Abstract
This paper examines the potential of using ammonia (NH\(_3\)) as a primary fuel in heavy-duty engines for decarbonization, with some challenges yet to be addressed. It presents a numerical study of a Reactivity Controlled Compression Ignition engine, where pilot diesel is used to ignite the premixed ammonia/air mixture. The numerical model and combustion mechanism are validated against engine experimental results using methanol and iso-octane fuels and ignition delay times of ammonia/n-heptane mixtures measured in a rapid compression machine. The findings show that the engine can effectively operate with up to 50% of the total energy supplied by premixed ammonia, albeit with slightly elevated NO emissions compared to a diesel-fueled engine. Increasing ammonia further leads to lower combustion efficiency. Hydrogen can be utilized in the ammonia engine to enhance ammonia combustion; however, NO emissions increase further. Ammonia leakage primarily originates from regions near the cold wall, the center of the cylinder, and the crevice. N\(_2\)O mainly forms at the ammonia flame front. Emission of N\(_2\)O is therefore mainly due to flame front quenching near the wall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In compliance with progressively stringent pollutant emission targets established in international agreements (Horowitz 2016), research in combustion has shifted its focus towards low-carbon fuels such as ammonia, hydrogen, methane, and renewable alcohols. A comparison of the physical properties of these low-carbon fuels and fossil fuels is provided in Table 1. Gasoline and diesel, the predominant fuels for current vehicles, release substantial amounts of carbon monoxide (CO), hydrocarbon (HC), nitrogen oxides (NO\(_x\)), particulate matters (PM, e.g., soot), and greenhouse gases (e.g., carbon dioxide, CO\(_2\)) into the atmosphere. Renewable alcohol fuels offer a cleaner alternative to fossil fuels with the added benefit of reduced CO\(_2\) emissions. The first four aliphatic alcohols (methanol, ethanol, propanol, and butanol) are particularly appealing as internal combustion engine (ICE) fuels due to their chemical or biological synthesis potential. Additionally, these four major alcohol fuels possess high octane ratings, which tend to enhance engine thermal efficiency. A recent study demonstrated that methanol can achieve higher engine efficiency and lower NO\(_x\) emissions compared to gasoline fuel in a direct-injection compression ignition (DICI) engine (Xu et al. 2022). However, a significant drawback of alcohol fuels is their production from corn, sugarcane, and grains, which may exacerbate the global food crisis. Given the pressing need for carbon neutrality, research on the utilization of carbon-free fuels in ICEs is of utmost importance. Hydrogen has been identified as a promising energy vector for next-generation engineering applications, as its combustion product is solely water (H\(_2\)O). However, the primary challenge lies in hydrogen’s relatively low energy density in gaseous form, necessitating cryogenic temperatures of 20 K for high energy density liquid storage. Ammonia can serve as a hydrogen carrier and carbon-free energy vector, as it is produced globally in large volumes and can be easily liquefied under light pressure and cooling. Its storage can be accomplished using pressure vessels similar to those used for liquefied petroleum gas (LPG) in powered equipment. Previous studies have suggested that ammonia has the potential to provide zero carbon emissions power (Valera-Medina et al. 2018). However, the thermo-chemical characteristics of ammonia have hindered its widespread adoption and practical application as a fuel. Directly replacing fossil fuels (diesel/gasoline) with ammonia would cause engines to malfunction, as ammonia does not ignite in current engine configurations. The very low flame speed of ammonia (Rocha et al. 2021) poses a significant challenge for combustion. Additionally, when NH\(_{3}\) is burned under lean conditions, it may emit a large amount of nitrogen oxides (NO and N\(_2\)O) (Valera-Medina et al. 2018). It is important to note that nitrogen oxides are not the final product of ammonia combustion, as the complete combustion products of ammonia are N\(_2\) and H\(_2\)O. Advanced technologies can be utilized to increase combustion efficiency and decrease nitrogen oxide emissions.
Several strategies have been proposed to address the ignition issue of ammonia in internal combustion engines (ICEs). These include increasing spark energy, ultra-high compression ratio, supercharging, and dual-fuel combustion with a pilot-burning fuel jet. Ryu (Ryu et al. 2014) investigated the engine performance using a gaseous ammonia and gasoline fuel mixture in a spark-ignition (SI) engine. However, the engine performance was less satisfactory than that of pure gasoline due to the low flame-speed propagation of NH\(_{3}\). In contrast, compression ignition (CI) engines are more attractive for heavy-duty applications due to their clean combustion and high efficiency. The dual-fuel approach is one of the best solutions for the utilization of ammonia in CI engines. In this approach, ammonia is burned with a ‘pilot’ burning fuel jet, such as diesel, biodiesel, or dimethyl ether, to achieve reliable ignition. Reiter and Kong (2011) reported that ammonia (95% of the total energy) initially ignited by diesel (with an energy contribution of 5%) can operate well in a heavy-duty engine.
