Abstract
In Ecuador, the main tick species affecting cattle are Rhipicephalus microplus and Amblyomma cajennense sensu lato. Understanding their spatial distribution is crucial. To assess their distribution, data from 2895 farms visited between 2012 and 2017 were utilized. Ticks were collected during animal inspections, with each farm’s location georeferenced. Bioclimatic variables and vapor pressure deficit data were obtained from Climatologies at High resolution for the Earth´s Land Surface Areas (CHELSA) dataset. They were overlaid to develop predictive maps for each species using Random Forest (RF) models. The cross-validation results for RF prediction models showed high accuracy for both R. microplus and A. cajennense s.l. presence with values of accuracy = 0.97 and 0.98, sensitivity = 0.96 and 0.99, and specificity = 0.96 and 0.93, respectively. A carefully selected subset of bioclimatic variables was used to describe the presence of each tick species. Higher levels of precipitation had positive effect on the presence of R. microplus but a negative effect on A. cajennense s.l. In contrast, isothermality (BIO3) was more important for the presence of A. cajennense s.l. compared to R. microplus. As a result, R. microplus had a broader distribution across the country, while A. cajennense s.l. was mainly found in coastal areas with evident seasonality. The coexistence of both species in some regions could be attributed to transitional zones, whereas high altitudes limited tick presence. This information can aid in developing appropriate tick management plans, particularly considering A. cajennense s.l.’s broad host range species and R. microplus’s specificity for cattle. Moreover, the predictive models can identify areas at risk of associated challenging hemoparasite, requiring special attention and mitigation measures.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecuador is a megadiverse country with particular climatic characteristics due to the Andes mountain range. The agricultural sector is important for the economy, accounting for 7.7% of the gross domestic product (Primicias 2022). Around 70% of livestock activities take place in tropical and subtropical zones (Guillén and Muñoz 2013), but has been affected by the presence of ticks. Hard ticks of the Ixodidae family affect mammals. Cattle in Ecuador are frequently affected by ticks, and humans occasionally. These ectoparasites cause substantial economic losses in the livestock sector (Paucar-Quishpe et al. 2023). Widespread acaricide resistance has been identified, causing additional control challenge and loss (Pérez-Otáñez et al. 2023).
The main species affecting cattle in Ecuador are Rhipicephalus microplus and Amblyomma cajennense s.l. (Bustillos and Rodríguez 2016; Maya-Delgado et al. 2020; Paucar et al. 2022). R. microplus is widely distributed in tropical and subtropical areas of Australia, Africa, and Latin America (doubtfully established in Chile) (González-Acuña and Guglielmone 2005). It arrived around five centuries ago with the Spanish colonizers (Estrada-Peña 1999) and adapted well to Ecuador. A. cajennense s.l. is distributed from the southern USA to northern Argentina, and it is a multi-host ectoparasite in mammals including humans incidentally. These species are not controlled satisfactorily in many countries (Lima et al. 2000; Forero-Becerra et al. 2022) and cause direct and indirect losses (Alcala-Canto et al. 2018). In cattle, parasitism results in weight loss, reduction in milk production (Estrada-Peña et al. 2006b) estimated at around 90.2 L per cow per year (Marques et al. 2020), and weakness due to blood loss. Ticks also vector pathogens that affect cattle. R. microplus is an efficient vector for Babesia bovis and Babesia bigemina, and a suspected vector for Anaplasma spp. in Ecuador (Escobar et al. 2015; Insuaste Taipe 2021), A. cajennense s.l. is also known to transmits the bacterium Ehrlichia ruminantium, which has not yet been reported in Ecuador. For the farmers, costs are associated to control and morbidity as well as animal mortality (Alonso-Díaz et al. 2013; Pothmann et al. 2016; Kasaija et al. 2021). In addition, Paucar-Quishpe et al. (2023) associated cattle financial losses with tick acaricide-resistances in Ecuador.
The survival and development of cattle ticks depend on climatic and management conditions. While hosts are necessary for tick presence, we expect cattle tick survival and development to be affected by bioclimatic variables such as rainfall and temperature, that affect the number of generations per year, and thus, abundance. The most limiting factors for their presence, as well as the tick ecotype, can vary between regions (Lima et al. 2000; Estrada-Peña 2023) (Lima et al. 2000; Estrada-Peña et al. 2006b). At broad scales, climate factors have been found useful to delimit tick distribution (Estrada-Peña et al. 2006a, b).
To develop adequate control plans, it is necessary to understand the geographical distribution, and environmental suitability for ticks (Estrada-Peña 1999). Although both R. microplus and A. cajennense s.l. affect cattle, their biology differs. R. microplus fulfills its biological cycle on one host, and has high host specificity for cattle (Nava et al. 2013). A. cajennense s.l. fulfills its cycle on three hosts, infesting mainly equines (Alonso-Díaz et al. 2013).Current on-farm tick control often neglects essential aspects of tick biology and species-specific traits, for example both species, Amblyomma cajennense s.l. and Rhipicephalus microplus, exhibit unique patterns of acaricide resistance, requiring separate resistance analyses and rotation schedules. A. cajennense s.l. may complete early life stages in the wild, emphasizing the need to clear vegetation around pastures. For R. microplus, pasture height and type management is essential due to its life cycle. Manually removing ticks daily could be effective for R. microplus population control, but A. cajennense s.l.’s longer hypostome could damage skin cattle. Attention to these distinct characteristics is vital for effective tick control strategies. Application of control measures without consideration for tick biology can lead to poor acaricide efficacy and induce acaricide resistance which has been widely reported for R. microplus in Ecuador (Rodríguez-Hidalgo et al. 2017; Maya-Delgado et al. 2020; Dzemo et al. 2022; Pérez-Otáñez et al. 2023; Paucar-Quishpe et al. 2023). Only a few studies have addressed acaricide resistance in A. cajennense s.l., but resistance is probable as well (Alonso-Díaz et al. 2013).
