imaging
Chemiluminescence images were recorded simultaneously with phosphor images to visualize the flame front in conjunction with Twall. A high-speed camera (VEO 710L, Phantom) equipped with a 50 mm Nikon lens (f/1.2) was used to image chemiluminescence. For 0D Twall measurements, the camera directly imaged the chemiluminescence in the crevice region through the 110 mm × 60 mm area of the front window (Fig. 1b). For 2D Twall measurements, the camera imaged the same ROI, but a dichroic mirror (DMLP505L, Thorlabs) was used to reflect light less than 505 nm for chemiluminescence imaging, while light above 505 nm was transmitted through the dichroic mirror to the phosphor camera (see Fig. 1c). The imaged region for chemiluminescence included the 22 × 22 mm2 ROI captured by the phosphor images. The chemiluminescence images had a projected pixel size of 180 µm/pixel, matching that of the phosphor images. The chemiluminescence images and phosphor images were spatially mapped in MATLAB such that both images were spatially mapped with respect to each other.
In both setups, for CH4-air experiments, a 433 ± 14 nm bandpass filter was used in front of the camera lens to image CH* chemiluminescence. For H2-air experiments, a 310 ± 10 nm bandpass filter was placed in front of the camera lens in an attempt to image OH*. However, the lean H2-air mixtures in combination with non-optimal detection system in the UV (e.g., 70% filter transmission, combined ~ 1% 310 nm transmission from the Nikon lens and camera spectral throughput at 310 nm) yielded weak OH* signals. Therefore, the filter was removed, which allowed the camera to detect the OH* signal (with estimated ~ 1% detection efficiency), as well as an infrared emission from vibrationally excited H2O at wavelengths greater than 700 nm and a weak “blue continuum” as reported by Schefer et al. (2009). Without the use of the bandpass filter and accounting for the spectral throughput of our imaging system, it is estimated that the chemiluminescence signals are mainly from the H2O* emissions in the infrared, while OH* and the “blue continuum” contribute to a lesser extent. The infrared emissions are captured due to the reflectance of the dichroic mirror, which opens up above 800 nm. The resulting chemiluminescence signals provide sufficient information of the global flame features, which are helpful when interpreting the Twall images.
For 0D Twall measurements, the chemiluminescence camera operated at a 2 kHz frame rate with an exposure time of 500 µs. For the 2D Twall measurements involving the CH4-air experiments, the camera also operated at a 2 kHz frame rate and 500 µs exposure time, such that chemiluminescence was captured either during or after the phosphorescence. The 433 ± 14 nm bandpass filter removed most of the laser light, therefore, it was possible to excite the phosphor and capture its phosphorescence during the 500 µs exposure of the CH* image. With this timing, the phosphorescence images could be easily correlated with CH* images. For H2-air experiments, because a filter was not used to remove laser light, the camera was operated at a 1 kHz frame rate such that the 500 µs exposure of chemiluminescence was designated to occur 30 µs after the laser excitation to avoid capturing laser light into the chemiluminescence image. Figure 3b, c show these exposure timings relative to the phosphorescence decay. While this work did not employ the same chemiluminescence exposure timing relative to the phosphorescence decay for CH4 and H2 experiments, the flame images in both cases provided valuable information to understand the Twall and flame dynamics.
3 Results and Discussion
3.1 Overview of Flame Visualization in Crevice
Before evaluating the spatiotemporal features of Twall, it is first helpful to describe the general flame characteristics in the crevice for the CH4 and H2 mixtures. As the flame reaches the crevice region, it propagates into the narrow passage of the crevice. The flame traveling in the crevice emulates a closed Hele-Shaw like configuration (Al Sarraf et al. 2019). Figure 4 shows chemiluminescence images of the flame in the crevice at select timings for the CH4-air Φ = 1.0 and H2-air Φ = 0.56 mixtures. Images are shown with CS = 2 mm and the flame is shown within the entire span of the crevice to describe its overall geometry relative to the 22 × 22 mm2 phosphor ROI where Twall is measured. The difference in flame propagation speed is evident by the timing of the images, where t = 0 is the start of experiment (t = 8 ms is start of ignition). The H2-flame arrives nearly three times faster to the ROI than the CH4-flame. The difference in flame propagation speed is also evident by the differences in the pressure curves shown in Fig. 2.
For the CH4-air mixture, the slow flame propagation is more conducive for chemiluminescence to resolve the detailed flame features. In the crevice, the CH4-flame exhibits large-scale wrinkling, which varies spatially and temporally. In Fig. 4a, the flame shown by the CH* signal appears as a thickened or elongated band. As discussed in our previous work (Ojo et al. 2022, 2023), this band is best explained when imaging in a plane orthogonal to the wall. Such an orthogonal view is shown in Fig. 4b for the CH4-air mixture (note that CH* images in Fig. 4a and b correspond to different experiments). The flame in Fig. 4b depicts an arrow-head shape with a leading edge centered within the crevice and trailing edges located closest to the walls. This arrow-head flame shape ultimately gives the elongated CH* band in Fig. 4a, where the leading and trailing edges are explicitly labeled. As shown in our previous work (Ojo et al. 2021, 2022, 2023), Twall increases sharply at the trailing edge of the CH* signal because it is the flame’s trailing edge that is closest to the wall. Therefore, under the CH4-air conditions employed in this work, Twall conforms to the flame’s trailing edge geometry, which is clearly seen in Fig. 4a.
For the H2-air Ф = 0.56 mixture, the features of the flame front are not well resolved within the chemiluminescence images. This is primarily due to the speed of the flame relative to the long camera exposure of 500 µs. Within the 500 µs exposure, the flame travels ~ 10 mm on average, which makes it challenging to capture a “still” image of the flame front. The resulting chemiluminescence image shown in Fig. 4c resolves a smeared-like flame, where the leading edge of the flame is shown further downstream of the sharp increase in wall temperature. Laser-induced fluorescence (LIF) imaging of OH radicals with 100 ns exposure time would be better suited to capture a “still” image of the flame. Unfortunately, OH-LIF was unavailable for these experiments.
Despite not capturing a “still” image of the H2 flame features, the chemiluminescence images for H2 reveal distinct features worth noting. In particular, elongated vertical streak patterns are shown in the chemiluminescence image in Fig. 4c. The corresponding Twall image also shows a vertical streak pattern with intermittent high- and low-temperature streaks. These vertical streaks appear consistent with what is often referred to as “flame fingers”, which are relatively large-scale wrinkling features often present for lean premixed H2-flames (Fernández-Galisteo et al. 2018; Berger et al. 2019). These flame fingers can be induced by hydrodynamic instabilities (Frouzakis et al. 2015), which can be exacerbated by elevated gas pressures (Attili et al. 2021), or can be introduced by the redirection of the flame as it enters the crevice (i.e., disturbance of the flame). While it is not possible to match the spatiotemporal attributes of the flame and Twall for the H2-air Ф = 0.56 mixture, the chemiluminescence images provide sufficient insight into the Twall features resolved from phosphor thermometry.
It should also be mentioned that the Twall images do not exhibit a smearing effect associated with the rapid flame propagation of H2 flames. The phosphorescence lifetime decay of the ScVO4:Bi3+ phosphor used in this work is less than 3 µs at room temperature, and the lifetime decreases with increasing temperature (Abram et al. 2020; Ojo et al. 2022). With a flame progression of 20 m/s on average, the flame will move 60 µm within the maximum time of the phosphorescence lifetime. This 60 µm flame movement is below the projected pixel size of 180 µm/pixel. Therefore, the phosphor images suitably capture the wall temperature when the H2 flame is relatively stationary.