However, ammonia dual-fuel combustion currently suffers from relatively high unburned ammonia and NO\(_x\) emissions. When the energy substitution by ammonia is less than 40%, NO\(_x\) emissions using the dual-fuel operation are lower than those using 100% diesel fuel. If ammonia energy accounts for more than 60%, exhaust NO\(_x\) emissions increase significantly due to the fuel-bound nitrogen in ammonia. Yousefi et al. (2022a, 2022b) conducted experiments on an ammonia/diesel dual-fuel (ADDF) engine (also called Reactivity Controlled Compression Ignition (RCCI) engine) to investigate engine performance and emissions behaviors. In the ADDF combustion strategy, ammonia is injected into the port and premixed with the air during the intake stroke, then ignited by the direct injection (DI) of diesel at a desirable engine crank angle. The results show that a single diesel injection achieves a lower indicated thermal efficiency (ITE) compared to the corresponding diesel-only combustion mode, while the double diesel injection strategy yields a higher ITE than the diesel engine but with a penalty of 10% higher nitrogen oxides (NOx) emissions. The unburned ammonia concentration in the exhaust flow is always above the recommended exposure limit. Frankl et al. (2021) used the commercial software package CONVERGE to investigate ammonia combustion in a high-pressure-dual-fuel (HPDF) engine. They reported that ammonia requires a significant amount of activation energy to combust, and pre-heating the ammonia can increase combustion efficiency. However, combustion and emission behaviors in ICEs are a manifestation of the nonlinear interaction among several physical and chemical processes, which are not fully understood using experimental methods. Therefore, high-fidelity numerical simulations based on well-validated chemical kinetic models are needed to advance ammonia-based combustion technologies.
Aiming at simultaneously improving thermal efficiency and meeting more stringent emission regulations, a number of state-of-the-art advanced combustion strategies have been developed in recent years, such as the RCCI, partially premixed combustion (PPC), and homogeneous charge compression ignition (HCCI). These strategies aim to control the combustion process by controlling fuel stratification and reactivity. It has been demonstrated that satisfactory engine performance can be achieved under different operating conditions; for example, about 55% engine efficiency can be achieved in RCCI engines (Reitz and Duraisamy 2015). The use of advanced strategies for ammonia fuels can contribute to enhanced engine performance and overall emissions improvement. For ammonia fuel which has a lower reactivity and flame speed than gasoline, the issues of ignition and incomplete combustion are serious. Further studies (Lhuillier et al. 2019) showed that a small hydrogen fraction, which could also be generated in-situ through NH\(_{3}\) catalytic or heat-assisted dissociation, can be used as a combustion promoter. Pochet et al. (2017) reported using ammonia and hydrogen mixtures in HCCI combustion could improve the combustion of ammonia.
There is a lack of both experimental and simulation studies on fuel stratification and stable combustion in ammonia-fueled engines, despite the potential of ammonia as a fuel for heavy-duty engines. To improve engine performance, it is essential to conduct systematic investigations on ammonia-fueled engines through numerical simulation. In this context, the present study aims to gain deeper insight into the mechanisms of combustion, pollutant emissions, and engine performance fueled with NH\(_{3}\) under the Reactivity Controlled Compression Ignition (RCCI) concept. Specifically, the study comprehensively investigates the effect of ammonia energy fraction on combustion performance and emissions of an RCCI engine at low load. Additionally, the effect of hydrogen fraction in the premixed ammonia/hydrogen mixture is analyzed to identify the optimized operating condition for achieving clean and high-efficiency combustion of ammonia fuels in heavy-duty engines.
2 Case Setup and Numerical Methods
Numerical simulations were conducted on a six-cylinder Scania D13 heavy-duty direct-injection engine to investigate the combustion performance, pollutant emissions, and engine efficiency of ammonia as a fuel. The four-stroke engine has a bore/stroke length of 130/160 mm and a connecting rod of 255 mm. Fuel was supplied to a twelve-hole solenoid injector with a nozzle orifice diameter of 230 \(\mu\)m and an umbrella angle of 120\(^\text {o}\) via a common rail. A stepped lip piston profile with a compression ratio of 17:1 was used, as shown in Fig. 1. Previous experiments with this engine have been conducted using iso-octane (Aziz et al. 2020a), methanol (Aziz et al. 2020b), gasoline (Li et al. 2017; Aziz et al. 2019), and PRF (a mixture of iso-octane and n-heptane) fuels. Table 1 shows that methanol fuel has comparable physical properties to ammonia fuel, such as research octane number, the heat of vaporization, and auto-ignition temperature. Both fuels require preheating of the intake air to achieve ignition around top dead centre (TDC). Thus, engine experiments conducted using methanol as the directly injected fuel under the Direct Injection Compression Ignition (DICI) concept, without Exhaust Gas Recirculation (EGR), were used as a baseline to validate the numerical model for ammonia engine simulations. The injection timing was varied from \(-100 ^\text {o}\) to \(-15 ^\text {o}\)CA ATDC to achieve both Homogeneous Charge Compression Ignition (HCCI) and Partially Premixed Combustion (PPC) regimes (Xu et al. 2019). The engine speed was maintained at 1200 rpm, and the intake pressure was 1 bar. Combustion phasing, represented by CA50, was kept constant at 3 \(^\text {o}\)CA by tuning the intake temperature. The total energy in the cylinder was around 2 KJ per cycle for both fuels, with an engine load of approximately 4 bar for methanol and varying slightly with SOI and fuel. Further details on the engine geometry and operating parameters can be found in our previous study (Xu et al. 2022). The experimental results were used to validate the current simulation models and provide boundary conditions for ammonia fuels.