Various distribution models have been published in the past for R. microplus in South America and West Africa (Estrada-Peña 1999; Estrada-Peña et al. 2006c; De Clercq et al. 2015; Zannou et al. 2022). They mostly use data drawn from bibliographic sources that often do not represent well the Andes and the particular climatological conditions present in Ecuador. Estrada-Peña (1999) found no suitability for R. microplus in Ecuador. Estrada-Peña et al. (2005), using updated data and a different methodology, found suitability in few parts of the Coastal zone. Marques et al. (2020) in their distribution model of R. microplus found a high suitability in the Andean zone of Ecuador. As for A. cajennense s.l. there are few distribution prediction studies in South América including Ecuador. Aguilar-Domínguez et al. (2021) showed suitability in the occidental part of the Coastal zone of Ecuador for Amblyomma mixtum, a member of the A. cajennense complex, similar than Estrada-Peña et al. (2014).
This study utilized data from “Climatologies at High resolution for the Earth’s Land Surface Areas” (CHELSA). CHELSA provides high-resolution bioclimatic variables over the Earth’s land surfaces at a 1 km-resolution suitable for broad scale modelling of tick climatic suitability. The precision provided by CHELSA increases the accuracy of predictive models (Karger et al. 2017). Greater model accuracy could support targeted tick management strategies and contributes to the containment of tick-borne diseases.
Thus, the present study aims to update the distribution cattle ticks (R. microplus and A. cajennense s.l.) in Ecuador, considering that their presence in highlands remain rare (Chávez-Larrea et al. 2021), and international databases may not adequately capture the specificities of mountain conditions in the Andes. Therefore, the main objective of this study is to use an extensive national dataset of presence and absence of ticks on cattle (R. microplus and A. cajennense s.l.) to model associations with bioclimatic variables. Furthermore, we predict suitable areas where these ticks can affect cattle in continental Ecuador. The findings of this study will provide valuable information for future prevention and control plans.
Methods
Study area
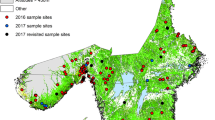
We assembled several cross-sectional studies carried out between 2012 and 2017 by the Instituto de Investigación en Zoonosis (CIZ) in Universidad Central del Ecuador (UCE). Specifically, the following studies were used: the “National survey about bovine Brucellosis, tuberculosis, and cattle ticks” (Maya-Delgado et al. 2020; Paucar et al. 2021), the “Spatial analysis and ecological-epidemiological aspects of Rhipicephalus microplus infestation and its resistance to acaricides”, the “Wild arthropod vectors and domestic reservoirs as indicators of vulnerability to re-emerging zoonotic diseases in the Ecuadorian Amazon", and the "Molecular epidemiology of parasites and microorganisms of zoonotic interest: cattle screwworm and ticks". Altogether, ticks were sampled from 22 of the 23 Ecuador continental provinces. In each farm three bovines selected randomly were sampled for ticks. 2895 cattle farms were visited in the three continental regions of the country. One Andean province (Cañar) was not included in any sample, as it has relatively fewer cattle farms. Table 1 and Fig. 1 describe the sampling in the provinces. Geographical coordinates were recorded in each farm with a Garmin GPS (WGS 84).
Tick collection and morphological identification.
The ticks collected were stored in tubes with absolute ethanol (100%) and taken to the Unidad de Entomología Aplicada of Instituto de Investigación en Zoonosis (CIZ). Ticks were morphologically identified using an Olympus SZ51 stereomicroscope with magnifications × 0.8–4.5 and taxonomic keys (Guerrero 1996; Voltzit 2007; Nava et al. 2014).Ticks were identified to genus and species level. We focused on R. microplus and A. cajennense s. l., as they were the most abundant (Rodríguez-Hidalgo et al. 2017; Maya-Delgado et al. 2020; Paucar et al. 2022).
Ethical considerations.
The attainment of the study’s objectives did not necessitate approval from research ethics committees, as the experimental procedures involved an unregulated invertebrate species.
Bioclimatic data
We used the 19 bioclimatic layers from CHELSA Bioclim, derived from the monthly mean, max, mean temperature, and mean precipitation values and the vapor pressure deficit over 1981–2010 (Table 2). This information is available at a horizontal resolution of 30 arc sec. Although relative humidity has been commonly used, vapor pressure deficit (VPD) is a more useful parameter for evaluating environmental conditions in relation to tick development, as it accounts for the air’s drying power (Wollaeger and Runkle 2015; Pascoe et al. 2019; Estrada-Peña 2023). The VPD data was calculated from hurs, considered a unitless fraction, and tas in ◦C as with this formula: VPD = esat(tas) × (1 − hurs)
where hurs is the relative humidity, esat(tas) is the saturation vapor pressure. To approximate esat(tas), the Magnus equation was used with the coefficients of Sonntag (1990):
VPD was calculated in R, using the package bigleaf (Brun et al. 2022). The area of continental Ecuador was extracted using the “mask” function from “raster” package under R environment. Each bioclimatic raster variable, as CHELSA recommends, was multiplied for the scale value and then added to the offset value (Karger et al. 2017).