3.2 Spatiotemporal Flame and T wall Dynamics with CS = 2 mm
This section presents temporal sequences of chemiluminescence and Twall images to describe the wall temperature distributions associated with the CH4 and H2 flame behavior in the crevice. Figure 5 shows select images from 1 kHz imaging sequences for the CH4-air Φ = 1 and H2-air Φ = 0.56 cases with CS = 2 mm. Although Fig. 5 shows images from individual experiments, the flame and Twall features shown are consistent with all experiments performed at these conditions. The red rectangle shown in the chemiluminescence images corresponds to the 22 × 22 mm2 region where Twall is measured. In the Twall images, spatially-averaged surface temperatures are extracted from rectangles R1, R2, and R3 (each of 2 × 2 mm2 area) to help quantify and compare spatial features of Twall between the CH4 and H2 flames. R1 is fixed in space and represents a traditional point-wise phosphor thermometry measurement, similar to what is reported in Sect. 3.3. R2 and R3 are selectively placed to track the hottest and coldest Twall locations (respectively) near the flame front. Identifying the exact location of the flame front for H2 cases is more challenging. For H2 cases, R2 and R3 are placed in hot and cold streaks, respectively. A rectangle is not shown if the particular feature is not present within the image.
Chemiluminescence and Twall image sequence for CH4-air Ф = 1 and H2-air Ф = 0.56 cases with CS = 2 mm. The CH4-air Φ = 1 case is shown in the top two rows, while the H2-air Φ = 0.56 case is shown in the bottom two rows. Rectangles report the spatially-averaged Twall at a fixed location (R1), at the highest temperature region (R2), and at the lowest temperature region near the flame (R3)
In the case of CH4-air Φ = 1, the flame exhibits large-scale wrinkling with distinct flame crests and cusps, which are defined to be convex and concave towards the reactants, respectively. The temperature images show that Twall conforms to the distinct flame geometry, including the large and fine scale wrinkling. Images show that Twall is highest immediately behind the flame’s trailing edge, and monotonically decreases with increasing distance from the flame. In Fig. 5, it is also shown that Twall is consistently highest behind flame crests, while flame cusps exhibit appreciably colder temperatures. Rectangles R2 and R3 track these features throughout the image sequence.
The lower temperatures at flame cusps has been discussed in our previous work (Ojo et al. 2023). As discussed in that work, the effects of the hydrodynamic instability associated with a wrinkled flame front is hypothesized to cause the lower temperature associated with flame cusps. The hydrodynamic instability (or the Darrieus-Landau instability) arises from thermal expansion across the flame front (Matalon 2007). This instability often creates an intrinsic flow pattern in relation to a wrinkled flame front. Figure 6 shows an exemplar flow field emulated after the work of Folga et al. (2017), which describes the flow features of a cusp-like flame resulting from the Darrieus-Landau instability for a flame propagating into a quiescent propane-air mixture. The generic flow field in Fig. 6 is presented to highlight common features resulting from the Darrieus-Landau instability. For a wrinkled flame front, the density jump across the flame front causes the streamlines ahead of flame crest regions to diverge, reducing the velocity in that region. The flow is consequently channelled into the flame cusp regions and the flow velocity increases in this region. It is hypothesized that the accelerated flow into the cusp region can lead to the lower Twall in flame cusp regions. For example, the increased unburned gas velocity is anticipated to slow the flame propagation in cusp regions. Slower flame propagation can be seen in the image sequence in Fig. 5, where the trough of the flame cusps propagates less quickly than other flame regions, making the flame cusp more pronounced (see also movie sequences in supplemental material). Longer residence times can be associated with the slower flame propagation, for which the cusp region can have more time for heat transfer at the wall. In addition, the flow velocity channelled into cusp regions can increase the strain rate at the surface of the flame cusp. The increased heat loss and strain rate can weaken the flame and lead to lower temperatures imparted onto the wall in cusp regions.
Example flow field emulated after the work of Folga et al (2017) describing the qualitative flow features associated with a cusp-like flame resulting from the Darrieus-Landau instability
Referring back to Fig. 5, at t = 52 ms, Twall is shown to be significantly colder at the flame cusp, almost as if a flame is not present at the trough of the cusp. Such features were also reported in Ojo et al. (2023). As the flame cusp becomes more pronounced it is suspected that the unburned gas is strongly channelled into the cusp region and this colder gas impinges onto the wall. It is suggested that this flow directly cools the wall or diverts the flame further from the wall, which results in colder Twall at the trough of the flame cusp. The supplemental material shows the high-speed movie of the full image sequence, which shows further details of the flame/wall dynamics. Additional measurements and numerical simulations emulating our conditions would are needed to explore the aforementioned hypotheses further.
For the H2-air Ф = 0.56 case shown in Fig. 5, the Twall signatures resemble a similar vertical streak pattern similar to those observed in the chemiluminescence images. As mentioned in Sect. 3.1, the flame has progressed further in the chemiluminescence images than the "temperature front" in the Twall images because the phosphor images are acquired before the chemiluminescence image and the flame progation is fast (~ 20 m/s). At t = 17 ms, the flame is near the bottom of the ROI. The border where Twall increases from its ambient value of ~ 293 K to a higher temperature is noticeably jagged in comparison to the CH -air flame (~ 20 m/s). Directly downstream of the jagged border, Twall is comparable in magnitude to the CH4-air case with Twall ranging from 307 – 312 K. Several hot vertical streaks are shown to extend upwards from this region. For example, a hot temperature streak extends from R2 to R1 and the temperatures in these rectangles are very similar despite having a large \(\Delta y\) separation. Overall, the walled regions covered by the flame exhibit a higher average Twall in the ROI than the CH4-air Φ = 1 cases. The higher average Twall is a direct consequence of the faster heat release (see Fig. 2) and less time for heat to dissipate in the wall from the time it was first exposed to burned gases. For example, it takes less than 2 ms for the H2-air flame to travel the \(\Delta y\) domain of the ROI, while it takes ~ 8 ms for the CH4-air flame to travel the same distance. For H2-air Φ = 0.56, the walled regions downstream the flame at t = 17 ms have just been exposed to the flame and the majority of this region exhibits temperatures above 308 K. For CH4-air Φ = 1, the equivalent wall region exposed to the flame and exhibiting Twall in excess of 308 K corresponds to a thin 1–2 mm region directly downstream the flame front.
At t = 18 ms within the H2-air Φ = 0.56 image sequence, the leading part of the flame is beyond the phosphor ROI and the bottom of the image contains the highest temperatures across its lower boarder with R2 reaching ~ 2 K higher than that reported for the CH4-air case. The remainder of the ROI exhibits an alternating pattern of hot and cold vertical streaks of width between 2–3 mm. This vertical streak pattern continues at t = 19 ms, but overall, Twall is colder due to more time for heat transfer and the streaks are shown to curve in the opposite direction as those shown at t = 18 ms. The change in curvature is likely due to hydrodynamic fluctuations associated with opening of the dump valve or the flame reaching the extent of the crevice region.
