Abstract
The European red squirrel (Sciurus vulgaris) is a common host for Ixodes ricinus ticks in urban and rural habitats, however, studies on ticks and tick-borne pathogens (TBPs) of squirrels have not been conducted in Poland yet. Thus, the aims of the current study were to assess and compare the prevalence and abundance of ticks on red squirrels trapped at two sites in the Warsaw area (in an urban forest reserve and an urban park) and using molecular tools, to assess the genetic diversity of three pathogens (Borrelia burgdorferi sensu lato, Rickettsia and Babesia spp.) in I. ricinus ticks collected from squirrels. For the detection of Rickettsia spp. a 750 bp long fragment of the citrate synthase gltA gene was amplified; for B. burgdorferi s.l. 132f/905r and 220f/824r primers were used to amplify the bacterial flaB gene fragments (774 and 605 bp, respectively) and for Babesia spp., a 550 bpfragment of 18S rRNA gene was amplified. In total, 91 red squirrels were examined for ticks. There were differences in tick prevalence and mean abundance of infestation in squirrels from the urban forest reserve and urban park. Three species of B. burgdorferi s.l., Rickettsia spp., and Babesia microti were detected in ticks removed from the squirrels. Our results broaden knowledge of S. vulgaris as an important host for immature I. ricinus stages and support the hypothesis that red squirrels act as a reservoir of B. burgdorferi. Moreover, we conclude that red squirrels may also play a role in facilitating the circulation of other pathogens causing serious risk of tick-borne diseases in natural and urban areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks and tick-borne pathogens (TBPs) constitute serious medical and veterinary problems not only in the natural rural environment but also in urban areas (Rizzoli et al. 2014; Kowalec et al. 2017, 2019). Ixodes ricinus (the castor been tick, Linnaeus, 1758) is the most common tick species occurring in Europe with important consequences for human health (Medlock et al. 2013; Cayol et al. 2017). The mean density of I. ricinus in urban public green areas in Europe can reach 6.9 ticks/100 m² or up to 13.2 ± 0.8/100 m2 adult plus nymphs, to even 306 nymphs/100 m2 (Kazimírová et al. 2016; Kowalec et al. 2017; Hansford et al. 2022). Ixodes ricinus ticks have been collected in highly-populated metropolitan areas, including London, Hannover, Berlin, Lyon, Zagreb, Ostrava, Košice or Zurich (Dobson et al. 2011; Pangrácová et al. 2013; Venclíková et al. 2014; Oechslin et al. 2017; Vucelja et al. 2020; Hauck et al. 2020; Mathews-Martin et al. 2020; Rubel et al. 2022). The presence of this tick species has been documented also in numerous Polish cities (Stanczak et al. 2004; Cisak et al. 2006; Kiewra and Lonc 2010; Buczek et al. 2014; Król et al. 2016; Kubiak et al. 2019; Liberska et al. 2021). In urban parks of Warsaw (Royal Łazienki Museum, Kabacki and Bielański Forests) mean tick density has been recorded as exceeding 10.1 ± 0.9 ticks/100 m2 (Kowalec et al. 2017).
Lyme borreliosis (LB) is the most important TBD and is caused by bacteria from Borrelia burgdorferi s.l. complex (Burn et al. 2023). In Poland, the incidence of Lyme borreliosis varies between years, however, an increasing trend has been observed in recent years. According to data published by the National Institute of Public Health - National Institute of Hygiene, in 2023 the incidence rate was 66.9 cases per 100,000 inhabitants, in 2022 − 45.9 and in 2021 − 32.7. In 2022, LB constituted 99% of human cases of tick-borne diseases (TBDs) (data from the Website of the Ministry of Health and the National Health Fund, https://pacjent.gov.pl/aktualnosc/uwazaj-na-kleszcze), and the incidence of borreliosis in previous years ranged from 33.1 in 2013 to 56.0 in 2017, although the figure for 2020 was lower at 33.7 cases.
Warsaw, the capital of Poland, is located in the Mazowieckie Voivodeship, where the incidence of LB is one of the highest in the country. To date, eight species of Borrelia burgdorferi s.l. have been detected in I. ricinus in Poland, differing in pathogenicity and host specificity (Gryczyńska et al. 2016; Wodecka et al. 2016; Kowalec et al. 2017). Among these, Bo. burgdorferi sensu stricto, Bo. garinii and Bo. afzelii are the main causes of LB (Branda and Steere 2021). In Europe, the mean prevalence of Borrelia spp. in questing I. ricinus (adults plus nymphs) collected from green urban areas has been recorded as 17.3% (range: 3.1– 38.1%) (Hansford et al. 2022). However, higher prevalence values in I. ricinus (adults and nymphs) have also been recorded, for example 25.5% in Hannover, Germany, (Glass et al. 2023), 28.1% in Prague, Czechia (Richtrová et al. 2022) and 40% in urban parks in Bulgaria (Blazhev et al. 2022). In three locations in Warsaw (Royal Łazienki Museum, Bielański and Kabacki Forests) prevalence of Bo. burgdorferi s.l. in I. ricinus (adults and nymphs) has been recorded as approximately 11% (Kowalec et al. 2017).
The DNA of Bo. miyamotoi, which can cause tick-borne relapsing fever (TBRF), has been identified in I. ricinus collected in city parks in Warsaw and other cities in Poland (Kowalec et al. 2017; Kubiak et al. 2019). The castor bean tick is known also to be a vector of the spotted fever group rickettsiae (SFG), bacteria of the Rickettsia genus (Kowalec et al. 2019; Welc-Falęciak et al. 2014) and of species of Babesia (Ba. microti, Ba. divergens, Ba. venatorum) (Azagi et al. 2021; Bajer and Dwużnik-Szarek 2021), which can cause human babesiosis, a disease that is especially hazardous for the splenectomized, elderly or immunocompromised individuals and pregnant women (Hildebrandt et al. 2021; Bajer et al. 2022).
Small rodents play an important role as hosts of I. ricinus larvae and nymphs (high intensity of I. ricinus infestation) and may act as reservoirs of TBPs (i.a. Bo. miyamotoi, Bo. burgdorferi s.l., tick-borne encephalitis virus (Orthoflavivirus encephalitidis), Bartonella spp. Ba. microti, Neoehrlichia mikurensis; Mihalca and Sandor, 2013; Grzybek et al. 2018; Dwużnik et al. 2019; Galfsky et al. 2019; Dwużnik-Szarek et al. 2021; Postler et al. 2023; Tołkacz et al. 2023). However, the rodent fauna of city parks is often depauperized in species (Gortat et al. 2014), and thus, red squirrels whose populations can reach very high densities in urban habitats (Beliniak et al. 2022), may act as important hosts for I. ricinus in urban areas (Sormunen et al. 2023), including Warsaw.
Pursuant to the Regulation of the Polish Ministry of the Environment (Dziennik Ustaw 2016 item 2183) released on 16th December 2016, red squirrels are partially protected species in Poland. They are a synurbic species, adapting well to environmental changes, occurring not only in natural forests but also in urban green areas (Krauze-Gryz et al. 2016; Babińska-Werka and Żółw 2012; Fey et al. 2016; Hämäläinen et al. 2018; Lurz et al. 2017; Fingland et al. 2021). The mean density of S. vulgaris can be higher in urban areas, compared to natural habitats, because of better access to supplemental food (walnuts, hazelnuts, peanuts) and other anthropogenic food sources (Krauze-Gryz et al. 2021; Fingland et al. 2021). Red squirrels are very common in green areas of Warsaw, especially in the park of the Royal Łazienki Museum (Babińska-Werka and Żółw 2008; Krauze-Gryz et al. 2020; Beliniak et al. 2022).
Data on tick infestation in red squirrels and on the pathogens carried by ticks from squirrels are limited (Humair and Gern 1998; Luu et al. 2021). However, some species of spirochaetes, including Bo. garinii, Bo. afzelii, Bo. burgdorferi s.s., and even Bo. miyamotoi have been recorded in tissue samples from red squirrels (skin, ear tissue samples; Humair and Gern 1998; Paulauskas et al. 2008; Pisanu et al. 2014). Furthermore, the DNA of other important TBPs, Anaplasma phagocytophilum (Ruyts et al. 2017) and Hepatozoon sp. (Modrý et al. 2021) has also been found in ticks collected from squirrels. However, to date no studies of tick infestations on red squirrels or of pathogens carried by squirrel-borne ticks have been conducted in Poland. We hypothesise that the large population of squirrels in urban parks and suburban forests contributes to the maintenance of high tick densities in these areas and, consequently, TBPs in the human environment. In urban areas, squirrels could significantly increase the transmission risk of TBPs (especially from the Borrelia genus) to humans and domestic animals (Pisanu et al. 2014; Ruyts et al. 2017).