Correlative analysis
In order to identify the strongest predictors of tick presence, generalized linear models (GLM) analyses were conducted using presence and absence data on R. microplus, and A. cajennense s.l., and as explanatory variables, the climatology data Bio1 to Bio19 and the vapor pressure deficit mean, minimum, and maximum. The "logit" function, part of the logistic model, was chosen as the link function for regression due to its preference for natural interpretations of coefficients in terms of odds ratios. The logit model is favored over the probit model because the interpretation of betas in probit regression is less intuitive. Although a comparison of both models based on their likelihood values could have been conducted, our preference was for the logit model to facilitate the interpretation of parameters and measurement of their effects. For the multivariable analyses, we included the explanatory variables with a P-value < 0.2 in the univariate analyses. Effect of collinearity in the model was reduced by removing variables presenting a variance inflation factor (VIF) higher than 8, with the exception of the variables known from literature to be important in tick biology. BIO1, the mean annual temperature, is associated to more favorable conditions for tick development Higher mean annual temperature shortens life cycle, lengthen seasonal activity (Alcala-Canto et al. 2018; Pascoe et al. 2019). Since all variables are derived from temperature and precipitation, they are expected to be collinear. Forward stepwise selection was used to build the final multiple GLM model, with the stepAIC function into the “MASS” package. We used the subset of variables producing the lowest Akaike information criteria (AIC). Finally, we computed the sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC-ROC), using the “CARET” and “Proc” R packages. The bioclimatic variables were standardized using “scale” function in R.
Predictive models for cattle tick’s habitat suitability
Random Forest (RF), a supervised model, was used to model habitat suitability of cattle ticks (Kopsco et al. 2022; Zannou et al. 2022). Using the presence and absence data and the results of the multiple GLM, the habitat suitability prediction model in continental Ecuador under bioclimatic conditions was modelled using the “Random Forest” package in R.
RF yields a value from 0 (completely unsuitable) to 1 (fully suitable). Using the "importance" function in the “Random Forest” package, we calculated the Mean Square Error (MSE), revealing elevated percentages for the most significant variables within the resulting RF model. The models were evaluated using sensitivity and specificity and area under the ROC curve (AUC-ROC). The models were trained with 80% of the data (randomly selected), and validated with the other 20% for 10 times, to obtain the mean sensitivity, specificity, and accuracy. Models were adjusted for: R. microplus, and A. cajennense s.l. The best model for each was mapped in QGIS, and reclassified into 5 categories: 0 to 0.2 (probability very low); 0.2 to 0.4 (probability low); 0.4 to 0.6 (moderate); 0.6 to 0.8 (high) and 0.8 to 1 (very high) (Namgyal et al. 2021).
In order to mask out areas recently not used for agriculture, we use the land cover map of Ecuador (Ministerio de Agricultura y Ganadería [MAG] 2014) as in Table 3. This data from 2014 is the most recent version. We applied a 50% transparency mask so that areas that may become used for cattle raising in the future are included, even though prediction accuracy may be lower in unsampled areas.
Finally, we made a map of the probability of having both species by combining the suitability maps of R. microplus and A. cajennense s.l. Suitability values were reclassified as: 0 to 0.5 = 0, and 0.51 to 1 = 1. The reclassified maps were combined in a new raster with the following classes: 0 = no ticks, 1 = one tick species, and 2 = two tick species.
Results
Presence data
2895 farms were included, of which 1780 farms had R. microplus presence. 460 farms had A. cajennense s.l., and 378 both species (Table 4).
Distribution maps
The distribution of Rhipicephalus microplus and Amblyomma cajennense sensu lato. is presented in Figs. 2 and 3. Farms with presence of one and both are shown in Fig. 4. R. microplus is widely distributed in the Coastal, foothills of the Andean zone and Amazon. A. cajennense s.l. is distributed in the Coastal zone and in the western foothills of the Andean zone.
Bi-variable generalized linear models
The individual association between bioclimatic factors and the presence of R. microplus and A. cajennense s.l. showed P values lower than 0.2 for all variables tested (Table 5).
Multivariate generalized linear model
The final explanatory GLM after removing colinear variables, and with the lowest AIC for R. microplus and A. cajennense s.l., included 10 bioclimatic variables: Bio1, Bio2, Bio3, Bio4, Bio12, Bio13, Bio14, Bio18, VPD_max, and VPD_min (Table 6).
The AUC-ROC, sensitivity, and specificity are above 0.68 for both models (Table 7).
Predictive model
Figure 5 shows the potential distribution of R. microplus obtained by RF. The Amazon and Coastal zones are highly suitable, while only some Andean areas were suitable, particularly in Andean valleys and foothills. Figure 6 is showing the suitability map for R. microplus with areas where cattle is currently absent masked out. For R. microplus the highest value of Percentage Increase in MSE (Regression) (%IncMSE) corresponds to Bio 14 followed by Bio 1 with 42.44 and 42.18 respectively (Fig. 7). Bio 4 and Bio 12 were also important variables. Thus, a combination of factors like temperature, humidity, and reduced change in seasonality were determinant factors for R. microplus presence.