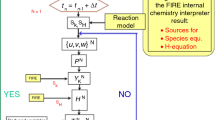
The simulations are carried out using the Reynolds-Averaged Navier Stokes (RANS) model. The fuel spray process is modeled based on the well-established Lagrangian–Eulerian approach. The continuum gas phase is treated by the Eulerian system of equations, and the discrete liquid phase parcel is treated in the Lagrangian framework. Spray droplets are subject to several processes from the time of injection until the time of vaporization, shown on the left of Fig. 1. The detailed submodel description is referred to our previous work (Xu et al. 2022). The injection profiles for different fuels and operation conditions are described by the model of Xu et al. (2018), which was widely validated and successfully applied to different injection conditions. Based on the experiment results of Andruskiewicz et al. (2018) and the engine cooling conditions, the cylinder wall, and the flat cylinder head had a temperature of 430K, while the piston wall temperature was 450K. Since the injector has 12 evenly distributed holes and is positioned in the center of the cylinder head, a 30\(^\text {o}\) sector mesh with periodic boundaries was accordingly chosen, as shown in Fig. 1. The case is modeled from intake valve closing (IVC, -141\(^\text {o}\) CA) to exhaust valve opening (EVO, 137\(^\text {o}\) CA). To maintain mesh quality (size and shape of cells) and density throughout the entirety of the simulation, a dynamic mesh layer algorithm (Lucchini et al. 2007; Xu et al. 2022) was developed based on OpenFOAM, which incorporates adding and removing of cells in accordance with the motion of the engine piston. The mesh has an average cell size of 1.2 mm in the squish region and a finer mesh of 0.5 mm in the bowl region, cf. Fig. 1.
3 Validation of Numerical Simulations with the Experimental Results
3.1 Validation of the Methanol and Iso-octane Fuels
The use of ammonia as the primary fuel in engine experiments is rare, as stated in the introduction, with only a few groups having attempted it but with incomplete information on the experimental setup. As an alternative, methanol, and iso-octane fuels were used to operate the DICI engine with different fuel injection timings to validate the numerical models and to identify the potential of current simulation models to capture engine performance for different fuels. Experimental results, including in-cylinder pressure and apparent heat release rate (AHRR) traces, intake temperature to maintain the same CA50 at different SOIs, and emissions (NO, CO, and UHC), were used to validate the numerical models. Figure 2 presents a comparison of the experimentally measured and simulated in-cylinder pressure, and AHRR traces with the SOI of -15\(^\text {o}\) CA for methanol and iso-octane fuels, demonstrating excellent agreement between the simulation and experimental results. Further information regarding these two cases can be found in reference (Xu et al. 2022).
Figure 3 displays the required intake temperature (symbol) in the experiment and IVC temperature (solid line) in the simulation to maintain CA50 at around 3 \(^\text {o}\)CA ATDC for each case. The overall trend of the intake/IVC temperature at various SOI for both fuels exhibits a ’spoon’ shape, where the ’handle of the spoon’ starts from the early SOI and ends around -60\(^\text {o}\)CA ATDC. This pattern is due to the spray/wall interaction and the fuel evaporation/mixing with air, leading to different fuel/air stratification and spatial distribution for the various SOIs. The simulation demonstrates good agreement with the experimental data and can effectively capture the trend, which validates that the current models can accurately represent the different fuel/air mixing processes. Figure 4 illustrates the emission characteristics during the SOI sweep for the two fuels. The simulation results also show reasonable agreement with the experimental results, confirming that the current numerical models can predict engine emissions for different operating conditions. The successful validation of different fuels demonstrates the reliability and predictive capability of our numerical simulation tools in assessing engine performance and combustion processes in ammonia/diesel RCCI engines.