Materials and methods
Study area and tick collection
Red squirrels were live-trapped at two sites within the administrative borders of Warsaw, the capital city of Poland.
An urban park: the Royal Łazienki Museum
The Royal Łazienki Museum is a historic park created by King Stanislaw II August Poniatowski in the 18th century. The park area (approximately 76 ha) is fenced (Kowalec et al. 2017) and located on the Vistula escarpment close to the city centre of Warsaw (52°12′53″N, 21°01′58″E; Fig. 1). According to an annual report, the number of tourists visiting the Royal Łazienki Museum has been increasing annually, from 3.5 million people in 2018 to more than 4 million visitors in 2022 (the Warsaw Tourism Organization, https://wot.waw.pl/Wiedza/). The park is a well-managed area, with numerous species of deciduous and coniferous trees, i.e. red oak (Quercus rubra), pedunculate oak (Q. robur), common hornbeam (Carpinus betulus), Norway spruce (Picea abies), silver fir (Abies alba), European larch (Larix deciudua) and open mowed areas (https://www.lazienki-krolewskie.pl/pl/ogrody/fauna-i-flora) (Fig. 2). Despite the lack of large animals, the presence of different potential hosts for I. ricinus has been observed, i.e. yellow necked mouse (A. flavicollis), striped field mouse (A. agrarius), the European hedgehog (Erinaceus roumanicus), the red fox (Vulpes vulpes) and passerine birds, especially black birds Turdus merula (Krauze-Gryz et al. 2016; Gryczyńska and Kowalec 2019; Dwużnik et al. 2020; Dwużnik-Szarek et al. 2021; https://www.lazienki-krolewskie.pl/pl/ogrody/fauna-i-flora). Companion animals are not allowed in the park.
Study areas: (1) the Royal Łazienki Museum and (2) the Natolin Forest Reserve, where red squirrels were live-trapped (from: Body condition and breeding of urban red squirrels: comparison of two Populations affected by different levels of urbanization; Beliniak et al. 2022)
The Royal Łazienki Museum (from https://www.lazienki-krolewskie.pl)
An urban forest reserve: the Natolin Forest Reserve
The Natolin Forest Reserve is a remnant of the former natural Masovian forest. It is located about 10 km south of the Warsaw city centre (52°8′20″ N, 21°4′25″ E; Fig. 1), covering an area of 105 ha. The forest has been under protection since 1991 according to the Ordinance of the Minister of Environmental Protection, Natural Resources and Forestry, (M.P. 1991 No 38, item 273) and access to the park is limited (http://warszawa.rdos.gov.pl/las-natolinski-jest-udostepniony-spoleczenstwu-3). Special permission is required to enter the reserve. There are no paths/trails within the Natolin Forest Reserve. The tree stand consists of pedunculate oak, hornbeam, ash (Fraxinus excelsior), elms (Ulmus spp.), hazel (Corylus sp.), and black alder (Alnus glutinosa). It is a breeding place for foxes, badgers (Meles meles), and raccoon dogs (Nyctereutes procyonoides). Weasels (Mustela nivalis), European polecats (M. putorius), voles (Microtus spp.), common shrews (Sorex araneus), Eurasian pygmy shrews (S. minutus), brown hares (Lepus europaeus), roe deer (Capreolus capreolus), beech martens (Martes foina) and hedgehogs have been observed (https://iwaw.pl/obiekt.php?p=312805017; Krauze-Gryz D., pers. obs; Jackowiak et al. 2021).
Red squirrel trapping methods
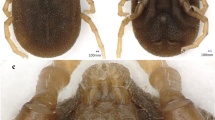
The methods utilised for trapping red squirrels have been fully described by Beliniak et al. (2021). In short, squirrels were live-trapped with 40 traps in the Natolin Forest Reserve and 30 traps in the Royal Łazienki Museum. Standard wire mesh live traps (51 × 15 × 15 cm) (manufactured by “Jerzyk” Jerzy Chilecki, Białowieża, Poland) were used. The traps were partly covered by dark plastic to provide shelter from rain and snow and were located on the ground or trees on a wooden platform. Live traps were pre-baited with hazelnuts and English walnuts for seven days. Then we trapped squirrels for four (in most cases) to nine days. The traps were baited and set in the morning (around 6–7 a.m., depending on the time of dawn), checked after 2–4 h, and secured for the night in a manner that prevented them from being closed. Every trapped squirrel was flushed into a wire mesh handling cone (Lurz et al. 1997; Fig. 3) to minimize stress during handling. Each newly trapped squirrel was individually marked with a numbered ear-tag 2 × 8 mm (National Tag&Band, Newport, KY, USA). Sex and age of trapped animals were recorded (Santicchia et al. 2018). Ticks were collected from handled animals and placed in 1.5 ml Eppendorf tubes, then transported to the laboratory of the Department of Eco-Epidemiology of Parasitic Diseases, Faculty of Biology, University of Warsaw, and frozen at -20ºC. Identification of collected ticks to species and developmental stage levels was facilitated by a morphological key (Estrada-Peña et al. 2018) and the use of a stereoscopic microscope Zeiss Stemi 508.
DNA extraction
The commercial Genomic Mini AX Tissue Spin kit (A&A Biotechnology, Gdańsk, Poland), was used for DNA extraction according to the manufacturer’s instructions. To increase the efficiency of the molecular component, larvae were processed in pools, comprising 2–10 larvae from a single host, while adult ticks and nymphs were processed individually.
In order to detect infection with Bo. burgdorferi s.l. and/or Bo. miyamotoi, nested PCR was performed using two sets of primers: 132f (5’-TGGTATGGGAGTTTCTGG-3’)/905r (5’-TCTGTCATTGTAGCATCTTT-3’) for amplification of 774 bp and 220f (5’CAGACAACAGAGGGAAAT-3’)/824r (5’-TCAAGTCTATTTTGGAAAGCACC-3’) for amplification of 605 bp product for the flagellin (flaB) gene (Wodecka et al. 2009). Reaction conditions were as provided by Kowalec et al. (2017) and Dwużnik-Szarek et al. (2021). For bacteria of the genus Rickettsia, primers CS409 (5’-CCTATGGCTATTATGCTTGC-3’) and Rp1258 (5’-ATTGCAAAAAGTACAGTGAACA-3’) were used, amplifying a 750 bp fragment of the citrate synthase (gltA) gene (Roux et al. 1997) with modified reaction conditions as described by Kowalec et al. (2019).
Nested PCR was performed to detect Babesia DNA. In the first reaction, primers CryptoF (5’AACCTGGTTGATCCTGCCAGT3’)/ CryptoR (5’GCTTGATCCTTCTGCAGGTTCACCTAC3’) were used to amplify about 1200 bp gene fragment of small subunit ribosomal RNA (18S rRNA gene). In the second reaction, primers BabGR2 (5’-CCAAAGACTTTGATTTCTCTC-3’)/ BabGF2 (5’-GYYTTGTAATTGGAATGATGG-3’) were used to amplify a 550 bp fragment (Bonnet et al. 2007; Tołkacz et al. 2017). The reaction conditions were the same for both PCR steps and described in details by Tołkacz et al.(2017).
Selected PCR products were commercially sequenced by Genomed company (Warsaw, Poland). Sequence alignments and analyses were carried out using BLAST-NCBI and MEGA X software (Kumar et al. 2018). Phylogenetic analyses were performed using the Maximum Likelihood method of tree construction. The evolutionary model was chosen in accordance with the data (following implemented model test in MEGA X) and bootstrapped over 1000 randomly generated sample trees (Dwużnik-Szarek et al. 2021). For Borrelia spp., the evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano + G model) based on the approximately 605 bp fragment of flaB gene, incorporating seven sequences obtained in this study and 18 reference sequences from GenBank. For Rickettsia spp. analyses, the evolutionary history was inferred by using the Maximum Likelihood method a Hasegawa-Kishino-Yano + G model based on the approximately 750 bp fragment of gltA gene The analysis included 22 nucleotide sequences (13 obtained in this study and 9 sequences from GenBank).
Statistical analyses
Statistical analyses were conducted for evaluation of the effect of host intrinsic factors (sex and age), and extrinsic factors including study site, year and month of trapping on tick infestation and presence of pathogen DNA in ticks collected from squirrels.
All statistical analyses were conducted in PS IMAGO PRO Academic v.7 (IBM SPSS Statistics software, institutional license purchased by the University of Warsaw, Warsaw, Poland). The statistical approach analysis has been documented in our previous papers (Behnke et al. 2001; Mierzejewska et al. 2020; Dwużnik-Szarek et al. 2021).