Overall, the H2-air Φ = 0.56 case exhibits wildly varying spatiotemporal flame and Twall features. In the burned gas region, Twall can exhibit strong variations in temperature depending on the transient nature of the hot and cold streaks. The transient spatiotemporal nature of these streaks can best be seen by the high-speed video in the supplemental material. The image sequences shown in Fig. 5 reveal that the 1 kHz imaging rate is too slow to resolve the transient Twall features sufficiently for the H2-air Φ = 0.56 case. This is in contrast to the CH4-air Φ = 1 case, where the 1 kHz imaging rate resolves the transient Twall features in appropriate detail.
3.3 Temporally-Resolved 0D T wall Measurements at 10 kHz
The 1 kHz imaging rate is insufficient to resolve the temporal flame-wall dynamics for the H2-air Φ = 0.56 mixtures. Twall images show large changes between successive measurements, which makes it difficult to understand FWI and measure the transient wall heat flux. To resolve the temporal evolution of Twall in sufficient detail, experiments employing point-wise (0D) phosphor thermometry at 10 kHz were performed. As mentioned in Sect. 2.2, the 0D measurement resolves Twall within a 7 mm2 area in the central \(\Delta y\) location on the phosphor coating.
Figure 7a, b shows the ensemble-average Twall evolution for the CH4-air Φ = 1 and H2-air Φ = 0.56 cases with CS = 2 mm. The ensemble-average is reported from 7 experiments at each condition and the standard deviation (σ) is shown by the shaded region. In Fig. 7a, Twall is reported as a function of the experiment time duration, where t = 0 refers to the start of experiment. Figure 7b reports the same data, but now Twall is reported relative to the flame arrival time, where t* = 0 refers to the time the flame arrives at the phosphor measurement location, which is determined as the time Twall rises to 10% of its maximum temperature.
a Ensemble-average Twall with respect to experiment duration, t = 0 refers to the start of ignition. b Ensemble-average Twall with respect to the time the flame arrives at the measurement location (t* = 0). Ensemble-average is reported from 7 repeated experiments. The shaded region indicates one standard deviation (σ). c Single-experiment wall heat flux calculated from Duhamel integral. Crevice spacing is 2 mm for the results shown
In Fig. 7b, it is shown that Twall increases more quickly for H2 than for CH4 cases. For H2, Twall increases from its ambient temperature (~ 294 K) to its maximum at a heating rate of 34.6 K/ms, while the heating rate for CH4 is 14.5 K/ms. The faster rise in Twall is expected due to the faster heat release of H2 as shown by the pressure curves in Fig. 2. Despite the faster temperature rise, the maximum Twall reached in each case is similar. After Twall reaches a maximum, the H2 cases are shown to exhibit larger temperature variations, which are arguably due to the spatially varying hot and cold temperature streaks in the burned gas region. As Twall decreases after FWI, Fig. 7a shows that Twall cools faster for H2 than for CH4. For example, Twall decreases from its maximum to a near ambient value of 298 K at a rate of 0.71 K/ms for H2, while this cooling rate is 0.33 K/ms for CH4. The faster wall cooling is a result of faster gas expansion during the exhaust event. As shown in Fig. 2, the H2 cases exhibit a sharper pressure decrease from the higher peak pressure. The rapid gas expansion will reduce gas temperature more quickly, which promotes faster wall cooling for H2 cases.
The Twall measurements resolved at a sufficient temporal resolution enable the calculation of the wall heat flux (Qwall). In this work, Qwall is calculated based on the time-dependent surface temperature of a thermally semi-infinite solid. Qwall is calculated assuming 1D transient heat conduction using a Duhamel integral to provide the time evolution of Qwall given by (Taler and Duda 2006):
In Eq. 4, the variables\(\rho\),\({C}_{p}\), and \(k\) are the density, specific heat capacity, and thermal conductivity of the solid material, respectively, while \(t\) is time and \(\overline{\tau }\) is the time variable of integration. It is often appropriate to consider the physical properties of the phosphor when calculating the wall heat flux (Witkowski and Rothamer 2023). The density and heat capacity of the host material ScVO4 are \(\rho\) = 3650 kg/m3 (Persson 2014) and \({C}_{p}\) = 693 J/kgK (Gavrichev et al. 2012). The thermal conductivity of ScVO4 was not found in the literature. Thermal conductivity values for phosphors such as HoVO4 and Pr:YAG have been reported in the literature as \(k\) = 2 W/mK (Kondrat’eva et al. 2021; Witkowski and Rothamer 2023). Given that ScVO4 and HoVO4 belong to the same group of orthovanadate compounds with similar tetrahedral zircon-type structure, and the fact that phosphors of different compounds can yield similar conductivity values, the value of \(k\) = 2 W/mK is used to calculate Qwall in Eq. 4.
Figure 7c shows temporally resolved Qwall traces for individual experiments with H2-air Φ = 0.56 and CH4-air Φ = 1. The H2-air Φ = 0.56 case exhibits a faster rise in Qwall and reaches a higher Qwall,max value of 1.39 MW/m2, while the CH4-air Φ = 1 case reaches a Qwall,max of 0.99 MW/m2. The difference in Qwall,max is nearly directly proportional to the difference in flame power (QΣ) reported for the two mixtures (see Table 1). The maximum non-dimensional heat flux (Qwall,max/QΣ) is 0.22 and 0.24 for the H2-air Φ = 0.56 and CH4-air Φ = 1 cases, respectively. The similar Qwall,max/QΣ values indicates that the wall heat loss is similar for each flame in these experiments. These trends are qualitatively similar for all experiments performed in this work under these conditions. It is also worth noting that the measurements for H2-air Φ = 0.56 are still likely under-resolved even at the 10 kHz repetition rate. As shown in Fig. 7c, only a few datapoints are resolved during the Qwall rise for H2-air Φ = 0.56. As such, Qwall,max may be under-reported in Fig. 7c. Nonetheless, the 10 kHz data provide a suitable understanding of the transient Twall response, which can be used in combination with the 2D Twall images.
3.4 Effect of Surface Area to Volume (SA/V) on Heat Transfer
Although the 1 kHz repetition rate of the 2D measurements is ill-suited to resolve the fine temporal progression of the flame and Twall for the H2-air Φ = 0.56 cases, access to 2D information provides useful insight into the FWI and its associated heat transfer to the wall. This section presents temporal sequences of flame and Twall images at a smaller crevice spacing of 1.2 mm to demonstrate the increased wall heat loss due to a larger surface area to volume ratio. Ojo et al. (2022) evaluated Twall as a function of CS for CH4 flames using 0D measurements at 6 kHz. This work complements that analysis by describing the effect of heat transfer on the spatial Twall distribution for both CH4 and H2 flames. The surface area to volume ratio (SA/V) for CS = 1.2 mm is 1.69 mm−1. The images shown in this section will be compared to those presented in Sect. 3.2 with CS = 2 mm (SA/V = 1.02 mm−1).
Figure 8 shows the temporal sequence of the flame and Twall for CH4-air Φ = 1 and H2-air Φ = 0.56 cases with CS = 1.2 mm. It is noted that CH* signals for the CH4-air flame are thinner in several regions, particularly in regions that pertain to flame cusps. The thinner CH* regions occur when the flame progression slows and the distance between the flame’s leading edge and trailing edge (see Fig. 4b) is small. These features have been discussed in detail in Ojo et al. (2022, 2023).