Areas with high predicted suitability for A. cajennense s.l. are all found in the Coastal zone (Fig. 8). Predicted suitability is low in the Andean and Amazon zones. However,the foothills of the western cordillera also have a degree of predicted suitability. Figure 9 presents the suitability with non-agricultural areas masked out. With respect to the importance of the variables, the highest %incMSE values correspond to Bio14 and Bio4 with 36.74 and 33.71 respectively (Fig. 10).Areas with marked seasonality, reduced isothermality are related to A. cajennese s.l. presence.
All provinces of the Coastal zone have a high suitability for both tick species, as well as Loja province in the Andean zone (Fig. 11).
All accuracy metrics for the Random Forest models were above 0.93 (Table 8).
The models were also trained and tested with 80% and 20% of the entire data, respectively. The average of 10 random models is presented in Table 9.
Discussion
The results of this study highlight the widespread distribution of R. microplus and A. cajennense s.l. ticks in tropical and subtropical areas of Ecuador, as supported by multiple studies (Escobar et al. 2015; Bustillos and Rodríguez 2016; Rodríguez-Hidalgo et al. 2017; Maya-Delgado et al. 2020; Chávez-Larrea et al. 2021; Guglielmone et al. 2021; Paucar et al. 2022; Pérez-Otáñez et al. 2023). Earlier studies had identified isolated presence of R. microplus in the provinces of Los Ríos (Escobar et al. 2015), Pichincha, Tungurahua, Manabí (Diazalulema 2015; Rodríguez-Hidalgo et al. 2017; Chávez-Larrea et al. 2021), Napo, Sucumbíos, Orellana (Quezada and Quezada 2015; Insuaste Taipe 2021). A. cajennense s.l. was documented in El Oro (Nava et al. 2014), Pichincha and Manabí (Beati et al. 2013; Paucar et al. 2022). However, this information is scattered and needs to be assembled to get a consolidated understanding of the distribution of these species in continental Ecuador.
In this study, we assembled various datasets to build a complete picture of the current distribution of R. microplus and A. cajennense s.l. in continental Ecuador, and we evaluated associations with bioclimatic variables to build a spatially continuous habitat suitability map.
Climate conditions, particularly temperature, humidity, and precipitation, were found to be crucial factors influencing the distribution and development of ticks, which is in line with previous research (Pfäffle et al. 2013). For this study, bioclimatic variables like Bio1, Bio2, Bio3, Bio4, Bio12, Bio13, Bio14, Bio18, VP_min, and VP_max were highly significant in the GLM, with Bio1, Bio12, Bio14 and VPD variables having the highest (above 1) and lowest (below 1) odds ratio. This is coherent with Marques et al. (2020) who in their study determined that the bioclimatic variables from WorldClim, such as Bio1, Bio4, Bio12, Bio14, and relative humidity, are useful predictors for R. microplus. Likewise, Bio 18 contributed to the models developed by Namgyal et al. (2021) in addition to elevation and land cover, which were not evaluated in this study as we focused on climatic factors. These results are coherent with tick biology for both species studied (Estrada-Peña et al. 2006c, 2014; Pascoe et al. 2019). In the case of A. cajennense s.l., the study of Aguilar-Domínguez et al. (2021) with A. mixtum, a species belonging to the cajennense complex, showed four important bioclimatic factors: Bio4, Bio6, Bio7 and Bio12, of which Bio4, and Bio12 are coherent with this study. A. cajennense s.l. has a suitable habitat in the coastal zone of Ecuador where seasonality of precipitations is marked but limited for temperature. Thus, is thermality played an important role in the distribution of A. cajennense s.l.
The present study utilized a Random Forest model to predict suitable areas for R. microplus and A. cajennense s.l. based on bioclimatic factors. Random Forest was used because we have both presence and absence data. In West Africa, Zannou et al. (2022) tested several models and found that Random Forest was an accurate model for habitat suitability of R. microplus.
Our models suggest that R. microplus could potentially occur in most areas of Ecuador, except the Andes Mountains, with the Amazon and Coastal zones had high suitability. Ecuador’s diverse ecological formations associated with varied microclimates create an intricate landscape for tick distribution (Galeas et al. 2013). However, caution is needed when considering areas that are currently unused for agricultural or livestock purposes, and as a result cannot be sampled for ticks dependent on cattle (Fig. 6 and 9), like the Amazon. Our model predicted suitability in those areas, suggesting tick may rapidly become a problem if those forest areas are cleared for pasture and cattle introduction. However, we focused on climatic determinants of tick abundance, and could not account for cattle, a factor necessary for tick presence. While the Amazon zone is known for its extensive forested regions and 16 Natural Protected Areas spanning 30,514 km2 (López. et al. 2012; Galeas et al. 2013), it is facing increasing pressure from anthropic activities, such as oil extraction, mining, deforestation, road construction, colonization, and disorganized rural settlements. Large forested habitats are becoming fragmented landscapes, potentially leading to changes in tick distribution (López et al. 2012; Galeas et al. 2013; Alemán Gaínza et al. 2014; Cicuttin 2019; Vale et al. 2019; Galeas et al. 2013). Access to accurate cattle distribution data, currently unavailable at a fine enough resolution in Ecuador, is a must for R. microplus. The question is different Amblyomma ticks, which parasite diverse wild hosts as well as cattle. Some such hosts are abundant in the Amazon zone abundant, such as mammals, birds, reptiles, and amphibians live. They serve as hosts for other species of ticks such as Amblyomma latepunctatum Tonelli Rondelli, Amblyomma humerale Koch, Amblyomma dissimile Koch, etc. (Guglielmone et al. 2021). Should the Amazon undergo extensive deforestation for cattle husbandry, the model may need to be updated locally for the association changes in climate conditions, including increased temperatures and reduced humidity (Pfäffle et al. 2013). As cattle mobility has been identified as a primary factor in the spread of cattle ticks (Chávez-Larrea et al. 2021), if cattle husbandry expands in the Amazon, great vigilance for tick issue should be applied, and surveillance started early.