For analysis of prevalence (% positive-tested samples), we applied maximum likelihood techniques based on log-linear analysis of contingency tables in IBM SPSS software. SITE of red squirrel trapping (two levels: an urban forest reserve and an urban park), SEX of squirrels (males and females), AGE (two levels: young, adult), YEAR (two levels: 2019 and 2020), MONTH (five levels: March, May, June, July, September), were used as the factors in models with the presence or absence of ticks and referred to as INFESTATION or PRESENCE/ABSENCE of pathogen DNA considered as a binary factor (0, 1). For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest-level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of χ2 was not significant, indicating that the model was sufficient in explaining the data. The importance of each term in interactions involving INFESTATION or PRESENCE/ABSENCE of pathogen in the final model was assessed by the probability that its exclusion would alter the model significantly and these values are given in the text, assessed by a likelihood ratio test between nested models with and without each factor of interest.
General linear models (GLMs; analyses also conducted in IBM SPSS software) were used for the analysis of mean intensity of tick infestation on squirrels, using models with normal errors, incorporating SEX and AGE of trapped squirrels, YEAR, SITE and MONTH in which the squirrels were trapped as fixed factors. Means are presented with the standard error of the mean (S.E.).
Ethical approval
The study was performed with the approval of the Regional Director for Environmental Protection (license number: WPN-I.6205.124.2018.AS and WPN-I.6401.208.2018.PF). Trapping and handling squirrels complied with current regulations on conducting experimental studies on animals in Poland and Europe and were carried out under a license obtained from the Second Local Ethical Committee in Warsaw (license number WAW2/072/2018).
Results
In total, 91 (52 males and 39 females; 12 young and 79 adults) red squirrel individuals were examined for ticks (some were re-trapped, resulting in 185 checks), including records of 105 males and 80 females; 173 records of adults and 12 records of juvenile individuals (Table 1). Sixty red squirrels were from the urban forest reserve and 31 from the urban park. In total, 458 I. ricinus ticks (72 larvae, 372 nymphs, 12 females, and 2 males) were collected (Table 2A).
Prevalence of tick infestation
The general infestation rate was 56.8% [95% CI: 49.56–63.74]. In 2019, the prevalence of tick infestation was lower than in 2020 (48.4% [95%CI: 38.41–58.46] vs. 65.2% [95%CI: 55.13–74.36]) (tick presence/absence × YEAR: χ21 = 5.37, P < 0.02). Significantly higher tick infestation was observed in May and June in comparison to the remaining months (tick presence/absence × MONTH: χ24 = 27.36, P < 0.001; Table 3).
The prevalence of I. ricinus infestation among squirrels trapped in the urban forest reserve (87.3%), was twice higherin the urban park (34%) (tick presence/absence × SITE: χ21 = 57.22, P < 0.001). Similar tick prevalence was observed for male and female red squirrels (60.4% vs. 51.4%; (P > 0.05), and there was no significant difference in prevalence between age classes as well (P > 0.05).
Mean intensity of tick infestation
Overall, the mean intensity of tick infestation was 4.19 ± 0.48 ticks/individual. As with prevalence, significantly higher mean intensity was observed in animals trapped in 2020 (5.38 ± 0.76 ticks/individual) in comparison to 2019 (3.00 ± 0.44 ticks/individual; main effect of YEAR on I. ricinus intensity of infestation: F1,104 = 6.28, P = 0.01). Mean intensity was two times higher among squirrels from the urban forest reserve compared to those from the urban park (5.25 ± 0.67 and 2.67 ± 0.49 ticks/individual, respectively; main effect of SITE on I. ricinus intensity of infestation: F1,104 = 6.80, P = 0.01) and it was higher for red squirrels trapped in June and September compared to the remaining months (main effect of MONTH on I. ricinus intensity of infestation: F4,104 = 10.36, P < 0.001; Table 3).
Molecular detection of pathogen DNA in ticks
In total, 377 I. ricinus ticks from 55 rodents were examined by molecular techniques, encompassing 9 females, one male, 306 nymphs, and 61 larvae in 34 pools (350 samples) (Table 2B).
The DNA of a single pathogen was identified in 15.9% [95% CI: 12.45–20.11]of ticks, and co-infection was detected only in one nymph from the urban park (Ba. microti + Bo. afzelii; prevalence of co-infection = 0.3%). A similar prevalence of pathogen DNA was observed in ticks collected in 2019 and 2020 (18%[95% CI: 12.07–25.3] and 14.9% [95% CI: 10.65–19.99], respectively; P > 0.05). There were also no differences in the prevalence of pathogens detected in ticks collected from squirrels trapped in different months. A higher prevalence of pathogen DNA was found in ticks collected from the urban forest reserve (18.3%)[95% CI: 14.13–23.31] compared to ticks from the urban park (7.5%; [95% CI: 3.27–15.19]; pathogen DNA presence/absence × SITE: χ21 = 6.22, P = 0.01). Pathogen DNA was identified in 20% of larval pools, 14% of nymphs, and 10% of adults (P > 0.05).
Detection of Borrelia spp. DNA in ticks from squirrels
Total prevalence of Bo. burgdorferi s.l. was 2.5% [95% CI:1.28–4.64] (9/350; one larva, eight nymphs and one female collected from nine rodents with two retrapped two times). Prevalence differed between years of tick collection and was 5.4% [95% CI: 2.48–10.44] in 2019 but only 0.9% [95%CI: 0.19–2.86] in 2020 (YEAR × Borrelia spp. presence/absence: χ21 = 6.46, P = 0.01). Borrelia spp. DNA was detected only in ticks collected in May (6.3%) [95% CI: 2.85–12.3] and in September (3.3%)[95% CI: 0.69–10.1] (MONTH × Borrelia spp. presence/absence: χ24 = 13.97, P = 0.01). There were no differences in prevalence between sites and tick stages (P > 0.05).
Seven sequences from nine positive samples of Borrelia spp. (four from nymphs from the urban forest reserve and one nymph, one female, and one larval pool from the urban park) were obtained. Three species were identified, Bo. afzelii, Bo. garinii, and Bo. burgdorferi s.s. Five Bo. afzelii sequences with a 99.9% similarity to B. afzelii from I. ricinus, Turkey (GenBank Acc. No. MK922620) were detected in nymphs from the urban forest reserve as well as in a nymph and a female from the urban park. One Bo. garinii (100% identity to Bo. garinii from I. ricinus, Poland (MK604263)) was found in a nymph from the urban forest, and one Bo. burgdorferi s.s. (99.8% identity to Bo. burgdorferi s.s. from I. ricinus, Poland (MW791413)) was detected in a larval pool from the urban park. The phylogenetic tree of Borrelia spp. is presented in Fig. 4.
Molecular phylogenetic analysis of flaB gene fragment of Borrelia burgdorferi s.l. (605 bp). The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree with the highest log likelihood (-1557.49) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+ G, parameter = 0.4006)). This analysis involved 25 nucleotide sequences. There were a total of 472 positions in the final dataset. Evolutionary analyses were conducted in MEGA X
Detection of Babesia sp. DNA in ticks from squirrels
The DNA of Babesia sp. was found only in one nymph from a squirrel from the urban park (total prevalence 0.3% [95% CI: 0.03–1.33]), and it had 100% identity to B. microti from I. ricinus from Turkey (OM066130).
Detection of Rickettsia spp. DNA in ticks from squirrels
Overall prevalence of Rickettsia spp. was 13.9% [95%CL: 10.67–17.93] 49/350; six pooled larvae, forty nymphs and and three females collected from 26 rodents with 12 of individuals were retrapped two to four times), and was similar in both years, 13.1% [95%CI: 8.24–19.96] in 2019 and 14.3% [95%CI: 10.27–19.48] in 2020, and in the months of trapping (P > 0.05). Prevalence of Rickettsia was three times higher among ticks from squirrels from the urban forest reserve than those from the urban park (16.5% [95%CI: 12.49–21.3] vs. 5.0%[95%CI: 1.76–11.73]) (SITE × Rickettsia spp. presence/absence: χ21 = 8.22, P = 0.004). Rickettsia DNA was detected in 30% [95%CI: 9.27–60.58] of adult ticks, 17% [95%CI: 7.72–32.81] of nymphs and 13% of larvae pools [95%CI: 9.65–17.19] (P > 0.05).
Thirteen Rickettsia PCR-positive products were sequenced (from five larval pools, three nymphs, and one female from the urban forest reserve as well as from two nymphs and one female from the urban park). Three Rickettsia species were identified: R. raoultii from larval pool from the urban forest reserve showed the 100% identity to the sequence from D. reticulatus from France (GenBank DQ365803); eleven R. helvetica sequences (from two females and one nymph from the urban park as well as four larvae, three nymphs and one female from the urban forest reserve) were 100% identical with a sample from A. agrarius from Poland (KY488349); and one R. monacensis from larval pool from the urban forest reserve had the highest identity (99.81%) with the sequence from tick collected from cat in Japan (GenBank Acc. No. LC507574; Fig. 5).