Chemiluminescence and Twall image sequences for CH4-air Ф = 1 and H2-air Ф = 0.56 cases with CS = 1.2 mm. The top two rows show CH4-air Φ = 1, while the bottom two rows show H2-air Φ = 0.56. Rectangles report the spatially-averaged Twall at a fixed location (R1), at the highest temperature region (R2), and at the lowest temperature region near the flame (R3). The CH4 sequence is shown 2 ms later than the CS = 2 mm case in Fig. 5 because the flame arrives ~ 2 ms later in the ROI
Overall, the spatial features of the flame and Twall for CS = 1.2 mm in Fig. 8 are qualitatively similar to those shown for CS = 2 mm in Fig. 5. For CH4, the flame exhibits large-scale wrinkling and Twall is highest at the trailing edge of the flame. In addition, Twall is consistently higher for flame crest regions compared to flame cusp regions as was shown in Fig. 5 for the CH4 flame. However, for CS = 1.2 mm, the temperatures in R2 and R3 regions are often 1–3 K lower than the CS = 2 mm case. In addition, wall temperatures downstream of the flame front are shown to cool more rapidly for CS = 1.2 mm than for CS = 2 mm. For example, within the image sequence shown, Twall reported within R1 cools at a rate of 1.13 K/ms for CS = 1.2 mm, while for CS = 2 mm, the wall cools at a rate of 0.58 K/ms. That is, for an increase in SA/V of ~ 65%, the cooling rate is shown to increase by ~ 95%. The disproportionate increase in heat loss with SA/V is consistent with Ojo et al. (2022), where cooling rates were measured using 0D phosphor thermometry for CS ranging from 3.5 mm to 1.2 mm.
For the H2-air Ф = 0.56 image sequence with CS = 1.2 mm, the Twall images exhibit hot and cold temperature streaks, which emulate the vertical streak pattern in the chemiluminescence images. The size of the temperature streaks is narrower for CS = 1.2 mm than for CS = 2 mm, which may be a consequence of the narrower CS. As the flame travels through the ROI at t = 17 ms, the wall temperature distribution is similar to that shown for CS = 2 mm with the temperatures in the hot and cold streaks being comparable in value. Again, the walled regions covered by the flame have a higher average Twall in the ROI compared to the CH4-air Ф = 1 images, which is a result of the faster heat release and less time for heat to dissipate in the wall for H2-air Ф = 0.56. After the flame travels past the ROI, the increased wall heat loss due to the smaller CS becomes more apparent. For example, Twall exhibits lower overall temperatures and the highest temperatures are 3–4 K colder than those at CS = 2 mm.
The greater cooling effect at larger SA/V (i.e., narrower CS) is described further in Fig. 9, which shows PDF distributions of Twall at select timings within image sequences of Figs. 5 and 8. The PDFs evaluate temperatures above 300 K to isolate wall locations that have been heated by the flame.
For the CH4-air Φ = 1 mixtures in Fig. 9a, PDFs are extracted when the flame is approximately half-way into the ROI. This timing corresponds to t = 48 ms for the CS = 2 mm case and t = 50 ms for the CS = 1.2 mm case. The slightly later arrival of the flame occurs for CS = 1.2 mm because the flame experiences more resistance penetrating into the narrower CS as described in Ojo et al. (2022). For the PDFs at t = 48 ms and 50 ms, Twall extends up to 312 K and the distributions from 309 to 312 K are very similar for each CS. These temperatures represent the higher temperatures in close proximity to the flame’s trailing edge. Temperatures below 309 K represent regions further away from the flame front where temperature dissipates. The PDFs at t = 48 ms and 50 ms exhibit large differences below 309 K, where CS = 1.2 mm shows a substantial shift towards colder Twall, which demonstrates the greater cooling associated with higher SA/V. The PDFs 4 ms later for each CS show a shift towards colder temperatures, which is largely due to flame regions exiting the imaging region. The greater cooling effect continues at these later timings with PDFs at t = 52 ms and 54 ms exhibiting a larger shift towards colder temperatures, particularly for CS = 1.2 mm.
For the H2-air Φ = 0.56 cases shown in Fig. 9b, the PDFs at t = 17 ms exhibit a very similar distribution for CS = 1.2 and 2 mm, where only a mild shift towards colder temperatures exists for CS = 1.2 mm. The Twall distribution at t = 17 ms is similar at both CSs despite the difference in SA/V because the heat imparted occurs very rapidly due to the fast flame propagation, and little time has progressed for heat transfer to have a strong effect on Twall for each CS. As more time progresses, the wall cools for both cases, but the cooling is greater for CS = 1.2 mm. The PDF for CS = 1.2 mm shows a 1-2 K shift towards colder temperatures revealing the faster cooling rate for the narrower SA/V.
The qualitative trends described in Fig. 9 are consistent for other image timings within the temporal sequence. However, in order to provide a fair comparison between each CS for a given fuel, the flame should penetrate a similar distance into the field of view so that a similar area downstream the flame is evaluated for each CS.
3.5 FWI Dynamics Associated with Thermodiffusive Instabilities of Lean H 2 Flames
The 1 kHz recording rate was too slow to capture temporally correlated Twall images for the H2-air Ф = 0.56 mixtures presented in Sects. 3.2 and 3.4. To slow the flame progression, a leaner H2-air mixture with Ф = 0.45 was used for which the 1 kHz repetition rate was sufficient to capture a temporally correlated progression of Twall imposed by the flame. At these lean mixtures, the flame is also highly susceptible to exhibit thermodiffusive instabilities, which will alter the flame dynamics (Frouzakis et al. 2015, Berger et al. 2019, Howarth and Aspden 2022). The thermodiffusive instability is a peculiar feature of lean H2 mixtures with low Lewis numbers (Le), where preferential diffusion of H2 leads to variations of the local equivalence ratio along the flame front. When this occurs, hydrogen diffuses into hotter regions, where the local reaction rate increases and the flame can reach super adiabatic temperatures (i.e., temperatures above the adiabatic temperature at the global equivalence ratio) (Frouzakis et al. 2015; Howarth et al. 2023). This preferential diffusion can therefore cause concern of high heat fluxes imposed onto surfaces.
This section presents image sequences for H2-air mixtures with Ф = 0.45 to study the Twall features and the consequential wall heat fluxes associated with thermodiffusive instabilities of lean H2-air flames. Experiments were conducted with CS = 1.2 mm, which aided in slowing the flame progression in the crevice. While thermodiffusive instabilities are studied for the H2-air Ф = 0.45 mixture, it should be emphasized that thermodiffusive instabilities may also occur for H2-air Ф = 0.56 cases. The Lewis numbers for both cases are low, and thermodiffusive instabilities may develop in both conditions. However, the fact that H2-air Ф = 0.56 cases provide a single image where the leading flame is detected in the ROI per experiment makes it very challenging to obtain definitive evidence of these unstable flame features and their influence on wall heat transfer. The slower propagation speed associated with the H2-air Ф = 0.45 CS = 1.2 mm operation allows us to visualize the development of a low Le flame in a more appropriate manner and record its unique heat signatures on the wall.