3.2 Validation of the Ammonia/Hydrogen Mechanism
Three-dimensional (3D) simulations of ammonia engines necessitate reliable chemical mechanisms with a limited number of species and reactions. However, the current ammonia and ammonia/hydrocarbon blend mechanisms are restricted to the C2 hydrocarbon fuels. Therefore, we developed a skeletal chemical kinetic mechanism for n-heptane/ammonia blend fuels using a joint decoupling methodology and optimization algorithm. The n-heptane skeletal mechanism is derived from the recent work of Chang et al. (Chang et al. 2022), and the ammonia mechanism is adapted from the original work of Stagni et al. (2020), which was optimized by Bertolino et al. (2021) using a machine learning method. The skeletal mechanism comprises 69 species and 389 reactions. A more detailed description of the skeletal mechanism is given in the reference (Xu et al. 2023).
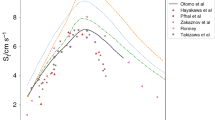
Experimental studies on the fundamental combustion behavior of NH\(_3\)/n-heptane blends are scarce. The ignition delay times (IDTs) of four ammonia/n-heptane mixtures with various NH\(_3\)/n-heptane blending ratios and equivalence ratios at pressures of 10 \(\sim\) 15 bar and temperatures ranging from 600 \(\sim\) 1000 K, as studied by Yu et al. (2020), are employed to validate the present mechanism, depicted in Fig. 5. The current chemical kinetic mechanisms captured the negative temperature coefficient (NTC) ignition behaviors and pressure dependence behavior for different ammonia/n-heptane mixtures. Additionally, the mechanism is also validated against various experimental datasets of pure ammonia and ammonia/hydrogen fuel blends, including IDTs, species profiles, and LBVs. Satisfactory predictions of these quantities are achieved using the skeletal mechanism. More results are described elsewhere (Xu et al. 2023). The present skeletal mechanism can effectively predict the ammonia/n-heptane combustion processes for a wide range of conditions.
4 Results and Discussion
Due to the low flame speed and high octane number, the ammonia/diesel RCCI engine operates with preheated intake air and supercharged conditions to enhance ammonia ignition. The boundary conditions are the same as the methanol SOI of \(-15^\text {o}\)CA ATDC case, with an IVC temperature of 458K and an IVC pressure of 1.17 bar. To control the CA50 around the TDC, the SOI of the pilot fuel, n-heptane, is set to \(-7^\text {o}\)CA ATDC, the same as the conventional diesel combustion (CDC) engine (Xu et al. 2018). Taking into account the injection delay time, the actual injection timing in the simulation is \(-4^\text {o}\)CA ATDC. The injection pressure and injector are identical to those in the methanol case. The total energy in the cylinder is 2000 J/cycle for all cases, corresponding to an IMEP of 4 bar. Two key parameters, the energy fraction of the premixed fuel (EP) and the mole fraction of ammonia in the ammonia/hydrogen blend (\(X_{NH_3}\)), are defined as follows:
where \(m_{NH_3}\) and \(m_{H_2}\) are the respective masses of ammonia and hydrogen in the cylinder. LHV is the low heat value of the specified fuel. The \(C_{NH_3}\) and \(C_{H_2}\) are the mole fractions of ammonia and hydrogen in the premixed ammonia/hydrogen/air mixture. In the simulation cases, the EP varies from 0.3 to 0.8 to investigate the critical required diesel amount in the ammonia engine. Co-combustion of ammonia with hydrogen is a potential approach to enhance engine performance since the high reactivity of hydrogen can compensate for the low reactivity of ammonia. Thus, the effect of the hydrogen fraction in the premixed fuels on engine performance is also studied, with the \(X_{NH_3}\) varying from 1 to 0.5. Additionally, a pure diesel case operating under the CDC concept is simulated as the reference case. Detailed parameters in the simulation cases are listed in Table 2. The injection duration of the pilot n-heptane fuel under different conditions is modeled using the injection model of Xu et al. (2018), as shown in Fig. 6. The equivalence ratio of the premixed ammonia/air mixture ranges from 0.106 (EP=0.3) to 0.283 (EP=0.8). More hydrogen in the ammonia/hydrogen mixture leads to a lower equivalence ratio under the same energy condition.
Injection profiles for the different engine operation cases in Table 2
Figure 7 displays the in-cylinder pressure and AHRR trace for the ammonia engine under different operating conditions. For the pure ammonia premixed cases (Fig. 7a), it is observed that compared to the pure diesel cases, the engine operates well with up to 50% of the total energy replaced by the premixed ammonia. The in-cylinder pressure is similar to the diesel CDC case. When further increasing the premixed ammonia energy (EP=0.8), the engine operation worsens, and the cylinder pressure is much lower due to a large amount of unburned ammonia. For the hydrogen/ammonia blend premixed cases, the onset of ignition advances significantly with the increase of hydrogen fraction in the premixed fuels. The combustion of the premixed ammonia improves considerably, and the in-cylinder pressure is much higher than in the diesel case. For the pure ammonia premixed cases (\(X_{NH_3}\)=1.0), the heat release rate profile is similar to the diesel case, with a peak heat release rate due to the ignition of the diesel, followed by a heat release stage from the diesel driven combustion of the premixed ammonia. Details of the reaction process will be discussed below.