The study’s findings diverge from some previous research. In our study, the Amazon zone and Coastal zone are highly suitable, as well as Andean valleys, and the eastern and western foothills of the Andes for R. microplus. This differs from results obtained at the regional level, for example, by Marques et al. (2020) who report high suitability in the Andean zone and medium suitability in the Coastal zone and the Amazon zone. Estrada-Peña (1999) showed Ecuador as non-suitable for R. microplus. In addition (Estrada-Peña et al. 2005) show only the northwestern part of the country (Esmeraldas and Carchi) as zones with high suitability in 1999, the northern provinces of the three regions, and parts of the southern zone as suitable zones in 2025 and 2050. This study provides a good estimation of the habitat suitability, with sensitivity of 0.97 and specificity of 0.96, similar values to Estrada-Peña (1999) with sensitivity of 0.91 and specificity of 0.88. Overall, our sample covers the diverse Ecuadorian environment and its specificities much more exhaustively.
For A. cajennense s.l., the model in this study shows that the highly suitable areas are limited to the Coastal zone and areas near to the western foothills of the Andes. To compare the results of this study, we will focus on studies conducted on Amblyomma mixtum, which is part of the Amblyomma cajennense s. l. complex. This choice is based on the description by other authors, suggesting that A. mixtum is the species present in Ecuador (Alcala-Canto et al. 2018; Pascoe et al. 2019; Aguilar-Domínguez et al. 2021). The model for Amblyomma mixtum Koch proposed by Nava et al. (2014) shows also highly suitability for the Coastal zone, similar to this study. Aguilar-Domínguez et al. (2021) describe the potential distribution of A. mixtum and show the Coastal zone of Ecuador as suitable as well, and for the coming 50 years. Our study also shows a very low suitability in some areas of the Amazon where the presence of this species has not been reported and most of them correspond to non-livestock (agricultural) areas. A. mixtum is known from western Ecuador (provinces of El Oro, Guayas, Los Ríos, Manabí and Pichincha) (Orozco Álvarez 2018; Paucar et al. 2022), A. cajennense s.l. was found in various environments, including dry and semiarid areas as well as riparian forests and savanna lowlands. (Estrada-Peña et al. 2014). However, its survival within these habitats is contingent on specific microclimatic conditions, particularly a relative humidity not dropping below 80% for extended periods (Pfäffle et al. 2013). A. cajennense s.l. spend more time and energy in finishing its life cycle because its way to feed (Polanco Echeverry and Ríos Osorio 2016). Due to species-specific differences, the abundance of Rhipicephalus microplus surpasses that of Amblyomma cajennense s.l. in the studied farms. This can be explained by the one-host cycle of R. microplus and its hostspecificity. R. microplus is also well adapted to the tropical and subtropical climate of Ecuador. Over 80% of farms surveyed had R. microplus, whereas A. cajennense s. l. accounts for only approximately 15% of farms infested the total tick population in these farms.
Figure 11 highlights areas where both tick species are likely to coexist on the same farm, all of the 43 farms surveyed by Alonso-Díaz et al. (2013) in Mexico had both species present. We found that 378 out of 2895 farms had both species, primarily in the Coastal zone and western foothills of the Andean Mountains. R. microplus and A. cajennense s.l. are closely associated with their respective hosts, with R. microplus primarily infesting cattle and A. cajennense s.l. primarily infesting equines, but also cattle (Guglielmone et al. 2021).
The model operates at the national scale and gives the broad spatial trends in suitability, but at the fine scale, other factors will determine tick presence and abundance. At the farm level, factors such as the presence and abundance of hosts (cattle), as well as control practices, such as use of acaricide, cattle resistance to ticks, and organization of grazing systems, play a significant role in the distribution and abundance of cattle ticks (Estrada-Peña et al. 2005; Alemán Gaínza et al. 2014; Paucar et al. 2022). Local models may be able to capture such factor, that are poorly documented at the national scale.
This research is the first to provide a national-level assessment of tick distribution in continental Ecuadorian, offering valuable insights into tick presence and absence across the country. This comprehensive understanding of tick distribution can aid in the development of effective tick control and management plans, considering the differences in tick species, their distribution, and their biological characteristics.
The study emphasizes the importance of continuous research to monitor tick populations as tick distribution may change over time due to factors including climate change and human activities. Moreover, knowing the main tick species and their spatial distribution is crucial for developing targeted strategies to mitigate tick-related problems and protect livestock health and productivity. The study’s findings call for the implementation of tick prevention/control plans considering the specific ecological contexts and host interactions, which could help reduce the negative impacts of ticks on livestock farming. Moreover, it is crucial to consider diverse approaches for their prevention/control due to the broad host range of A. cajennese s.l., and the specificity of R. microplus for cattle. Additionally, it highlights the importance of addressing the challenges posed by R. microplus resistance to acaricides, which has become a significant concern for livestock farmers in Ecuador (Rodríguez-Hidalgo et al. 2017; Maya-Delgado et al. 2020; Paucar-Quishpe et al. 2023).