Molecular phylogenetic analysis of citrate synthase (gltA) gene fragment of Rickettsia (750 bp). The evolutionary history was inferred by using the Maximum Likelihood method and Hasegawa-Kishino-Yano model. The tree with the highest log likelihood (-1343,27) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+ G, parameter = 0,2041)). This analysis involved 22 nucleotide sequences. There were a total of 667 positions in the final dataset. Evolutionary analyses were conducted in MEGA X
Discussion
In the present study, we confirmed the role of red squirrels as important hosts for I. ricinus ticks and recovered the DNA of seven pathogens from the ticks collected from these animals captured in the urban areas.
The prevalence of infestation of red squirrels by the castor bean tick was 56.8%. A much higher infestation by I. ricinus (88%) was observed in squirrels caught in Norway (Mysterud et al. 2021). This difference may be due to the higher number of animals tested in our study (91 squirrels in our study and 17 from Norway) and the longer period of sampling, including months of lower tick activity. High tick infestation of red squirrels has also been recorded on two islands, Brownsea (88.9%) and Arran (53.2%) in the UK (Luu et al. 2021). In similar studies conducted in France, the recorded prevalence of I. ricinus infestation on squirrels reached 34% (Romeo et al. 2013). However, even a lower infestation of I. ricinus (25%) was identified among road-killed red squirrels in the UK (Simpson et al. 2013). Examining a host under anaesthesia and/or live trapped animals as in our case, reduces the likelihood of postmortem loss of ectoparasites, and consequently, ectoparasite burden can be expected to be higher compared with road-killed animals (Tahir et al. 2020).
In our study, we trapped red squirrels in two urban settings. A significantly higher prevalence of I. ricinus infestation was noted among squirrels from the urban Natolin Forest Reserve compared to those from the Royal Łazienki Museum. The Royal Łazienki Museum is a managed park, located near the city centre, and the lawns are regularly mowed, which may reduce the prevalence of tick infestation in squirrels. It has been demonstrated previously that regular mowing and maintenance of green areas can significantly contribute to reducing tick densities (Medlock et al. 2012; Bajer et al. 2017), thus the number of infested hosts. In the study conducted by Kowalec et al. (2017), higher tick density (collected from vegetation) was recorded in the natural Białowieża Forest in comparison to three urban parks in Warsaw (Kabacki and Bielański forests and the Royal Łazienki Museum; Kowalec et al. 2017).
The overall prevalence of Bo. burgdorferi s.l. infection in I. ricinus collected from squirrels in our study was low (2.5%). More than five times higher prevalence of Borrelia spp. (12.8%) was found in ticks collected from red squirrels in the UK (Luu et al. 2021). The high prevalence of Borrelia-positive I. ricinus ticks collected from vegetation can be a source of infection for many host species, including red squirrels occurring in Warsaw parks. In 2009–2010, a prevalence of 5.7% for Borrelia spp. was observed in adult ticks collected from vegetation in the city park of the Royal Łazienki Museum (Chmielewski et al. 2011). During the study conducted in 2012–2015, the prevalence of Borrelia-positive I. ricinus (adults plus nymphs) reached more than 17% (Kowalec et al. 2017). Those studies show that infection of Borrelia spp. could fluctuate between years. Similar to the prevalence of Babesia canis in Dermacentor reticulatus tick populations, that may indicate the incidence of babesiosis in dogs, prevalence of Borrelia in I. ricinus collected from vegetation may indicate the risk of infection of potential hosts (Dwużnik-Szarek et al. 2021).
The sequencing of our samples revealed the presence of three genospecies of Borrelia: Bo. afzelii, Bo. garinii, and Bo. burgdorferi s.s. Borrelia afzelii and Bo. burgdorferi s.s. were detected in ticks collected from rodents trapped in Royal Łazienki Museum, while in Natolin Forest Reserve Bo. garini and Bo. afzelii were detected. All of Borrelia genospecies found in our study were previously noted in I. ricinus collected from vegetation in urban and natural areas (Ruyts et al. 2018; Heylen et al. 2019, Grochowska et al. 2020). Research conducted by Kowalec et al. (2017) revealed dominance of B. afzelii (69.3%) in urban and B. garinii (48.1%) in natural areas in ticks collected from vegetation (Kowalec et al. 2017). In Switzerland, two Borrelia species (Bo. afzelii and Bo. burgdorferi s.s) were identified in ticks collected from red squirrels (Humair and Gern 1998). In that study, 15 squirrel skin samples were also tested, in which both Borrelia species were detected (Humair and Gern 1998). Many other studies conducted in France, Belgium and Hungary have confirmed the presence of Bo. afzelii, Bo. miyamotoi, Bo. garinii, Bo. burgdorferi s.s., Bo. bissetti, and Bo. carolinensis in red squirrel tissues/blood samples with prevalence ranging from 3.5% to even 45% (Pisanu et al. 2014; Ruyts et al. 2017; Szekeres et al. 2019; Majerová et al. 2020). Interestingly, in our study, the sequences of Bo. garinii (from I. ricinus nymph collected from red squirrels trapped in the Natolin Forest Reserve; Fig. 4) clustered close with the sequence identified as Bo. garinii subsp. bavariensis (KU372549). Both genospecies differ in their suspected reservoir hosts (B. garinii: birds, B. bavariensis: rodents) and both have been detected in I. ricinus in Europe (Margos et al. 2019). Borrelia bavariensis (previously known as Bo. garinii OspA serotype 4) was raised to species level in 2009 and thus separated from its sister species B. garinii, however, using just one gene marker might not allow differentiation between them (Margos et al. 2019).
We detected only one Ba. microti-positive I. ricinus tick, a nymph from the red squirrel from the urban park. To the best of our knowledge, there are just two reports of Babesia presence in red squirrels and one finding of Babesia DNA in ticks feeding on squirrels (Tsuji et al. 2006; Lipatova et al. 2017). In Japan, a B. microti-like strain was found in three of six examined squirrels (Tsuji et al. 2006). Surprisingly, in red squirrels in Lithuania, the DNA of Babesia spp. was detected in 57.6% of squirrels and13.4% of ticks collected from these hosts (Lipatova et al. 2017). Our result (prevalence 0.3%) however, was similar to the very low prevalence of Babesia spp. (usually below 2%) recorded in I. ricinus ticks collected from vegetation in many countries in Europe, including Poland (Cotte´ et al. 2010; Schorn et al. 2011; Egyed et al. 2023; Øines et al. 2012; Sytykiewicz et al. 2015; Hamšíková et al. 2016; Karlsson and Andersson 2016; Wilhelmsson et al. 2021; Grochowska et al. 2022; Wondim et al. 2022). Interestingly, the Ba. microti positive nymph was co-infected with Bo. afzelii.
The role of the red squirrel as a reservoir of Rickettsia is poorly investigated. We found only one report, based on work conducted in Lithuania, where the DNA of Rickettsia spp. was found in red squirrel tissues (prevalence 12.1%) and in ticks collected from this host (prevalence about 23%; Lipatova et al. 2017). In our study, the overall prevalence of the Rickettsia spp. detected in ticks collected from red squirrels was the highest of all tested pathogens and reached almost 14%. The prevalence in the urban park was much lower (5%) than in the urban forest (16%). Higher rate of infection of ticks with bacteria of the Rickettsia genus may be caused by the higher prevalence and intensity of tick infestation observed in squirrels from the natural forest than from the urban park. Bacteria from the Rickettsia genus are usually prevalent in I. ricinus populations including ticks collected from vegetation in the Warsaw areas. Previous studies from the Royal Łazienki Museum revealed Rickettsia prevalence of 6.5-7.7% or even 26% in questing I. ricinus (Chmielewski et al. 2009; Welc-Falęciak et al. 2014; Kowalec et al. 2019), which is in line with our findings from this location.