For the hydrogen/ammonia blend cases, the heat release rates exhibit two stages for the ammonia/hydrogen blend premixed cases. The first stage of the AHRR results from the auto-ignition of the premixed ammonia/hydrogen fuels, which occurs before the injection of n-heptane (-4\(^\text {o}\)CA ATDC). The second stage is the heat release from the injected diesel and post-flame residual premixed fuels. It should be noted that this result is particular for the present engine setup, e.g., the use of an elevated intake temperature taken from the methanol PPC engine experiments.
For the low hydrogen fraction cases, \(X_{NH_3}\)=0.9 (Fig. 7b), before the diesel injection, there is a small heat release from the premixed ammonia/hydrogen fuels. After the diesel injection into the cylinder, it is immediately ignited, resulting in a heat release peak. With the increase in the mole fraction of hydrogen in the premixed fuels (Figs. 7c, d), the premixed fuel burns substantially before the diesel injection, leading to a high heat release from the premixed fuel. Further discussion on the combustion process is given below.
Figure 8 shows the variation of the residual mass of NH\(_3\) in cylinder normalized by its initial mass, and mole fractions of NO, N\(_2\)O, and NO\(_2\) during the combustion process for the pure ammonia premixed cases (\(X_{NH_3}=1\)). The mole fractions of these species are based on the total mole of these gases in the cylinder. The residual mass of ammonia is 1 at the unburned stage and 0 when it is completely combusted. As shown in Fig. 8a, Ammonia starts to combust around TDC, and after 20 \(^{\circ }\)CA ATDC, the combustion of ammonia is very slow. The final ammonia efficiency (at exhaust valve opening time) is around 82% for the EP=0.3 cases. With an increase in ammonia energy share in the engine, the ammonia combustion efficiency decreases. For the EP of 0.8 cases, the ammonia efficiency is only 74.3%, and a large amount of unburned ammonia will leak into the exhaust gas. The results show that the current ammonia RCCI engine requires a large amount of diesel to support ammonia combustion. As listed in Table 2, the equivalence ratio of premixed ammonia ranges from 0.106 (EP=0.3) to 0.283 (EP=0.8); all cases are under very fuel-lean conditions. The ammonia flame speed is rather low and cannot support self-propagation. The ammonia flame is essentially a diesel flame-driven mode, i.e., the combustion of ammonia is driven by the mixing of diesel fuel or diesel combustion products with the ambient ammonia/air mixture. Thus, more diesel (low EP) achieves higher ammonia combustion efficiency.
The NO emission in the ammonia RCCI engine is higher than that of the conventional diesel engine, as seen in Fig. 8b. For the pure ammonia cases (EP=0.3, 0.5, and 0.6), the NO emission increases with the increase of ammonia energy share in the engine. For high ammonia energy cases (EP=0.7 and 0.8), the NO emission decreases with the increase of ammonia due to incomplete combustion of ammonia. The residual ammonia reacts with NO through the well-known deNOx reactions. It is worth noting that the N\(_2\)O emission is rather high in the current ammonia RCCI engine. From Fig. 8c, the diesel engine produces almost zero N\(_2\)O emissions. However, for the ammonia RCCI engine, the amount of N\(_2\)O emissions is comparable to the NO emissions. The peak N\(_2\)O mass fraction in the cylinder is around 10 \(^{\circ }\)CA ATDC. Thereafter, for the low ammonia energy cases (EP of 0.3), N\(_2\)O mass fraction decreases as the combustion progresses. For the high ammonia energy cases, From 10–15 \(^{\circ }\)CA ATDC, N\(_2\)O mass fraction decreases first, reaching a local minimum around 10–15 \(^{\circ }\)CA ATDC. The NO\(_2\) emissions are low for all cases but are relatively higher for the ammonia engine than for the diesel engine, as seen in Fig. 8d.