Figure 10 shows chemiluminescence and Twall images at select times as the flame progresses through the crevice region for a single experiment with H2-air Ф = 0.45. Due to the leaner mixture, the chemiluminescence has a low signal-to-noise ratio (SNR). Despite the lower SNR, it is possible to visualize the leading flame front location, while the corresponding Twall images from phosphor thermometry provide insight into the geometrical flame features near the wall. In contrast to the H2-air Ф = 0.56 cases, the chemiluminescence and Twall images do not reveal long finger-like flame structures, but instead the Twall images show small cellular-like temperature structures at the leading “temperature front”. These cellular-like temperature structures take time to develop as the flame penetrates into the crevice region. At t = 26 ms, the cellular structures are relatively small in the Twall image with temperatures reaching as high as 307 K within the cellular region. As time progresses, these cellular structures become more pronounced and the wall temperatures associated with these structures increase, reaching as high as 314 K. Although these high temperature regions occupy small regions on the wall, it is remarkable that Twall reaches values as high as the CH4-air Ф = 1.0 and H2-air Ф = 0.56 CS = 1.2 mm cases, despite having a flame power that is 2.5 to 3.6 times lower, respectfully.
The cellular structures seen in the Twall images in Fig. 10 are congruent with trademark features of a thermodiffusively unstable flame (Yu et al. 2017; Berger et al. 2019, 2022; Howarth and Aspden 2022; Howarth et al. 2023). Given the fact that Twall images can closely resemble the near-wall flame features, it is argued that the spatial features of Twall are qualitatively similar to the near-wall flame distribution as the flame becomes thermodiffusively unstable. The regions of high temperature are considered to be flamelets with locally richer H2 concentrations and higher flame temperatures. These flamelets, while being hotter, will also burn closer to the wall due to their higher flame speed. Both of these aspects will contribute to the higher wall temperatures. This description is qualitatively similar to DNS findings from Ghai et al. (2023), where it was reported that positively curved flame regions of low Le flames yield smaller flame quenching distances, which were a result of locally richer and hotter flame structures that could withstand greater heat loss before quenching.
With the increasing demand for hydrogen in combustion-based applications, it is important to understand the implications of the thermodiffusive flame instabilities on the wall heat flux. Using the 2D Twall images, which are now sufficiently resolved temporally, Qwall is calculated according to Eq. 4 at each image pixel. Qwall,max is calculated using a 3-point temporal moving average to reduce measurement noise. Figure 11 (top row) reports the maximum wall heat flux (Qwall,max) recorded at each pixel as the flame travels through the ROI. Qwall,max is shown for the full image sequences corresponding to CH4-air Φ = 1 with CS = 2.0 mm, CH4-air Φ = 1 with CS = 1.2 mm, and H2-air Φ = 0.45 with CS = 1.2 mm. The bottom row of Fig. 11 reports the maximum heat flux in its non-dimensional form (Qwall,max/QΣ), where QΣ is the average flame power reported in Table 1. Qwall,max/QΣ provides a measure of the energy transferred to the wall relative to the theoretical available energy of the flame at the global mixture conditions. Although mixture stratification is expected when thermodiffusive instabilities occur, QΣ associated with the global mixture condition is used for normalization, which helps emphasize the deviation resulting from thermodiffusive instabilities.
The Qwall,max images in Fig. 11 describe the spatially distributed wall heat flux associated with distinct flame features. The CH4-air cases reveal the Qwall,max relative to the large-scale flame wrinkling. For example, the CH4-air Ф = 1 CS = 2.0 mm image shows the trajectory of two flame crests and two flame cusp regions as the flame travels vertically downwards. The Qwall,max image for CH4-air Ф = 1 CS = 1.2 mm shows the dynamics of two flame crests merging and the formation of a large flame cusp in the center of the ROI. The trajectories of flame crest regions consistently exhibit the highest Qwall,max reported in the image (~ 0.75 MW/m2), resulting from higher Twall associated with flame crests (see Figs. 5 and 8). The trajectories of flame cusp regions exhibit Qwall,max values that are approximately half of crest regions (0.4–0.5 MW/m2), which result from the lower Twall in flame cusp regions. The maximum Qwall values reported in Fig. 11 are ~ 25% lower than those reported in Fig. 7, which is a result of the lower temporal resolution of 1 kHz. Although Qwall,max values in Fig. 11 are under-resolved, the images provide a useful spatial comparison between the different operating conditions. For the CH4-air Ф = 1 cases, both crevice spacings exhibit qualitatively similar heat flux distribution. The region of zero Qwall,max at the bottom of the ROI for the CH4-air Ф = 1 CS = 1.2 mm case is a result of the flame not progressing over this area before the majority of the gas is evacuated during the exhaust phase.
The Qwall,max image for the H2-air Ф = 0.45 shows the heat flux associated with the smaller-scale cellular flame structures. Qwall,max is much lower over the majority of the ROI, except in the regions where flame instabilities result in locally high Twall values. In these regions, Qwall,max reaches the same magnitude as the CH4-air Ф = 1 cases, which is noteworthy given the lower global flame power for H2-air Ф = 0.45. The bottom row of Fig. 11 shows the normalized heat flux, which emphasizes the stark contrast in Qwall relative to the available global flame power between the CH4-air Ф = 1 and H2-air Ф = 0.45 cases. For the CH4-air Ф = 1 mixture, Qwall,max/QΣ reach values up to 0.17 and is consistent for each CS. However, for the H2-air Ф = 0.45 mixture, the majority of the ROI exhibit Qwall,max/QΣ values above 0.2 (i.e., above the highest value reported for CH4-air Ф = 1), and the maximum Qwall,max/QΣ values reach 0.43. As mentioned previously, the Qwall,max/QΣ values reported are anticipated to be under-estimated due to the lower temporal resolution of 1 kHz, but this underestimation is expected to be the same for each case.
From the evaluation of Fig. 11, it is clear that the overall Qwall imposed onto the wall is greater for CH4-air Ф = 1 than for H2-air Ф = 0.45.Footnote 1 For CH4-air Ф = 1, nearly all wall regions exposed by the flame reach a value between 0.65 and 0.75 MW/m2, while for H2-air Ф = 0.45, only a small region where burning is most intense reaches such values. However, it should be mentioned that the experimental conditions employed in this work are modest in comparison to technically relevant environments that would employ lean hydrogen mixtures. For example, in this work, there is a limited amount of time for the thermodiffusive instabilities to develop within the chamber. Evidence of the thermodiffusive instabilities exists for a 10–15 ms time period in these experiments. The thermodiffusive instabilities appear to reach their most intense stage around t = 35 ms, where Twall and Qwall reach their peak. At t = 37 ms, the chamber pressure is already decreasing rapidly as gases are evacuated during the exhaust phase (see Fig. 2). The decreasing pressure induces gas expansion, which reduces heat release and weakens the thermodiffusive instabilities. Consequently, Twall and Qwall reduce substantially after t = 35 ms.
In the experiments, if the chamber pressure was maintained, it is reasonable to anticipate that the thermodiffusive instabilities would persist and that Qwall,max values could occupy a larger region on the wall. Qwall,max values may even exceed those reported if the instabilities had more time to develop. This scenario may exist in gas turbine engines, where a flame burns more continuously. In reciprocating engines, the timescales of pressure rise/decay are comparable or faster than those in the present study. However, the timescales of flame development would be much faster at the higher pressures and temperatures achieved in an IC engine. Therefore, for lean burn H2 IC engines, thermodiffusive instabilities may require less time to develop to a mature stage, and may influence heat fluxes on engine surfaces.