In the present study, three species of Rickettsia were identified: R. raoultii, R. helvetica, and R. monacensis, all pathogenic to humans. Every year the National Institute of Public Health reports several cases of rickettsiosis in Poland (https://www.pzh.gov.pl/) but the number of cases seems to be underestimated due to the non-specific symptoms of the infection (Kirczuk et al. 2021). Rickettsia monacensis was first identified in 1989 in Munich, Germany (Simser et al. 2002). In Poland, the first finding of R. monacensis in I. ricinus was published in 2012 (Rymaszewska and Piotrowski 2013). Rickettsia raoultii is highly prevalent in D. reticulatus populations (Dwużnik-Szarek et al. 2022) but has been also noted in I. ricinus (Stańczak et al. 2012). The presence of R. raoultii and R. monacensis DNA in I. ricinus larvae collected from red squirrels may indicate the source of these pathogens from the host. However, as bacteria from the genus Rickettsia can be transmitted via transstadial and transovarial routes, the infections could be inherited (Nováková and Šmajs 2018). Another possibility is via the meal contamination phenomenon described by Dwużnik et at. (2019). We cannot exclude that Rickettsia-positive larvae co-fed with other ticks infected with these pathogens on the same hosts. This is an interesting observation that confirms the value of conducting research on tick-host-pathogen interactions. Rickettsia helvetica, the most commonly detected species in our samples (in all life stages), has been detected frequently in questing I. ricinus in Poland, also from the area of Warsaw (Wodecka et al. 2014; Stańczak et al. 2012; Kowalec et al. 2019). All three Rickettsia species were detected in ticks from the urban forest, while in the urban park, only R. helvetica was found.
Conclusions
Red squirrels constitute a permanent element of the urban fauna and are a significant host for I. ricinus, especially for larvae and nymphs. Considering the large populations of red squirrels in Warsaw, this rodent may act as an important reservoir of tick-borne pathogens, mainly bacteria of the B. burgdorferi s.l. complex. The detection of the DNA of Babesia and Rickettsia in ticks collected from squirrels indicates the persistence of these pathogens in the urban environment. It is now necessary to verify whether, as in the case of Borrelia, the red squirrel can be a reservoir of both Babesia and Rickettsia spp. and thus contribute to the circulation of these pathogens in Warsaw.
Data availability
We declare all data is being provided within this manuscript.
References
Azagi T, Jaarsma RI, van Docters A, Fonville M, Maas M, Franssen FFJ, Kik M, Rijks JM, Montizaan MG, Groenevelt M, Hoyer M, Esser HJ, Krawczyk AI, Modrý D, Sprong H, Demir S (2021) Circulation of Babesia species and their exposure to humans through Ixodes ricinus. Pathogens 10:386. https://doi.org/10.3390/pathogens10040386
Babińska-Werka and Żółw M (2008) Urban populations of the red squirrel (Sciurus vulgaris) in Warsaw. Ann Zool Fenn 45:270–276. https://doi.org/10.5735/086.045.0405
Babińska-Werka J, Żółw M (2012) Urban populations of the red squirrel (Sciurus vulgaris) in Warsaw. Ann Zool Fenn 45:270–276. https://doi.org/10.5735/086.045.0405
Bajer A, Dwużnik-Szarek D (2021) The specificity of babesia-tick vector interactions: recent advances and pitfalls in molecular and field studies. Parasit Vectors 14:507. https://doi.org/10.1186/s13071-021-05019-3
Bajer A, Rodo A, Alsarraf M, Dwużnik D, Behnke JM, Mierzejewska EJ (2017) Abundance of the tick Dermacentor reticulatus in an ecosystem of abandoned meadows: experimental intervention and the critical importance of mowing. Vet Parasitol 246:70–75. https://doi.org/10.1016/j.vetpar.2017.09.004
Bajer A, Beck A, Beck R, Dwużnik-Szarek D, Eichenberger RM, Farkas R, Fuehrer HP, Heddergott M, Jokelainen P, Leschnik M, Oborina V, Paulauskas A, Radzijevskaja J, Ranka R, Schnyder M, Springer A, Strube C, Tolkacz K, Walochnik J (2022) Babesiosis in Southeastern, central and Northeastern Europe: an emerging and re-emerging tick-borne disease of humans and animals. Microorganisms 10:945. https://doi.org/10.3390/microorganisms10050945
Behnke JM, Barnard CJ, Bajer A, Bray D, Dinmore J, Frake K, Osmond J, Race T, Sinski E (2001) Variation in the helminth community structure in bank voles (Clethrionomys glareolus) from three comparable localities in the Mazury Lake District region of Poland. Parasitology 123:401–414. https://doi.org/10.1017/S0031182001008605
Beliniak A, Krauze-Gryz D, Jasińska K, Jankowska K, Gryz J (2021) Contrast in daily activity patterns of red squirrels inhabiting urban park and urban forest. Hystrix 32:159–164. https://doi.org/10.4404/hystrix-00476-2021
Beliniak A, Gryz J, Klich D, Krauze-Gryz D, Jasińska K (2022) Body condition and breeding of urban red squirrels: comparison of two populations affected by different levels of urbanization. Animals 12:3246. https://doi.org/10.3390/ani12233246
Blazhev A, Stanilov I, Miteva LD, Atanasova M, Blazheva S, Stanilova S (2022) Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from Kaylaka Park in Pleven, Bulgaria. Microorganisms 10, 772. https://doi.org/10.3390/microorganisms10040772
Bonnet S, Jouglin M, L’Hostis M, Chauvin A (2007) Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg Infect Dis 13:1208. https://doi.org/10.3201/eid1308.061560
Branda JA, Steere AC (2021) Laboratory diagnosis of Lyme borreliosis. Clin Microbiol Rev 34:10–1128. https://doi.org/10.1128/cmr.00018-19
Burn L, Pilz A, Vyse A, Gutiérrez Rabá AV, Angulo FJ, Tran TMP, Fletcher MA, Gessner BD, Moïsi JC, Stark JH (2023) Seroprevalence of lyme borreliosis in Europe: results from a systematic literature review (2005–2020). Vector Borne Zoonotic Dis 23:195–220. https://doi.org/10.1089/vbz.2022.0069
Buczek A, Ciura D, Bartosik K, Zając Z, Kulisz J (2014) Threat of attacks of Ixodes ricinus ticks (Ixodida: Ixodidae) and Lyme borreliosis within urban heat islands in south-western Poland. Parasit Vectors 7:562. https://doi.org/10.1186/s13071-014-0562-y
Cayol C, Koskela E, Mappes T, Siukkola A, Kallio ER (2017) Temporal dynamics of the tick Ixodes ricinus in Northern Europe: epidemiological implications. Parasit Vectors 10:1–11. https://doi.org/10.1186/s13071-017-2112-x
Chmielewski T, Podsiadly E, Karbowiak G, Tylewska-Wierzbanowska S (2009) Rickettsia spp. in ticks, Poland. Emerg Infect Dis 15:486–488. https://doi.org/10.3201/eid1503.080711
Chmielewski T, Andrzejewski K, Mączka I, Fiecek B, Radlińska M, Tylewska-Wierzbanowska S (2011) Ticks infected with bacteria pathogenic to humans in municipal parks in Warsaw. Przegl Microbiol 65:577–581
Cisak E, Wójcik-Fatla A, Stojek N, Chmielewska-Badora J, Zwoliński J, Buczek A, Dutkiewicz J (2006) Prevalence of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from Lublin Region (Eastern Poland). Ann Agric Environ Med 13:2
Cotté V, Bonnet S, Cote M, Vayssier-Taussat M (2010) Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis 10:723–730. https://doi.org/10.1089/vbz.2009.0066
Dobson A, Taylor JL, Randolph SE (2011) Tick (Ixodes ricinus) abundance and seasonality at recreational sites in the UK: hazards in relation to fine-scale habitat types revealed by complementary sampling methods. Ticks Tick Borne Dis 2:67–74. https://doi.org/10.1016/j.ttbdis.2011.03.002
Dwużnik D, Mierzejewska EJ, Drabik P, Kloch A, Alsarraf M, Behnke JM, Bajer A (2019) The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of ‘meal contamination’. Exp Appl Acarol 78:181–202. https://doi.org/10.1007/s10493-019-00380-6
Dwużnik D, Mierzejewska E, Kowalec M, Alsarraf M, Stańczak Ł, Opalińska P, Krokowska-Paluszak M, Górecki G, Bajer A (2020) Ectoparasites of red foxes (Vulpes vulpes) with a particular focus on ticks in subcutaneous tissues. Parasitology 147:1359–1368. https://doi.org/10.1017/S003118202000116X
Dwużnik-Szarek D, Mierzejewska E, Alsarraf M, Alsarraf M, Bajer A (2021) Pathogens detected in the tick Haemaphysalis concinna in Western Poland: known and unknown threats. Exp Appl Acarol 84:769–783. https://doi.org/10.1007/s10493-021-00647-x
Dwużnik-Szarek D, Mierzejewska EJ, Kiewra D, Czułowska A, Robak A, Bajer A (2022) Update on prevalence of Babesia canis and Rickettsia spp. in adult and juvenile Dermacentor reticulatus ticks in the area of Poland (2016–2018). Sci Rep 12:5755. https://doi.org/10.1038/s41598-022-09419-y