To understand the combustion process and NO formation process, spatial distributions of key species and temperature in the cylinder are examined. Figure 9 shows the distribution of temperature and mass fractions of NH\(_3\), NO, N\(_2\)O and NO\(_2\) in the cylinder at 10 \(^\text {o}\)CA ATDC for the pure ammonia (\(X_{NH_3}=1.0\)) cases with an EP of 0.8. To show the distribution of n-heptane in the cylinder, Fig. 9f shows also the mixture fraction defined based on the injected n-heptane (\(Z=1\) indicates that the mixture is originated from pure n-heptane; \(Z=0\) indicates that the mixture is originated from the pure ambient gas mixture). As shown in Fig. 9, combustion starts from the n-heptane mixture region, and the flame propagates to the ammonia/air mixture. However, due to the very fuel-lean ammonia/air mixture, the ammonia flame only follows the diesel flame and hardly self-propagates to the ammonia/air mixture, i.e., the reaction front follows that of the mixture fraction. NH\(_3\) is distributed near the cylinder wall, in the center of the cylinder, and the crevice, and it cannot burn completely, which are the main sources of ammonia leakage. The piston profile and/or injection strategies should be further optimized to improve n-heptane spray development, thus increasing ammonia combustion efficiency.
N\(_2\)O at the ammonia flame front is very high. N\(_2\)O is indeed oxidized in the burned side of the ammonia flame. Since the ammonia flame is quenched near the wall and in the center of the cylinder, the oxidation of N\(_2\)O is also quenched, which results in a high N\(_2\)O concentration near the wall and in the center of the cylinder, Fig. 9d.
NO\(_2\) is formed as a result of NO oxidation processes, primarily through the reaction NO+HO\(_2\)=NO\(_2\)+OH (Li et al. 2019). The distribution of NO\(_2\) (as shown in Fig. 9e) is similar to that of N\(_2\)O at the ammonia flame front. Studies (Li et al. 2019) have shown that the concentration of NO\(_2\) is dominant under lean conditions. In current engine operation conditions, the premixed fuel is in very fuel-lean conditions, which promotes the formation of NO\(_2\), resulting in higher NO\(_2\) concentrations compared to pure diesel combustion.
As mentioned earlier, the combustion efficiency of ammonia is improved for the hydrogen and ammonia blend cases. Figure 10 shows the comparison of the pure ammonia case and hydrogen/ammonia cases, in terms of the normalized residual ammonia and emissions (NO, N\(_2\)O, and NO\(_2\)) during the combustion process, for the EP of 0.8 cases. From Fig. 10a, the premixed ammonia/hydrogen/air mixture auto-ignites around -8 \(^\text {o}\)CA ATDC due to the high cylinder temperature (above 1000K). For the high hydrogen cases (\(X_{NH_3}=0.5\) and 0.7), the ammonia burns substantially, i.e., more than 90%, before the diesel is injected. For the 10% hydrogen case (\(X_{NH_3}=0.9\)), the ignition of the premixed mixture is along with the injection of n-heptane injection. The onset of ignition in this case is still substantially earlier than in the pure ammonia case.
The NO emissions for the hydrogen/ammonia premixed cases are much higher than those of the pure ammonia case and the diesel CDC case, as shown in Fig. 10b. The reason is that the in-cylinder pressure and temperature are very high due to the combustion of the premixed ammonia/hydrogen. The diesel is injected into extremely high-temperature hot gases, leading to high NO production. Besides, ammonia combustion under fuel-lean conditions produces a high amount of NO (Valera-Medina et al. 2018). The engine operates at a low load, and the equivalence ratio of the premixed ammonia/air mixture is lower than 0.3. The N\(_2\)O profile for the ammonia engine cases has a very high peak value due to the combustion of the fuel-lean premixed mixture, as seen in Fig. 10c. The N\(_2\)O concentration decreases during the expansion stroke due to further oxidation. The final N\(_2\)O emissions are lower than those of the pure ammonia case due to the high combustion efficiency of ammonia in the hydrogen/ammonia blend cases. The NO\(_2\) emissions for the ammonia engines are slightly higher than those of the diesel CDC case and are only marginally affected by the hydrogen fraction.
Figure 11 shows the distributions of temperature, mixture fraction, and mass fractions of NH\(_3\), NO, N\(_2\)O, and NO\(_2\) in the cylinder at 10 \(^\text {o}\)CA ATDC for the 10% hydrogen blend case (\(X_{NH_3}=0.9\)) with an EP of 0.8. For the ammonia/hydrogen premixed case (Fig. 11), ammonia is almost completely burned at 10 \(^\text {o}\)CA ATDC. Only a minor amount of ammonia near the cold wall and in the crevice cannot burn well. NO species are present in the region where NH\(_3\) is burned. N\(_2\)O is mainly from the near wall region and the center of the cylinder, as well as the crevice region, where NH\(_3\) cannot be completely burned. Compared with the pure ammonia cases, as shown in Fig. 9, it is clear that the complete combustion of ammonia in the near-wall region is important for reducing N\(_2\)O emissions. As shown in Fig. 9e, NO\(_2\) is formed in the ammonia flame front. In the ammonia/hydrogen premixed case, since ammonia is rather completely combusted, there is no NO\(_2\) front visible in the cylinder. Complete combustion of ammonia in the near-wall region is crucial for minimizing the formation of NO\(_2\).