In conclusion, this study significantly contributes to the understanding of tick distribution in Ecuador, shedding light on the climatic factors influencing their presence and abundance. The results offer valuable insights for policymakers, farmers, and researchers to develop effective tick preventive/control plans and protect livestock health in the region. However, continuous research and monitoring are necessary to keep abreast of the evolving tick distribution patterns and make informed decisions for tick management and livestock production in the future.
Data availability
The data that support the results of this study are available from the corresponding author upon request.
References
Aguilar-Domínguez M, Moo-Llanes DA, Sánchez-Montes S et al (2021) Potential distribution of Amblyomma mixtum (Koch, 1844) in climate change scenarios in the Americas. Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2021.101812
Alcala-Canto Y, Figueroa-Castillo JA, Ibarra-Velarde F et al (2018) Development of the first georeferenced map of Rhipicephalus (Boophilus) spp. in Mexico from 1970 to date and prediction of its spatial distribution. Geospat Health 13:110–117. https://doi.org/10.4081/gh.2018.624
Alemán Gaínza Y, Martínez Marrero S, Corona González B (2014) Ticks of veterinary interest in Cuba, and its importance in the changing climatic conditions. Rev Electron Vet 15:1–22
Alonso-Díaz MA, Fernández-Salas A, Martínez-Ibáñez F, Osorio-Miranda J (2013) Amblyomma cajennense (Acari: Ixodidae) tick populations susceptible or resistant to acaricides in the Mexican Tropics. Vet Parasitol 197:326–331. https://doi.org/10.1016/j.vetpar.2013.06.004
Beati L, Nava S, Burkman EJ et al (2013) Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: phylogeography and evidence for allopatric speciation. BMC Evol Biol. https://doi.org/10.1186/1471-2148-13-267
Brun P, Zimmermann NE, Hari C et al (2022) Global climate-related predictors at kilometer resolution for the past and future. Earth Syst Sci Data 14:5573–5603. https://doi.org/10.5194/essd-14-5573-2022
Bustillos R, Rodríguez R (2016) Ecologia parasitaria de Riphicephalus microplus en bovinos, 1st edn. Editorial Académica Española, Madrid
Chávez-Larrea MA, Cholota-Iza C, Medina-Naranjo V et al (2021) Detection of Babesia spp. in high altitude cattle in Ecuador, possible evidence of the adaptation of vectors and diseases to new climatic conditions. Pathogens. https://doi.org/10.3390/pathogens10121593
Cicuttin GL (2019) Estudio epidemiológico de las garrapatas presentes en un área urbana protegida en la Ciudad Autónoma de Buenos Aires. Universidad Nacional del Litoral, Santa Fe
De Clercq EM, Leta S, Estrada-Peña A et al (2015) Species distribution modelling for Rhipicephalus microplus (Acari: Ixodidae) in Benin, West Africa: Comparing datasets and modelling algorithms. Prev Vet Med 118:8–21. https://doi.org/10.1016/j.prevetmed.2014.10.015
Diazalulema S (2015) Identificación Taxonómica De Garrapatas En Ganado Bovino De La Parroquia La Matriz Del Cantón Patate. Universidad Técnica de Ambato, Ambato
Dzemo WD, Thekisoe O, Vudriko P (2022) Development of acaricide resistance in tick populations of cattle: a systematic review and meta-analysis. Heliyon 8:e08718. https://doi.org/10.1016/j.heliyon.2022.e08718
Escobar A, Cevallos O, Villarreal P et al (2015) (2015) Prevalence and detection by nested PCR of Anaplasma marginale in cattle and tick in the center of the coast of Ecuador. Cienc y Tecnol 8:11–17
Estrada-Peña A (1999) Geostatistics and remote sensing using NOAA-AVHRR satellite imagery as predictive tools in tick distribution and habitat suitability estimations for Boophilus microplus (Acari: Ixodidae) in South America. Vet Parasitol 81:73–82. https://doi.org/10.1016/S0304-4017(98)00238-6
Estrada-Peña A (2023) The climate niche of the invasive tick species Hyalomma marginatum and Hyalomma rufipes (Ixodidae) with recommendations for modeling exercises. Exp Appl Acarol. https://doi.org/10.1007/s10493-023-00778-3
Estrada-Peña A, Sánchez Acedo C, Quílez J, Del Cacho E (2005) A retrospective study of climatic suitability for the tick Rhipicephalus (Boophilus) microplus in the Americas. Glob Ecol Biogeogr 14:565–573. https://doi.org/10.1111/j.1466-822X.2005.00185.x
Estrada-Peña A, Bouattour A, Camicas JL et al (2006a) The known distribution and ecological preferences of the tick subgenus Boophilus (Acari: Ixodidae) in Africa and Latin America. Exp Appl Acarol 38:219–235. https://doi.org/10.1007/s10493-006-0003-5
Estrada-Peña A, Corson M, Venzal JM et al (2006b) Changes in climate and habitat suitability for the cattle tick Boophilus microplus in its southern Neotropical distribution range. J Vector Ecol 31:158–167. https://doi.org/10.1645/0022-3395(2001)087[0978:cwacih]2.0.co;2
Estrada-Peña A, García Z, Sánchez HF (2006c) The distribution and ecological preferences of Boophilus microplus (Acari: Ixodidae) in Mexico. Exp Appl Acarol 38:307–316. https://doi.org/10.1007/s10493-006-7251-2