Egyed L, Nagy D, Lang Z (2023) Features of engorgement of Ixodes ricinus ticks infesting the northern white-breasted hedgehog in an urban park. Microorganisms 11:881. https://doi.org/10.3390/microorganisms11040881
Estrada-Peña A, Mihalca AD, Petney TN (2018) Ticks of Europe and North Africa: a guide to species identification. Springer. https://doi.org/10.1007/978-3-319-63760-0
Fauna i Flora of the Royal Łazienki Museum https://www.lazienki-krolewskie.pl/pl/ogrody/fauna-i-flora
Fey K, Hämäläinen S, Selonen V (2016) Roads are no barrier for dispersing red squirrels in an urban environment. Behav Ecol 27:741–747. https://doi.org/10.1093/beheco/arv215
Fingland K, Ward SJ, Bates AJ, Bremner-Harrison S (2021) A systematic review into the suitability of urban refugia for the eurasian red squirrel Sciurus vulgaris. Mammal Rev 52:26–38. https://doi.org/10.1111/mam.12264
Galfsky D, Król N, Pfeffer M, Obiegala A (2019) Long-term trends of tick-borne pathogens in regard to small mammal and tick populations from Saxony. Ger Parasit Vectors 12:1–14. https://doi.org/10.1186/s13071-019-3382-2
Glass A, Springer A, Raulf MK, Fingerle V, Strube C (2023) 15-year Borrelia prevalence and species distribution monitoring in Ixodes ricinus/inopinatus populations in the city of Hanover, Germany. Ticks Tick Borne Dis 14:102074. https://doi.org/10.1016/j.ttbdis.2022.102074
Gortat T, Barkowska M, Gryczyńska-Siemiątkowska A, Pieniążek A, Kozakiewicz A, Kozakiewicz M (2014) The effects of urbanization — small mammal communities in a gradient of human pressure in Warsaw city, Poland. Pol J Ecol 62:163–172. https://doi.org/10.3161/104.062.0115
Grochowska A, Milewski R, Pancewicz S, Dunaj J, Czupryna P, Milewska AJ, Róg-Makal M, Gryczoruk Sambor, Moniuszko-Malinowska A (2020) Comparison of tick-borne pathogen prevalence in Ixodes ricinus ticks collected in urban areas of Europe. Sci Rep 10:6975. https://doi.org/10.1038/s41598-020-63883-y
Grochowska A, Dunaj-Małyszko J, Pancewicz S, Czupryna P, Milewski R, Majewski P, Moniuszko-Malinowska A (2022) Prevalence of tick-borne pathogens in questing Ixodes ricinus and Dermacentor reticulatus ticks collected from recreational areas in northeastern Poland with analysis of environmental factors. Pathogens 11:468. https://doi.org/10.3390/pathogens11040468
Gryczyńska A, Kowalec M (2019) Different competence as a Lyme Borreliosis causative agent reservoir found in two thrush species: the blackbird (Turdus merula) and the Song Thrush (Turdus philomelos). Vector Borne Zoonotic Dis 19:450–452. https://doi.org/10.1089/vbz.2018.2351
Gryczyńska A, Welc-Falęciak R (2016) Long-term study of the prevalence of Borrelia burgdorferi s.l. infection in ticks (Ixodes ricinus) feeding on blackbirds (Turdus merula) in NE Poland. Exp Appl Acarol 70:381–394. https://doi.org/10.1007/s10493-016-0082-x
Grzybek M, Alsarraf M, Tołkacz K, Behnke-Borowczyk J, Biernat B, Stańczak J, Strachecka A, Guz L, Szczepaniak K, Paleolog J, Behnke J, Bajer A (2018) Seroprevalence of TBEV in bank voles from Poland—a long-term approach. Emerg Microbes Infect 7:1–8. https://doi.org/10.1038/s41426-018-0149-3
Hämäläinen S, Fey K, Selonen V (2018) Habitat and nest use during natal dispersal of the urban red squirrel (Sciurus vulgaris). Landsc Urban Plan 169:269–275. https://doi.org/10.1016/j.landurbplan.2017.09.011
Hamšíková Z, Silaghi C, Rudolf I, Venclíková K, Mahríková L, Slovák M, Mendel J, Blažejová H, Berthová L, Kocianová E, Hubálek Z, Schnittger L, Kazimírová M (2016) Molecular detection and phylogenetic analysis of Hepatozoon spp. in questing Ixodes ricinus ticks and rodents from Slovakia and Czech Republic. Parasitol Res 115:3897–3904. https://doi.org/10.1007/s00436-016-5156-5
Hansford KM, Wheeler BW, Tschirren B, Medlock JM (2022) Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: a review. Zoonoses Public Health 69:153–166. https://doi.org/10.1111/zph.12913
Hauck D, Jordan D, Springer A, Schunack B, Pachnicke S, Fingerle V, Strube C (2020) Transovarial transmission of Borrelia spp., Rickettsia spp. and Anaplasma phagocytophilum in Ixodes ricinus under field conditions extrapolated from DNA detection in questing larvae. Parasit Vectors 13:176. https://doi.org/10.1186/s13071-020-04049-7
Heylen D, Lasters R, Adriaensen F, Fonville M, Sprong H, Matthysen E (2019) Ticks and tick-borne diseases in the city: Role of landscape connectivity and green space characteristics in a metropolitan area. Sci Total Environ. 670:941–949. https://doi.org/10.1016/j.scitotenv.2019.03.235
Hildebrandt A, Zintl A, Montero E, Hunfeld KP, Gray J (2021) Human babesiosis in Europe. Pathogens 10:1165. https://doi.org/10.3390/pathogens10091165
Humair PF, Gern L (1998) Relationship between Borrelia burgdorferi sensu lato species, red squirrels (Sciurus vulgaris) and Ixodes ricinus in enzootic areas in Switzerland. Acta Trop 69:213–227. https://doi.org/10.1016/S0001-706X(97)00126-5
Jackowiak M, Gryz J, Jasińska K, Brach M, Bolibok L, Kowal P, Krauze-Gryz D (2021) Colonization of Warsaw by the red fox Vulpes vulpes in the years 1976–2019. Sci Rep 11:13931. https://doi.org/10.1038/s41598-021-92844-2
Karlsson ME, Andersson MO (2016) Babesia species in questing Ixodes ricinus, Sweden. Ticks Tick Borne Dis 7:10–12. https://doi.org/10.1016/j.ttbdis.2015.07.016
Kazimírová M, Hamšíková Z, Kocianová E, Marini G, Mojšová M, Mahríková L, Berthová L, Slovák M, Rosá R (2016) Relative density of host-seeking ticks in different habitat types of south-western Slovakia. Exp Appl Acarol 69:205–224. https://doi.org/10.1007/s10493-016-0025-6
Kiewra D, Lonc E (2010) Geograficzne systemy informacji o kleszczach i boreliozie na Dolnym Śląsku. Praca i Studia Geograficzne 44:209–216
Kirczuk L, Piotrowski M, Rymaszewska A (2021) Detection of tick-borne pathogens of the genera Rickettsia, Anaplasma and Francisella in Ixodes ricinus ticks in Pomerania (Poland). Pathogens. 10, 901. https://doi.org/10.3390/pathogens10070901
Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A (2017) Ticks and the city - are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasit Vectors 10:573. https://doi.org/10.1186/s13071-017-2391-2
Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G, Bajer A (2019) Rickettsiales occurrence and co-occurrence in Ixodes ricinus ticks in natural and urban areas. Microb Ecol 77:890–904. https://doi.org/10.1007/s00248-018-1269-y
Krauze-Gryz D, Mazur K, Gryz J (2016) Density of red squirrels and their use of non-native tree species in the Rogow Arboretum. Leśne Prace Badawcze 77:42. https://doi.org/10.1515/frp-2016-0005
Krauze-Gryz D, Gryz J, Wrotek N, Zosicz M (2020) Występowanie Wybranych gatunków ssaków na terenie parku Pole Mokotowskie w Warszawie. Prace i Studia Geograficzne 65:97–103
Krauze-Gryz D, Gryz J, Brach M (2021) Spatial organization, behaviour and feeding habits of red squirrels: differences between an urban park and an urban forest. J Zool 315:69–78. https://doi.org/10.1111/jzo.12905
Król N, Obiegala A, Pfeffer M, Lonc E, Kiewra D (2016) Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasit Vectors 9:351. https://doi.org/10.1186/s13071-016-1632-0
Kubiak K, Dziekońska-Rynko J, Szymańska H, Kubiak D, Dmitryjuk M, Dzika E (2019) Questing Ixodes ricinus ticks (Acari, Ixodidae) as a vector of Borrelia burgdorferi Sensu Lato and Borrelia miyamotoi in an urban area of north-eastern Poland. Exp Appl Acarol 78:113–126. https://doi.org/10.1007/s10493-019-00379-z
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Liberska J, Michalik J, Pers-Kamczyc E, Wierzbicka A, Lane RS, Rączka G, Opalińska P, Skorupski M, Dabert M (2021) Prevalence of Babesia canis DNA in Ixodes ricinus ticks collected in forest and urban ecosystems in west-central Poland. Ticks Tick Borne Dis 12:5. https://doi.org/10.1016/j.ttbdis.2021.101786
Lipatova I, Razanske I, Jurgelevicius V, Paulauskas A (2020) Bartonella Washoensis infection in red squirrels (Sciurus vulgaris) and their ectoparasites in Lithuania. Comp Immunol Microbiol Infect Dis 68:101391. https://doi.org/10.1016/j.cimid.2019.101391
Lurz P, Garson P, Wauters L (1997) Effects of temporal and spatial variation in habitat quality on red squirrel dispersal behaviour. Anim Behav 54:427–435. https://doi.org/10.1006/anbe.1996.0486