Although the modest operating conditions employed in this work do not allow us to evaluate the impact of thermodiffusive instabilities on FWI and wall heat flux for all scenarios, the findings clearly demonstrate the ability of thermodiffusive instabilities to impose heat fluxes well beyond their expected value onto surfaces. This information is important when considering thermal fatigue and the cooling processes required in next-generation hydrogen applications. The experimental measurements presented are intended to help guide numerical simulation work studying FWI and hydrogen fuels. The presented measurements, as well as our those presented in our previous work in the FVC provide a valuable database of transient FWI and heat transfer processes (Escofet-Martin et al. 2021; Ojo et al. 2021, 2022, 2023). These measurements include gas- and solid-phase temperature measurements, and are available for interested modelling partners.
4 Conclusions
Phosphor thermometry was used to measure the spatiotemporal distribution of wall temperature (Twall) resulting from flame-wall interactions of lean H2-air flames and stoichiometric CH4-air flames. Experiments were performed in a two-walled crevice passage in an optically accessible fixed volume chamber. The phosphor ScVO4:Bi3+ was used, which offers high temperature sensitivity and measurement precision. The lifetime-based intensity ratio method was used to image Twall in a 22 × 22 mm2 wall region with 180 µm/pixel resolution at a repetition rate of 1 kHz. Point-wise (0D) phosphor thermometry was also performed at 10 kHz using the lifetime method to resolve the evolution of Twall and transient wall heat flux (Qwall) with better temporal precision. Chemiluminescence imaging was performed simultaneously with Twall measurements to correlate the spatiotemporal heat signatures measured on the wall with their corresponding flame features.
Experiments were first performed for CH4-air Ф = 1 and H2-air Ф = 0.56 mixtures with a crevice spacing (CS) of 2 mm. The CH4 flames exhibit large scale wrinkling for which Twall conforms remarkably well to the flame geometry. Twall images reveal that flame crest regions yield higher Twall and wall heat flux (Qwall) than flame cusp regions. The lower Twall in flame cusp regions is argued to be caused by the hydrodynamic instability that can strain the flame and lead to longer residence times. These features will weaken the heat signatures of the flame imposed on the wall. For H2-air Φ = 0.56, the flame exhibited elongated finger-like structures, which yielded a vertically alternating high and low temperature streak pattern within the Twall images. Due to the faster heat release for the H2 flame, a larger region of the wall was exposed to the flame in a shorter time duration. With less time for the heat to dissipate in the wall, a larger fraction of the region of interest exhibited elevated wall temperatures (e.g., Twall > 308 K). For the slower CH4-air flames, these elevated temperature regions are restricted to a 1–2 mm region downstream of the flame front, as more time elapses allowing heat to dissipate beyond this region. The underlying differences in Twall distribution, flame morphology, and flame propagation between CH4-air and lean H2-air mixtures are attributed to the differences in their Lewis numbers.
Experiments were performed with a smaller CS of 1.2 mm and compared to CS = 2 mm to study the wall heat transfer associated with larger surface are to volume ratios (SA/V). The spatiotemporal flame and Twall features were in qualitative agreement between CS = 1.2 mm and 2 mm, but a clear shift towards colder Twall existed for the smaller crevice spacing. The wall cooling rate was disproportionally larger than the increase in SA/V, which demonstrated the substantial wall heat loss associated with higher SA/V.
Leaner H2-air mixtures with Ф = 0.45 were employed with CS = 1.2 mm where the flame progression was slow enough to be captured by the 1 kHz repetition rate of the phosphor measurements. The 2D Twall images captured the intrinsic FWI features of a thermodiffusively unstable flame as it progressed through the imaged region. The temperature front exhibited strong cellular structures, which grew in size and increased in temperature as the flame progressed in the crevice. These instabilities imposed wall temperatures and wall heat fluxes as high as those seen for flames with 2–3 times greater flame power. While these flame instabilities occur within a small space/time domain in this study, they demonstrate the unique ability to impose appreciable heat flux on surfaces. Such aspects are important to consider in terms of surface durability and required cooling demand for lean hydrogen flame applications.
Data Availability
Data will be made available upon request.
Notes
Note that H2-air Ф = 0.56 mixture explored in this work impose higher wall heat fluxes than the CH4-air Ф = 1 mixture (see Fig. 7c). However, it was not possible to resolve Qwall from the 2D images for H2-air Ф = 0.56, due to the poorer temporal resolution associated with the faster flame propagation. H2-air Φ = 0.56 cases are therefore not considered in this discussion.
References
Abram, C., Panjikkaran, I.W., Ogugua, S.N., Fond, B.: ScVO4:Bi3+ thermographic phosphor particles for fluid temperature imaging with sub-oC precision. Opt. Lett. 45, 3893–3896 (2020)
Agostinelli, P.W., Laera, D., Chterev, I., Boxx, I., Gicquel, L., Poinsot, T.: On the impact of H2-enrichment on flame structure and combustion dynamics of a lean partially-premixed turbulent swirling flame. Combust. Flame 241, 112120 (2022)
Al Saffar, E., Almarcha, C., Quinard, J., Radisson, B., Denet, B., Garcia-Ybarra, P.: Darrieus-Landau instability and Markstein numbers of premixed flames in Hele-Shaw cell. Proc. Combust. Inst. 37, 1783–1789 (2019)
Aldén, M., Omrane, A., Richter, M., Särner, G.: Thermographic phosphors for thermometry: a survey of combustion applications. Prog. Energy Combust. Sci. 37, 422–461 (2011)
Alkidas, A.C.: Combustion-chamber crevices: the major source of engine-out hydrocarbon emissions under fully warmed conditions. Prog. Energy Combust. Sci. 25, 253–273 (1999)
Aniello, A., Poinsot, T., Selle, L., Schuller, T.: Hydrogen substitution of natural-gas in premixed burners and implications for blow-off and flashback limits. Int. J. Hydrogen Energy 47, 33067–33081 (2022)
Attili, A., Lamioni, R., Berger, L., Kleinheinz, K., Lapenna, P.E., Pitsch, H., Creta, F.: The effect of pressure on the hydrodynamic stability limit of premixed flames. Proc. Combust. Inst. 38, 1973–1981 (2021)
Baum, E., Peterson, B., Böhm, B., Dreizler, A.: On the validation of LES applied to internal combustion engine flows: part 1: comprehensive experimental database. Flow Turbul. Combust. 92, 269–297 (2014)