5 Conclusions
Ammonia shows promise as a prospective candidate for achieving zero greenhouse gas emissions (CO\(_2\)) in next-generation engineering applications. However, its utilization faces challenges related to difficult ignition and high nitrogen oxide emissions (NO and N\(_2\)O) due to its unique thermo-chemical characteristics, including extremely low flame speed and fuel-bound nitrogen content. To address these issues, the reactivity-controlled compression ignition (RCCI) concept, employing dual-fuel combustion, emerges as a potential solution. This paper presents a numerical study on the use of ammonia as the primary fuel in an RCCI engine. The main fuels (ammonia) are homogeneously premixed with air in the intake port and ignited by a small amount of direct-injected pilot diesel fuel (modeled as n-heptane) around TDC. The numerical models are validated against methanol and iso-octane fuel operation in a DICI engine. A skeletal chemical kinetic mechanism for n-heptane/ammonia blend fuels was developed and validated using the IDTs of different ammonia/n-heptane mixtures at various pressures measured in a rapid compression machine. The CFD model and combustion mechanism employed in the current simulation are shown to provide qualitatively accurate results for using ammonia as the main fuel. The performance and emission behaviors of the ammonia/diesel RCCI engine are then investigated. The main conclusions are as follows:
-
For the ammonia usage in heavy-duty engines, the engine can operate effectively with up to 50% of the total energy replaced by premixed ammonia, with a penalty of slightly higher NO emissions compared to the diesel CDC engine and significant N\(_2\)O emissions.
-
Under low load conditions, the premixed ammonia/air mixture exhibited fuel-lean equivalence ratios, resulting in a low ammonia flame speed incapable of self-propagation. Instead, the ammonia combustion process relied on the mixing of diesel fuel or diesel combustion products with the surrounding ammonia/air mixture, essentially operating in a diesel flame-driven mode.
-
Unburned ammonia is another issue, as nearly 20% of ammonia cannot completely burn even for the EP of 0.3 case. For the EP of 0.8 case, the unburned ammonia is around 25%. Ammonia leakage primarily originates from areas near the cold wall, the center of the cylinder, and the crevice.
-
Blending hydrogen into the premixed ammonia significantly improves ammonia combustion efficiency, but with a trade-off of increased NO emissions.
-
N\(_2\)O mainly forms at the ammonia flame front. Since the ammonia flame is quenched near the wall and in the center of the cylinder, the oxidation of N\(_2\)O is also quenched, which results in a high N\(_2\)O concentration near the wall and in the center of the cylinder.
References
Andruskiewicz, P., Najt, P., Durrett, R., Biesboer, S., Schaedler, T., Payri, R.: Analysis of the effects of wall temperature swing on reciprocating internal combustion engine processes. Int. J. Engine Res. 19(4), 461–473 (2018)
Aziz, A., Garcia, A., Pinto Dos Santos, C., Tuner, M.: Impact of multiple injection strategies on performance and emissions of methanol PPC under low load operation. SAE 2020-01-0556 (2020)
Aziz, A., Li, C., Verhelst, S., Tuner, M.: The relevance of different fuel indices to describe autoignition behaviour of gasoline in light duty DICI engine under PPC mode. SAE 2019-01-1147 (2019)
Aziz, A., Xu, L., Garcia, A., Tunér, M.: Influence of injection timing on equivalence ratio stratification of methanol and isooctane in a heavy-duty compression ignition engine. SAE 2020-01-2069 (2020)
Bertolino, A., Fürst, M., Stagni, A., Frassoldati, A., Pelucchi, M., Cavallotti, C., Faravelli, T., Parente, A.: An evolutionary, data-driven approach for mechanism optimization: theory and application to ammonia combustion. Combust. Flame 229, 111366 (2021)
Chang, Y., Jia, M., Wang, P., Niu, B., Liu, J.: Construction and derivation of a series of skeletal chemical mechanisms for n-alkanes with uniform and decoupling structure based on reaction rate rules. Combust. Flame 236, 111785 (2022)
Frankl, S., Gleis, S., Karmann, S., Prager, M., Wachtmeister, G.: Investigation of ammonia and hydrogen as co2-free fuels for heavy duty engines using a high pressure dual fuel combustion process. Int. J. Engine Res. 22(10), 3196–3208 (2021)
Horowitz, C.A.: Paris agreement. Int. Legal Mater. 55(4), 740–755 (2016)
Lhuillier, C., Brequigny, P., Contino, F., Rousselle, C.: Performance and emissions of an ammonia-fueled SI engine with hydrogen enrichment. SAE 2019-24-0137 (2019)