Estrada-Peña A, Tarragona EL, Vesco U et al (2014) Divergent environmental preferences and areas of sympatry of tick species in the Amblyomma cajennense complex (Ixodidae). Int J Parasitol 44:1081–1089. https://doi.org/10.1016/j.ijpara.2014.08.007
Forero-Becerra E, Acosta A, Benavides E et al (2022) Amblyomma mixtum free-living stages: inferences on dry and wet seasons use, preference, and niche width in an agroecosystem (Yopal, Casanare, Colombia). PLoS ONE 17(4):e0245109
Galeas R, Guevara JE, Medina-Torres B et al (2013) Sistema de clasificación de los ecosistemas del Ecuador continental. Ministerio del Ambiente del Ecuador, Quito
González-Acuña D, Guglielmone AA (2005) Ticks (Acari: Ixodoidea: Argasidae, Ixodidae) of Chile. Exp Appl Acarol 35:147–163. https://doi.org/10.1007/s10493-004-1988-2
Guerrero R (1996) Las garrapatas de Venezuela (acarina: ixodoidea): listado de especies y claves para su identificación/ticks of Venezuela (acarina: ixodoidea): list of specific and clavichord for your classification. Bol Dir Malariol Saneam Ambient 36(1/2):1–24
Guglielmone AA, Nava S, Robbins RG (2021) Neotropical hard ticks (Acari: Ixodida: Ixodidae). Springer, Cham
Guillén NX, Muñoz LE (2013) Estudio taxonómico a nivel de género de garrapatas en ganado bovino de la parroquia Alluriquín - Santo Domingo de los Tsáchilas. Universidad de las Fuerzas Armadas (ESPE), Sangolquí
Insuaste Taipe EP (2021) Identificación molecular de Babesia bovis y B. bigemina en garrapatas Riphicephalus (Boophilus) microplus de la región norte amazonica del Ecuador. Universidad Internacional (SEK), Quito
Karger DN, Conrad O, Böhner J et al (2017) Climatologies at high resolution for the Earth’s land surface areas. Sci Data 4:1–20. https://doi.org/10.1038/sdata.2017.122
Kasaija PD, Estrada-Peña A, Contreras M et al (2021) Cattle ticks and tick-borne diseases: a review of Uganda’s situation. Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2021.101756
Kopsco HL, Smith RL, Halsey SJ (2022) A scoping review of species distribution modeling methods for tick vectors. Front Ecol Evol. https://doi.org/10.3389/fevo.2022.893016
Lima WS, Ribeiro MF, Guimaraes MP (2000) Seasonal variation of Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) in cattle in Minas Gerais state, Brazil. Trop Anim Health Prod 32:375–380. https://doi.org/10.1023/A:1005229602422
López AV, Espíndola F, Calles LJ (2012) Amazonía. Ecuatoriana Bajo Presión, Quito
Marques R, Krüger RF, Peterson AT et al (2020) Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Vet Res 51:1–10. https://doi.org/10.1186/s13567-020-00802-z
Maya-Delgado A, Madder M, Benítez-Ortíz W et al (2020) Molecular screening of cattle ticks, tick-borne pathogens and amitraz resistance in ticks of Santo Domingo de los Tsáchilas province in Ecuador. Ticks Tick Borne Dis 11:101492. https://doi.org/10.1016/j.ttbdis.2020.101492
Ministerio de Agricultura y Ganadería (MAG) (2014) 2013–2014—Mapa de Cobertura y Uso de la tierra en el Ecuador continental—Categoría: Tierra en transición, escala 1:100.000, año 2013–2014. http://geoportal.agricultura.gob.ec/geonetwork/srv/spa/catalog.search#/metadata/35ccb71a-c0a9-485a-84d4-965da38410f8
Namgyal J, Lysyk TJ, Couloigner I et al (2021) Identification, distribution, and habitat suitability models of ixodid tick species in cattle in eastern Bhutan. Trop Med Infect Dis. https://doi.org/10.3390/tropicalmed6010027
Nava S, Mastropaolo M, Guglielmone AA, Mangold AJ (2013) Effect of deforestation and introduction of exotic grasses as livestock forage on the population dynamics of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in northern Argentina. Res Vet Sci 95:1046–1054. https://doi.org/10.1016/j.rvsc.2013.09.013
Nava S, Beati L, Labruna MB et al (2014) Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1. Ticks Tick Borne Dis 5:252–276. https://doi.org/10.1016/j.ttbdis.2013.11.004
Orozco Álvarez GE (2018) Distribución espacial de garrapatas que afectan a las ganaderías ecuatorianas de las tres regiones, usando como referencia la línea equinoccial. Universidad Central del Ecuador, Quito
Pascoe EL, Marcantonio M, Caminade C, Foley JE (2019) Modeling potential habitat for Amblyomma tick species in California. Insects 10:1–19. https://doi.org/10.3390/insects10070201
Paucar V, Ron-Román J, Benítez-Ortiz W et al (2021) Bayesian estimation of the prevalence and test characteristics (sensitivity and specificity) of two serological tests (rb and sat-edta) for the diagnosis of bovine brucellosis in small and medium cattle holders in ecuador. Microorganisms 9:1–20. https://doi.org/10.3390/microorganisms9091815
Paucar V, Pérez-Otáñez X, Rodríguez-Hidalgo R et al (2022) The associated decision and management factors on cattle tick level of infestation in two tropical areas of Ecuador. Pathogens 11:1–20. https://doi.org/10.3390/pathogens11040403
Paucar-Quishpe V, Pérez-Otáñez X, Rodríguez-Hidalgo R et al (2023) An economic evaluation of cattle tick acaricide-resistances and the financial losses in subtropical dairy farms of Ecuador: a farm system approach. PLoS ONE 18:1–31. https://doi.org/10.1371/journal.pone.0287104