Lurz W, Krauze-Gryz D, Gryz J, Meredith A, Schilling A, Thain C, Heller E (2017) Invisible threats to native mammals-mercury levels in three eurasian red squirrel populations. Hystrix 28:280–283. https://doi.org/10.4404/hystrix-28.2-12335
Luu L, Palomar A, Farrington G, Schilling AK, Premchand-Branker S, McGarry J, Makepeace BL, Meredith A, Bell-Sakyi L (2021) Bacterial pathogens and symbionts harboured by Ixodes ricinus ticks parasitising red squirrels in the United Kingdom. Pathogens 10:458. https://doi.org/10.3390/pathogens10040458
Majerová K, Hönig V, Houda M, Papežík P, Fonville M, Sprong H, Rudenko N, Golovchenko M, Černá Bolfíková B, Hulva P, Růžek D, Hofmannová L, Votýpka J, Modrý D (2020) Hedgehogs, squirrels, and blackbirds as Sentinel hosts for active surveillance of Borrelia miyamotoi and Borrelia burgdorferi complex in urban and rural environments. Microorganisms 8(1908). https://doi.org/10.3390/microorganisms8121908
Margos G, Fingerle V, Reynolds S (2019) Borrelia bavariensis: vector switch, niche invasion, and geographical spread of a tick-borne bacterial parasite. Front Ecol Evol 7:401. https://doi.org/10.3389/fevo.2019.00401
Mathews-Martin L, Namèche M, Vourc’h G, Gasser S, Lebert I, Poux V, Barry S, Bord S, Jachacz J, ChalvetMonfray K, Bourdoiseau G, Pamies S, Sepúlveda D, ChambonRouvier S, RenéMartellet M (2020) Questing tick abundance in urban and peri-urban parks in the French city of Lyon. Parasit Vectors 13:576. https://doi.org/10.1186/s13071-020-04451-1
Medlock JM, Shuttleworth H, Copley V, Hansford KM, Leach S (2012) Woodland biodiversity management as a tool for reducing human exposure to Ixodes ricinus ticks: a preliminary study in an English woodland. J Vec Ecol 37:307–315. https://doi.org/10.1111/j.1948-7134.2012.00232.x
Medlock JM, Hansford K, Bormane A, Derdakova M, Estrada-Peña A, George JG, Golovljova I, Jaenson TGT, Jensen JK, Jensen PM, Kazimirova M, Oteo JA, Papa A, Pfister K, Plantard O, Randolph SR, Rizzoli A, Santos-Silva MM, Sprong H, Vial L, Hendrickx G, Zeller H, Van Bortel W (2013) Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6:1–11. https://doi.org/10.1186/1756-3305-6-1
Mierzejewska E, Dwużnik D, Tołkacz K, Bajer A, Panek M, Grzybek M (2020) The efficiency of live-capture traps for the study of red fox (Vulpes vulpes) cubs: a three-year study in Poland. Animals 10:374. https://doi.org/10.3390/ani10030374
Mihalca A, Sándor A (2013) The role of rodents in the ecology of Ixodes ricinus and associated pathogens in Central and Eastern Europe. Front Cell Infect Microbiol 3:56. https://doi.org/10.3389/fcimb.2013.00056
Modrý D, Hofmannová L, Papežík P, Majerová K, Votýpka J, Hönig V, Růžek D, Hrazdilová K (2021) Hepatozoon in eurasian red squirrels Sciurus vulgaris, its taxonomic identity, and phylogenetic placement. Parasitol Res 120:2989–2993. https://doi.org/10.1007/s00436-021-07229-1
Mysterud A, Hügli C, Viljugrein H (2021) Tick infestation on medium–large-sized mammalian hosts: are all equally suitable to Ixodes ricinus adults? Parasit Vectors 14:254. https://doi.org/10.1186/s13071-021-04775-6
Nováková M, Šmajs D (2018) Rickettsial endosymbionts of ticks. Ticks Tick Borne Pathogens 4. https://doi.org/10.5772/intechopen.80767
Oechslin C, Heutschi D, Lenz N, Tischhauser W, Péter O, Rais O, Beuret CM, Leib SL, Bankoul S, Ackermann-Gäumann R (2017) Prevalence of tick-borne pathogens in questing Ixodes ricinus ticks in urban and suburban areas of Switzerland. Parasit Vectors 10:558. https://doi.org/10.1186/s13071-017-2500-2
Øines Ø, Radzijevskaja J, Paulauskas A, Rosef O (2012) Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit Vectors 5(156). https://doi.org/10.1186/1756-3305-5-156
Pangrácová L, Derdáková M, Pekárik L, Hviščová I, Víchová B, Stanko M, Hlavatá H, Peťko B (2013) Ixodes ricinus abundance and its infection with the tick-borne pathogens in urban and suburban areas of Eastern Slovakia. Parasit Vectors 6:1–8. https://doi.org/10.1186/1756-3305-6-238
Paulauskas A, Ambrasiene D, Radzijevskaja J, Rosef O, Turcinaviciene J (2008) Diversity in prevalence and genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in Lithuania and Norway. Int J Med Microbiol 298:180–187. https://doi.org/10.1016/j.ijmm.2008.04.003
Pisanu B, Chapuis J, Dozières A, Basset F, Poux V, Vourc’h G (2014) High prevalence of Borrelia burgdorferi s.l. in the European red squirrel Sciurus vulgaris in France. Ticks Tick Borne Dis 5:1–6. https://doi.org/10.1016/j.ttbdis.2013.07.007
Postler TS, Beer M, Blitvich B.J, Bukh J, de Lamballerie X, Drexler D, Imrie A, Kaporo K, Karganova GG, Lemey P, Lohmann V, Simmonds P, Smith DB, Stapleton JT, Kuhn J (2023) Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol 168:224 https://doi.org/10.1007/s00705-023-05835-1
Report on the incidence of selected infectious diseases in Poland https://www.pzh.gov.pl/
Reports and research carried out by the Warsaw Tourism Organization or the Warsaw City Hall https://wot.waw.pl/Wiedza/
Richtrová E, Míchalová P, Lukavská A, Navrátil J, Kybicová K (2022) Borrelia burgdorferi sensu lato infection in Ixodes ricinus ticks in urban green areas in Prague. Ticks Tick Borne Dis 13:102053. https://doi.org/10.1016/j.ttbdis.2022.102053
Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, Plantard O, Vayssier-Taussat M, Bonnet S, Spitalská E, Kazimírová M (2014) Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health 2:251. https://doi.org/10.3389/fpubh.2014.00251
Romeo C, Pisanu B, Ferrari N, Basset F, Tillon L, Wauters LA, Martinoli A, Saino N, Chapuis JL (2013) Macroparasite community of the eurasian red squirrel (Sciurus vulgaris): poor species richness and diversity. Parasitol Res 112:3527–3536. https://doi.org/10.1007/s00436-013-3535-8
Roux V, Rydkγna E, Eremeeva M, Raoult D (1997) Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int J Syst Bacteriol 47:252–261. https://doi.org/10.1099/00207713-47-2-252
Rubel F, Dautel H, Nijhof A, Kahl O (2022) Ticks in the metropolitan area of Berlin, Germany. Ticks Tick Borne Dis 13:6. https://doi.org/10.1016/j.ttbdis.2022.102029
Ruyts SC, Landuyt D, Ampoorter E, Heylen D, Ehrmann S, Coipan EC, Matthysen E, Sprong H, Verheyen K (2008) Low probability of a dilution effect for Lyme borreliosis in Belgian forests. Ticks Tick Borne Dis 9:1143–1152. https://doi.org/10.1016/j.ttbdis.2018.04.016
Ruyts S, Frazer-Mendelewska E, Van Den Berge K, Verheyen K, Sprong H (2017) Molecular detection of tick-borne pathogens Borrelia afzelii, Borrelia miyamotoi and Anaplasma phagocytophilum in eurasian red squirrels (Sciurus vulgaris). Eur J Wildl Res 63:43. https://doi.org/10.1007/s10344-017-1104-7
Rymaszewska A, Piotrowski M (2013) Use of DNA sequences for Rickettsia identification in Ixodes ricinus ticks: the first detection of Rickettsia monacensis in Poland. Microbes Infect 15:140–146. https://doi.org/10.1016/j.micinf.2012.11.005
Santicchia F, Gagnaison C, Bisi F, Martinoli A, Matthysen E, Bertolino S, Wauters LA (2018) Habitat-dependent effects of personality on survival and reproduction in red squirrels. Behav Ecol Sociobiol 72:134. https://doi.org/10.1007/s00265-018-2546-y
Schorn S, Pfister K, Reulen H, Mahling M, Silaghi C (2011) Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in bavarian public parks, Germany. Parasit Vectors 4:135. https://doi.org/10.1186/1756-3305-4-135
Simpson VR, Hargreaves J, Butler HM, Davison NJ, Everest DJ (2013) Causes of mortality and pathological lesions observed post-mortem in red squirrels (Sciurus vulgaris) in Great Britain. BMC Vet Res 9:229. https://doi.org/10.1186/1746-6148-9-229
Simser JA, Palmer AT, Fingerle V, Wilske B, Kurtti TJ, Munderloh UG (2002) Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. App Environ Microbiol 68:4559–4566. https://doi.org/10.1128/AEM.68.9.4559-4566.2002
Sormunen J, Mäkelä S, Klemola T, Alale TY, Vesterinen EJ (2023) Voles, shrews and red squirrels as sources of tick blood meals and tick-borne pathogens on an island in southwestern Finland. Ticks Tick Borne Dis 14:102–134. https://doi.org/10.1016/j.ttbdis.2023.102134
Stanczak J, Gabre MR, Kruminis-Lozowska W, Racewicz M, Kubica -Bierat M (2004) Ixodes ricinus as a vector of Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti in urban and suburban forests. Ann AgricEnviron Med 11.