Li, C., Tunestal, P., Tuner, M., Johansson, B.: Comparison of Gasoline and Primary Reference Fuel in the Transition from HCCI to PPC. SAE 2017-01-2262 (2017)
Li, R., Konnov, A.A., He, G., Qin, F., Zhang, D.: Chemical mechanism development and reduction for combustion of NH3/H2/CH4 mixtures. Fuel 257, 116059 (2019)
Lucchini, T., D’Errico, G., Jasak, H., Tukovic, Z.: Automatic mesh motion with topological changes for engine simulation. SAE 2007-01-0170 (2007)
Pochet, M., Truedsson, I., Foucher, F., Jeanmart, H., Contino, F.: Ammonia-hydrogen blends in homogeneous-charge compression-ignition engine. SAE 2017-24-0087 (2017)
Reiter, A.J., Kong, S.-C.: Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 90(1), 87–97 (2011)
Reitz, R.D., Duraisamy, G.: Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines. Prog. Energy Combust. Sci. 46, 12–71 (2015)
Rocha, R.C., Zhong, S., Xu, L., Bai, X.-S., Costa, M., Cai, X., Kim, H., Brackmann, C., Li, Z., Alden, M.: Structure and laminar flame speed of an ammonia/methane/air premixed flame under varying pressure and equivalence ratio. Energy Fuels 35(9), 7179–7192 (2021)
Ryu, K., Zacharakis-Jutz, G.E., Kong, S.-C.: Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 116, 206–215 (2014)
Stagni, A., Cavallotti, C., Arunthanayothin, S., Song, Y., Herbinet, O., Battin-Leclerc, F., Faravelli, T.: An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. Reaction Chem. Eng. 5, 696–711 (2020)
Valera-Medina, A., Xiao, H., Owen-Jones, M., David, W., Bowen, P.: Ammonia for power. Prog. Energy Combust. Sci. 69, 63–102 (2018)
Xu, L., Bai, X.-S., Jia, M., Qian, Y., Qiao, X., Lu, X.: Experimental and modeling study of liquid fuel injection and combustion in diesel engines with a common rail injection system. Appl. Energy 230, 287–304 (2018)
Xu, L., Bai, X.-S., Li, C., Tunestål, P., Tunér, M., Lu, X.: Combustion characteristics of gasoline DICI engine in the transition from HCCI to PPC: Experiment and numerical analysis. Energy 185, 922–937 (2019)
Xu, L., Treacy, M., Zhang, Y., Aziz, A., Tuner, M., Bai, X.-S.: Comparison of efficiency and emission characteristics in a direct-injection compression ignition engine fuelled with iso-octane and methanol under low temperature combustion conditions. Appl. Energy 312, 118714 (2022)
Xu, L., Chang, Y., Treacy, M., Zhou, Y., Jia, M., Bai, X.-S.: A skeletal chemical kinetic mechanism for ammonia/n-heptane combustion. Fuel 331, 125830 (2023)
Yousefi, A., Guo, H., Dev, S., Lafrance, S., Liko, B.: A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel 316, 123412 (2022)
Yousefi, A., Guo, H., Dev, S., Liko, B., Lafrance, S.: Effects of ammonia energy fraction and diesel injection timing on combustion and emissions of an ammonia/diesel dual-fuel engine. Fuel 314, 122723 (2022)
Yu, L., Zhou, W., Feng, Y., Wang, W., Zhu, J., Qian, Y., Lu, X.: The effect of ammonia addition on the low-temperature autoignition of n-heptane: an experimental and modeling study. Combust. Flame 217, 4–11 (2020)
Acknowledgements
This work was supported by the Nordic Energy Research (NER) and Swedish Transport Administration (Trafikverket) through the CAHEMA project within the Nordic Maritime Transport and Energy Research Programme. L. Xu is partially supported by the ÅForsk foundation (ID:21–345). The simulations were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at NSC, HPC2N and PDC.
Funding
Open access funding provided by Lund University. This work was supported by the Nordic Energy Research (NER) and Swedish Transport Administration (Trafikverket) through the CAHEMA project within the Nordic Maritime Transport and Energy Research Programme. L. Xu is partially supported by the ÅForsk foundation (ID:21–345).
Author information
Authors and Affiliations
Contributions
Lx: Methodology, Investigation, Visualization, Writing-Original draft preparation. XSB: Funding acquisition, Writing-Reviewing and Editing, Resource, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval
This research did not contain any studies involving animal or human participants, nor did it take place on any private or protected areas. No specific permissions were required for corresponding locations.
Informed Consent
This research did not contain any studies involving animal or human participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, L., Bai, XS. Numerical Investigation of Engine Performance and Emission Characteristics of an Ammonia/Hydrogen/n-Heptane Engine Under RCCI Operating Conditions. Flow Turbulence Combust 112, 957–974 (2024). https://doi.org/10.1007/s10494-023-00453-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10494-023-00453-y