Berger, L., Kleinheinz, K., Attili, A., Pitsch, H.: Characteristic patterns of thermodiffusively unstable premixed lean hydrogen flames. Proc. Combust. Inst. 37, 1879–1886 (2019)
Berger, L., Attili, A.: Pitsch, H.: Intrinsic instabilities in premixed hydrogen flames: parametric variation of pressure, equivalence ratio, and temperature. Part 2—non-linear regime and flame speed enhancement, Combust. Flame 240, 111936 (2022)
Boust, B., Sotton, J., Labuda, S.A., Bellenoue, M.: A thermal formulation for single-wall quenching of transient laminar flames, Combust. Flame 149, 286–294 (2007)
Burnford, J., Morrisset, D., Ojo, A.O., Hadden, R.M., Law, A., Peterson, B.: Assessment and application of phosphor thermometry for spatially resolved surface temperature measurements during downward flame spread. Fuel 365, 131201 (2024)
Cheng, W.K., Hamrin, D., Heywood, J.B., Hochgreb, S., Min, K., Norris, M.: An overview of hydrocarbon emission mechanisms in spark-ignition engines, SAE Technical Paper 932708 (1993)
Chterev, I., Boxx, I.: Effect of hydrogen enrichment on the dynamics of a lean technically premixed elevated pressure flame. Combust. Flame 225, 149–159 (2021)
Demuynck, J., De Paepe, M., Huisseune, H., Sierens, R., Vancoillie, J., Verhelst, S.: On the applicability of empirical heat transfer models for hydrogen combustion engines. Int. J. Hydrogen Energy 36, 975–984 (2011)
Dreizler, A., Böhm, B.: Advanced laser diagnostics for an improved understanding of premixed flame-wall interactions. Proc. Combust. Inst. 35, 37–64 (2015)
Dronniou, N., Dec, J.: Investigation of development of thermal stratification from the near-wall regions to the bulk-gas in an HCCI engine with planar imaging thermometry. SAE Int. J. Engines 5(3), 1046–1074 (2012)
Escofet-Martin, D., Ojo, A.O., Mecker, N.T., Linne, M.A., Peterson, B.: Simultaneous 1D hybrid fs/ps rotational CARS, phosphor thermometry, and CH* imaging to study transient near-wall heat transfer processes. Proc. Combust. Inst. 38, 1579–1587 (2021)
Escofet-Martin, D., Ojo, A.O., Peterson, B.: Ultrafast multi-photon excitation of ScVO4:Bi3+ for luminescence thermometry. Opt. Lett. 47, 13–16 (2022)
Ferguson, C., Keck, J.C.: On laminar flame quenching and its application to spark ignition engines. Combust. Flame 28, 197–205 (1977)
Fernández-Galisteo, D., Kurdyumove, V.N., Ronney, P.: Analysis of premixed flame propagation between two closely-spaced parallel plates, Combust. Flame 190, 133–145 (2018)
Feuk, H., Pignatelli, F., Subash, A., Bi, R., Szász, R.Z., Bai, X.S., Lörstad, D., Richter, M.: Impact of methane and hydrogen-enriched methane pilot injections on the surface temperature of a scaled-down burner nozzle measured using phosphor thermometry. Int. J. Turbomach. Propuls. Power. 7(4), 29 (2022)
Folga, N., Creta, F., Matalon, M.: The turbulent flame speed for low-to-moderate turbulence intensities: hydrodynamic theory vs. experiments, Combust. Flame 175, 155–169 (2017)
Frouzakis, C.E., Folga, N., Tomboulides, A.G., Altantzis, C., Matalon, M.: Numerical study of unstable hydrogen/air flames: shape and propagation speed. Proc. Combust. Inst. 35, 1087–1095 (2015)
Gavrichev, K.S., Ryumin, M.A., Tyurin, A.V., Gurevich, V.M., Solov’ev, O.I., Komissarova, L.N.: Thermodynamic functions of ScVO4 at temperatures from 0 to 350 K. Inorg. Mater 48, 845–850 (2012)
Ghai, S.K., Ahmed, U., Chakraborty, N.: Effect of fuel Lewis number on wall heat transfer during Oblique flame-wall interaction of premixed flames within turbulent boundary layers, flow. Turbul. Combust. 111, 867–895 (2023)
Gruber, A., Sankaran, R., Hawkes, E.R., Chen, J.H.: Turbulent flame-wall interaction: a direct numerical simulation study. J. Fluid Mech. 658, 5–32 (2010)
Guiberti, T.F., Durox, D., Scouflaire, P., Schuller, T.: Impact of heat loss and hydrogen enrichment on the shape of confined swirling flames. Proc. Combust. Inst. 35, 1385–1392 (2015)
Heywood, J.B.: Internal Combustion Engine Fundamentals. McGraw-Hill Series in Mechanical Engineering, McGraw-Hill, New York (1988)
Howarth, T.L., Aspden, A.J.: An empirical characteristic scaling model for freely-propagating lean premixed hydrogen flames. Combust. Flame 237, 111805 (2022)
Howarth, T.L., Hunt, E.F., Aspden, A.J.: Thermodiffusively-unstable lean premixed hydrogen flames: phenomenology, empirical modelling, and thermal leading points. Combust. Flame 253, 112811 (2023)
Janas, P., Ribeiro, M.D., Kempf, A., Schild, M., Kaiser, S.A.: Penetration of the flame into the top-land crevice—large eddy simulation and experimental visualization, SAE Technical Paper 2015-01-1907 (2015)
Johe, P., Zentgraf, F., Greifenstein, M., Steinhausen, M., Hasse, C., Dreizler, A.: Characterization of flow field and combustion dynamics in a novel pressurized side-wall quenching burner using high-speed PIV/OH-PLIF measurements. Int. J. Heat Fluid Flow 94, 108921 (2022)
Ketelheun, A., Kuenne, G., Janicka, J.: Heat transfer modeling in the context of large edge simulation of premixed combustion with tabulated chemistry. Flow Turbul. Combust. 91, 867–893 (2013)
Kondrat’eva, O.N., Nikiforova, G.E., Tyurin, A.V., Smirnova, M.N., Gavrichev, K.S.: Thermodynamic and thermophysical properties of holmium orthovanadate. J. Alloys Compounds. 859, 157786 (2021)
Konnov, A.A., Mohammad, A., Kishore, V.R., Kim, N.I., Prathap, C., Kumar, S.: A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 68, 197–267 (2018)
Lai, J., Ahmed, U., Klein, M., Chakraborty, N.: A comparison between head-on quenching of stoichiometric methane-air and hydrogen-air premixed flames using direct numerical simulations. J. Heat Fluid Flow 93, 108896 (2022)
Lavoie, G.A.: Correlations of combustion data for SI engine calculations—laminar flame speed, quench distance and global reaction rates. SAE Trans. 780229, 1015–1033 (1978)
Leach, F., Kalghatgi, G., Stone, R., Miles, P.: The scope of improving the efficiency and environmental impact of internal combustion engines. Transp. Eng. 1, 100005 (2020)
Leite, C.R., Laignel, M., Brequigny, P., Borée, J., Foucher, F.: Experimental combustion analysis in a gasoline baseline hydrogen-fueled internal combustion engine at ultra-lean conditions, SAE Technical Paper 2023-24-0073 (2023a)
Leite, C.R., Oung, R., Brequigny, P., Borée, J., Foucher, F.: Combustion cycle-to-cycle variation analysis in diesel baseline hydrogen-fueled spark-ignition engines, SAE Technical Paper 2023-01-0290 (2023b)
Mari, R., Cuenot, B., Rocchi, J.P., Selle, L., Duchaine, F.: Effect of pressure on hydrogen/oxygen coupled flame-wall interaction. Combust. Flame 168, 409–419 (2016)
Masri, A.R.: Challenges for turbulent combustion. Proc. Combust. Inst. 38, 121–155 (2021)
Matalon, M.: Intrinsic flame instabilities in premixed and nonpremixed combustion. Annu. Rev. Fluid Mech. 39, 163–191 (2007)
Mejia, D., Selle, L., Bazile, R., Poinsot, T.: Wall-temperature effects on flame response to acoustic oscillations. Proc. Combust. Inst. 35, 3201–3208 (2015)