Stańczak J, Cieniuch S, Racewicz M, Biernat B (2012) The occurrence and activity of Ixodes ricinus and the risk of tick bites in the recreational areas of the Tricity and Kashubian Lake District. Arthropods. The medical and economic importance. 63–79
Sytykiewicz H, Karbowiak G, Chorostowska-Wynimko J, Szpechciński A, Supergan-Marwicz M, Horbowicz M, Szwed M, Czerniewicz P, Sprawka I (2015) Coexistence of Borrelia burgdorferi s.l. genospecies within Ixodes ricinus ticks from central and eastern Poland. Acta Parasitol 60:654–661. https://doi.org/10.1515/ap-2015-0093
Szekeres S, Docters van Leeuwen A, Tóth E, Majoros G, Sprong H, Földvári G (2019) Road-killed mammals provide insight into tick‐borne bacterial pathogen communities within urban habitats. Transbound Emerg Dis 66:277–286. https://doi.org/10.1016/j.ttbdis.2021.101865
Tahir D, Meyer L, Fourie J, Jongejan F, Mather T, Choumet V, Blagburn B, Straubinger RK, Varloud M (2020) Interrupted blood feeding in ticks: causes and consequences. Microorganisms 8:910. https://doi.org/10.3390/microorganisms8060910
Tołkacz K, Bednarska M, Alsarraf M, Dwużnik D, Grzybek M, Welc-Falęciak R, Behnke JM, Bajer A (2017) Prevalence, genetic identity and vertical transmission of Babesia microti in three naturally infected species of Vole, Microtus spp. (Cricetidae) Parasit Vectors 10:66. https://doi.org/10.1186/s13071-017-2007-x
Tołkacz K, Kowalec M, Alsarraf M, Grzybek M, Dwużnik-Szarek D, Behnke JM, Bajer A (2023) Candidatus Neoehrlichia mikurensis and hepatozoon sp. in voles (Microtus spp.): occurrence and evidence for vertical transmission. Sci Rep 13:1733. https://doi.org/10.1038/s41598-023-28346-0
Tsuji M, Zamoto A, Kawabuchi T, Kataoka T, Nakajima R, Asakawa M, Ishihara C (2006) Babesia microti-like parasites detected in eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido. Japan J Vet Med Sci 68:643–646. https://doi.org/10.1292/jvms.68.643
Venclíková K, Betášová L, Sikutová S, Jedličková P, Hubálek Z, Rudolf I (2014) Human pathogenic borreliae in Ixodes ricinus ticks in natural and urban ecosystem (Czech Republic). Acta Parasitol 59:717–720. https://doi.org/10.2478/s11686-014-0296-1
Vucelja M, Bjedov L, Boljfetić M, Vucelja M, Bjedov L, Boljfetić M, Klobucar A, Krčmar S, Borak S, Modrić M, Juričić K, Peleš V, Margaletić J, Vilibić-Čavlek T (2020) Monitoring of hard ticks at urban recreational sites in the city of Zagreb from 2016 to 2018. Infektol Glasn 39:33–39. https://doi.org/10.37797/ig.39.2.1
Welc-Falęciak R, Kowalec M, Karbowiak G, Bajer A, Behnke JM, Siński E (2014) Rickettsiaceae and Anaplasmataceae infections in Ixodes ricinus ticks from urban and natural forested areas of Poland. Parasit Vectors 7:121. https://doi.org/10.1186/1756-3305-7-121
Wilhelmsson P, Pawełczyk O, Jaenson T, Waldenström J, Olsen B, Forsberg P, Lindgren PE (2021) Three Babesia species in Ixodes ricinus ticks from migratory birds in Sweden. Parasit Vectors 14:183. https://doi.org/10.1186/s13071-021-04684-8
Wodecka B, Skotarczak B (2016) Identification of host blood-meal sources and Borrelia in field-collected Ixodes ricinus ticks in north-western Poland. Ann Agric Environ Med 23(1). https://doi.org/10.5604/12321966.1196853
Wodecka B, Rymaszewska A, Sawczuk M, Skotarczak B (2009) Detectability of tick-borne agents DNA in the blood of dogs, undergoing treatment for borreliosis. Ann Agric Environ Med 16:9–14
Wodecka B, Rymaszewska A, Skotarczak B (2014) Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp Appl Acarol 62:543–555. https://doi.org/10.1007/s10493-013-9763-x
Wondim MA, Czupryna P, Pancewicz S, Kruszewska E, Groth M, Moniuszko-Malinowska A (2022) Epidemiological trends of trans-boundary tick-borne encephalitis in Europe, 2000–2019. Pathogens 11:704. https://doi.org/10.3390/pathogens11060704
Acknowledgements
The authors would like to thank students of Warsaw University of Life Sciences-SGGW for help in fieldwork: Aleksandra Boreczek, Mateusz Cieślik, Karolina Jankowska, Jadwiga Kaczyńska, Natalia Kozłowiec, Monika Marguż, Magdalena Naber, Martyna Wirowska. The authors would like to express our gratitude to the Royal Łazienki Museum in Warsaw for site access and help whilst fieldwork in the park was being conducted. We also thank Professor Jerzy M Behnke, University of Nottingham, UK, for the linguistic proofreading of this article.
Funding
The study was financially supported by the Ministry of Science and Higher Education through the Faculty of Biology, University of Warsaw intramural grant 501-D114-01-1141000 and the Polish Ministry of Science and Higher Education with funds from the Institute of Forest Sciences, Warsaw University of Life Sciences (WULS), for the purpose of scientific research.
Author information
Authors and Affiliations
Contributions
DDS: conceptualization, laboratory studies, statistical and phylogenetic analysis, writing—original draft, project funding; WM: laboratory studies, statistical analysis; AB, DGK: project funding, data collection and field studies; JG, KJ: data collection and field studies; DW: drafting & editing the manuscript AB: conceptualization, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Access to the Natolin Forest Reserve and red squirrel capture was allowed with permission issued by the General and Regional Directorates for Environmental Protection (WPN-I.6205.124.2018.AS and WPN-I.6401.208.2018.PF). Trapping and handling squirrels complied with current laws on animal research in Poland and was carried out with a permit from the Local Ethical Committee (WAW2/072/2018).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dwużnik-Szarek, D., Beliniak, A., Malaszewicz, W. et al. Pathogens detected in ticks (Ixodes ricinus) feeding on red squirrels (Sciurus vulgaris) from city parks in Warsaw. Exp Appl Acarol (2024). https://doi.org/10.1007/s10493-024-00955-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10493-024-00955-y