Pérez-Otáñez X, Vanwambeke SO, Orozco-Alvarez G et al (2023) Widespread acaricide resistance and multi-resistance in Rhipicephalus microplus in Ecuador and associated environmental and management risk factors. Ticks Tick Borne Dis 15:102274. https://doi.org/10.1016/j.ttbdis.2023.102274
Pfäffle M, Littwin N, Muders SV, Petney TN (2013) The ecology of tick-borne diseases. Int J Parasitol 43:1059–1077. https://doi.org/10.1016/j.ijpara.2013.06.009
Polanco Echeverry DN, Ríos Osorio LA (2016) Aspectos biológicos y ecológicos de las garrapatas duras. Cienc Tecnol Agropec 17:81–95
Pothmann D, Poppert S, Rakotozandrindrainy R et al (2016) Prevalence and genetic characterization of Anaplasma marginale in zebu cattle (Bos indicus) and their ticks (Amblyomma variegatum, Rhipicephalus microplus) from Madagascar. Ticks Tick Borne Dis 7:1116–1123. https://doi.org/10.1016/j.ttbdis.2016.08.013
Primicias (2022) La inversión en el sector agrícola representa una apuesta para el futuro Para hacer uso de este contenido cite la fuente y haga un enlace a la nota original en Primicias.ec: https://www.primicias.ec/noticias/patrocinado/la-inversion-en-el-sector-agricola-. https://www.primicias.ec/noticias/patrocinado/la-inversion-en-el-sector-agricola-representa-una-apuesta-para-el-futuro/
Quezada L, Quezada N (2015) Efectividad de Beauveria spp. como controlador biológico de garrapatas Rhipicephalus spp. en ganado bovino en la provincia de Orellana. Escuela superior politécnica de Chimborazo sede Orellana, Francisco de Orellana
Rodríguez-Hidalgo R, Pérez-Otáñez X, Garcés-Carrera S et al (2017) The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 12:1–15. https://doi.org/10.1371/journal.pone.0174652
Sonntag D (1990) Important new values of the physical constants of 1986, vapour pressure formulations based on the ITS-90, and psychrometer formulae. Z Meteorol 70(5):340–344
Vale P, Gibbs H, Vale R et al (2019) The expansion of intensive beef farming to the Brazilian Amazon. Glob Environ Chang 57:101922. https://doi.org/10.1016/j.gloenvcha.2019.05.006
Voltzit VO (2007) A review of neotropical Amblyomma species (Acari: Ixodidae). Acarina 15:3–134
Wollaeger H, Runkle E (2015) VPD vs relative humidity. Inside Grower 28–29. https://www.canr.msu.edu/uploads/resources/pdfs/vpd-vs-rh.pdf
Zannou OM, Da Re D, Ouedraogo AS et al (2022) Modelling habitat suitability of the invasive tick Rhipicephalus microplus in West Africa. Transbound Emerg Dis 69:2938–2951. https://doi.org/10.1111/tbed.14449
Acknowledgements
Our thanks to the Belgian Development Cooperation (DGD), Ministry of Agriculture and Livestock of Ecuador (MAG), Universidad Central del Ecuador (UCE), and Instituto de Investigación en Zoonosis (CIZ), for funding this research. To the Academy of Research and Higher Education (ARES) for funding the PhD program and UCLouvain for hosting the PhD program. In addition, we thank all participating farmers, local veterinarians, and MAG technicians who supported the field collections. Special thanks to the Medical Geography team (ELI) for their valuable comments and contributions (Earth & Life Institute, UCLouvain).
Funding
Sample collection for this study was supported by Belgian Development Cooperation (DGD), Ministry of Agriculture and Livestock of Ecuador (MAG), and Universidad Central del Ecuador (UCE). The project was approved and received national consent by the Ministry of Agriculture and Livestock of Ecuador (MAG) (N◦ SENPLADES-SGPBV-2012-0237-OF—Programa Nacional de Cárnicos). The PhD program (XP) was funded by the Academy of Research and Higher Education (ARES) through the Research for Development Project (PRD) B1.31203.006-E6 entitled ‘Socio-eco-epidemiology of ticks, tick-borne parasites, acaricide resistance and residual effects of acaricides in tropical Ecuadorian livestock: environmental, animal and public health impacts’, which involves universities from Ecuador (CIZ, Universidad Central del Ecuador) and Belgium (UCLouvain and ULiège).
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission. (1) XP, SV, LR, RR, SE, made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; (2) XP, SV, LR, RR, SE, CS drafted the work or revised it critically for important intellectual content; (3) XP, SV, LR, RR, SE, FV, WB, CS, MC approved the version to be published; and (4) XP, SV, LR, RR, SE, FV, WB, CS, MC agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The participant farmers were properly informed and gave their consent prior to collect ticks over their animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Otáñez, X., Rodríguez-Hidalgo, R., Enríquez, S. et al. High-resolution prediction models for Rhipicephalus microplus and Amblyomma cajennense s.l. ticks affecting cattle and their spatial distribution in continental Ecuador using bioclimatic factors. Exp Appl Acarol 92, 439–462 (2024). https://doi.org/10.1007/s10493-023-00883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00883-3