Michl, J., Neumann, J., Rottengruber, H., Wensing, M.: Derivation and validation of a heat transfer model in a hydrogen combustion engine. Appl. Thermal Engineering 98, 502–512 (2016)
Miniero, L., Pandey, K., De Falco, G., D’Anna, A., Noiray, N.: Soot-free and low NO combustion of Jet A-1 in a lean azimuthal flame (LEAF) combustor with hydrogen injection. Proc. Combust. Inst. 39, 4309–4318 (2023)
Morrisset, D., Burnford, J., Ojo, A.O., Peterson, B., Law, A., Hadden, R.H.: The relative position of pyrolysis onset and flame front location for downward flame spread. Proc. Combust. Inst. 40, 105355 (2024)
Nau, P., Müller, A., Petry, N., Nilsson, S., Endres, T., Richter, M., Witzel, B.: Fiber-coupled phosphor thermometry for wall temperature measurements in a full-scale hydrogen gas turbine combustor. Meas. Sci. Technol. 34, 104003 (2023)
Ojo, A.O., Escofet-Martin, D., Collins, J., Falconetti, G., Peterson, B.: Experimental investigation of thermal boundary layers and associated heat loss for transient engine-relevant processes using HRCARS and phosphor thermometry. Combust. Flame 233, 111567 (2021)
Ojo, A.O., Escofet-Martin, D., Abram, C., Fond, B., Peterson, B.: Precise surface temperature measurements at kHz-rates using phosphor thermometry to study flame-wall interactions in narrow passages. Combust. Flame 240, 111984 (2022)
Ojo, A.O., Escofet-Martin, D., Peterson, B.: High-precision 2D surface phosphor thermometry at kHz-rates during flame-wall interaction in narrow passages. Proc. Combust. Inst. 39, 1455–1463 (2023)
Onorati, A., Payri, R., Vaglieco, B., Agarwal, A., Bruneaux, G., Canakci, M., Gavaises, M., Günthner, M., Hasse, C., et al.: The role of hydrogen for future internal combustion engines. Int. J. Engines Res. 23, 529–540 (2022)
Owston, R., Magi, V., Abraham, J.: Interactions of hydrogen flame with walls: influence of wall temperature, pressure, equivalence ratio and diluents. Int. J. Hydrogen Energy 32, 2094–2104 (2007)
Padhiary, A., Pilla, G., Sotton, J., Bellenoue, M.: Effect of pressure and turbulence intensity on the heat flux during flame wall interaction (FWI). Flow Turbul. Combust. 111, 1345–1370 (2023)
Padhiary, A., Collins, J., Ojo, A.O., Escofet-Martin, D., Dreizler, A., Peterson, B.: An experimental investigation of the thermal flame structure during side-wall quenching of a laminar premixed flame. Proc. Combust. Inst. 40, 105376 (2024)
Persson, K.: Materials Data on ScVO4 (SG: 141) by Materials Project. OSTI, https://www.osti.gov/dataexplorer/biblio/dataset/1194131 (2014). https://doi.org/10.17188/1194131
Peterson, B., Baum, E., Böhm, B., Sick, V., Dreizler, A.: High-speed PIV and LIF imaging of temperature stratification in an internal combustion engine. Proc. Combust. Inst. 34, 3653–3660 (2013)
Peterson, B., Baum, E., Böhm, B., Sick, V., Dreizler, A.: Evaluation of toluene LIF thermometry detection strategies applied in an internal combustion engine. Appl. Phys. B 117, 151–175 (2014)
Poinsot, T., Veynante, D.: Theoretical and numerical combustion. RT Edwards, Pennsylvania (2005)
Rakopoulos, C.D., Kosmadakis, G.M., Pariotis, E.G.: Critical evaluation of current heat transfer models used in CFD in-cylinder engine simulations and establishment of a comprehensive wall-function formulation. Appl. Energy 87, 1612–1630 (2010)
Schefer, R.W., Kulatilaka, W.D., Patterson, B.D., Setterseten, T.B.: Visible emission of hydrogen flames. Combust. Flame 156, 1234–1241 (2009)
Smith G.P., Golden, D.M., Frenklach, M., Moriarty, N.W., Eiteneer, B, Goldenberg M., et al.: GRI Mech 3.0, http://combustion.berkeley.edu/gri-mech/ (2011). Accessed 31 Jan 2024
Szybist, J.P., Busch, S., McCormick, R.L., Pihl, J.A., Splitter, D.A., Ratcliff, M.A., Kolodziej, C.P., Storey, J.M.E., Moses-Debusk, M., Vuilleumier, D., et al.: What fuel properties enable higher thermal efficiency in spark-ignited engines? Prog. Energy Combust. Sci. 82, 100876 (2021)
Taamallah, S., Vogiatzaki, K., Alzahrani, F.M., Mokheimer, E.M.A., Habib, M.A., Ghoniem, A.F.: Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: technology, fundamentals, and numerical simulations. Appl. Energy 154, 1020–1047 (2015)
Taler, J., Duda, P.: Solving direct and inverse heat conduction problems. Springer, Berlin-Heidelberg (2006)
Verhelst, S., Wallner, T.: Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 35, 490–527 (2009)
Witkowski, D., Rothamer, D.A.: Evaluation of 15-kHz high-speed Pr:YAG phosphor surface thermometry of a thermal barrier coating in a reciprocating IC engine. Proc. Combust. Inst. 39, 1289–1297 (2023)
Yu, J.F., Yu, R., Bai, X.S., Sun, M.B., Tan, J.G.: Nonlinear evolution of 2D cellular lean hydrogen/air premixed flames with varying initial perturbations in the elevated pressure environment. Int. J. Hydrogen Energy 42, 3790–3803 (2017)
Zhao, D., Hernández Peréz, F.E., Guo, C., Im, H.G., Wang, L.: Near-wall effects on the premixed head-on hydrogen/air flame. Combust. Flame 244, 112267 (2022)
Zhu, J., Zwires, T., Zhang, F.C., Li, Z.J., Zhang, Y., Pan, J.F.: Correlation of wall heat loss with quenching distance for premixed H2/Air flames during unsteady flame-wall interaction. Chem. Eng. Science 283, 119391 (2024)
Funding
We gratefully acknowledge funding from the European Research Council (ERC grant #759546) and EPSRC (EP/P020593/1, EP/V003283/1).
Author information
Authors and Affiliations
Contributions
AO: conceptualization, methodology, investigation, formal analysis, writing—review and editing; AP: conceptualization, methodology, experimental investigations, formal analysis, Cantera simulations, writing—review and editing; BP: conceptualization, formal analysis, funding acquisition, supervision, writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Videos of the Twall and chemiluminescence image sequences presented in this work are provided as supplemental material.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental video of T and chemiluminescence sequence for the CH -air Φ = 1.0 mixture and CS = 2 mm conditions shown in Fig. 5
Supplemental video of T and chemiluminescence sequence for the CH -air Φ = 1.0 mixture and CS = 2 mm conditions shown in Fig. 5
Supplemental video of T and chemiluminescence sequence for the CH -air Φ = 1.0 mixture and CS = 2 mm conditions shown in Fig. 8
Supplemental video of T and chemiluminescence sequence for the CH -air Φ = 1.0 mixture and CS = 2 mm conditions shown in Fig. 8
Supplemental video of T and chemiluminescence sequence for the CH -air Φ = 1.0 mixture and CS = 2 mm conditions shown in Fig. 10
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ojo, A.O., Padhiary, A. & Peterson, B. Spatiotemporal Surface Temperature Measurements Resolving Flame-Wall Interactions of Lean H2-Air and CH4-Air Flames Using Phosphor Thermometry. Flow Turbulence Combust (2024). https://doi.org/10.1007/s10494-024-00571-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10494-024-00571-1