Abstract
Rhipicephalus (Boophilus) microplus, an invasive species to Africa, and the endemic R. (B.) decoloratus are of high economic importance in the cattle industry. Invasion of the alien species in South Africa has mostly been reported for traditional communal grazing areas where it seemed to be rapid and, in some cases, even replaced the native species. The alien species is also assumed to already be resistant to acaricides upon invasion. The presence of R. (B.) microplus on commercial farms was therefore investigated and resistance screening of both species to field concentrations of cypermethrin, amitraz, and chlorfenvinphos was determined by means of the larval immersion test. Results showed that only 3.7% (of 383) tick collections submitted were R. (B.) microplus populations. A further 1.6% (of 383) showed co-existence of the two species. Comparing the level of resistance to the acaricides between the two species indicated a mean phenotypic resistance of 66.2 and 26.5% of R. (B.) decoloratus populations to cypermethrin and amitraz, respectively. This was significantly lower for R. (B.) microplus, with 23.0 and 4.1% of its populations resistant to cypermethrin and amitraz, respectively. Closed commercial farming areas seemed to have a preventative advantage for the invasion of R. (B.) microplus and displacement of R. (B.) decoloratus by R. (B.) microplus. Regular monitoring of these two species may be of high importance to prevent unnecessary financial losses due to insufficient control and increased awareness of the threat of Asiatic babesiosis vectored by R. (B.) microplus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhipicephalus (Boophilus) microplus (Canestrini) (Acari: Ixodidae) is one of the most economically important tick species worldwide and is also known as the Asiatic or pantropical tick (Horak et al. 2018) or southern cattle tick (Robbertse et al. 2016). In South Africa, Howard initially reported R. (B.) microplus near King Williamstown in the Eastern Cape Province in 1908. Isolated pockets of this species were later recorded in the southern parts of the Western Cape Province (Howell et al. 1978). More recent reports indicated its presence and invasion in some areas, mostly communal grazing areas, found in the Limpopo (Tønnesen et al. 2004), Eastern Cape (Ntondini et al. 2008; Horak et al. 2009; Nyangiwe et al. 2013), Northwest (Spickett et al. 2011) and Mpumalanga provinces (Robbertse et al. 2016). Communal grazing areas are common to African traditions where cattle, belonging to different owners, graze on unfenced communal grounds that do not represent a closed farming system. The cattle are normally not fed any additional concentrates, and in some cases, pastures tend to be overgrazed (Tønnesen et al. 2004). This is in contrast with commercial farms, where producers regulate the cattle herd and cattle of one owner are confined to one fenced-in farm (Bryson et al. 2002).
The closely related African blue tick, Rhipicephalus (Boophilus) decoloratus (Koch), is considered indigenous to Africa and South Africa. Although possible displacement of R. (B.) decoloratus by R. (B.) microplus seems to be evident in some communal areas (Tønnesen et al. 2004; Ntondini et al. 2008; Nyangiwe et al. 2013; Robbertse et al. 2016), R. (B.) decoloratus is still found to be more abundant on commercial farms in South Africa (Horak 1999; Schroder and Reilly 2013). A National Tick Resistance Survey (NTRS) in South Africa, conducted on commercial farms from 1998 to 2001, only recorded four populations of R. (B.) microplus compared to 180 R. (B.) decoloratus populations (Van Dalen and Jansen van Rensburg 2023a). However, Van Wyk et al. (2016) did report co-infestation of both species at 40% of 108 farms in a study where ticks were collected to investigate molecular pyrethroid resistance. The real threat and extent of invasion of R. (B.) microplus on commercial grazing fields of South African cattle do exist but still needs to be elucidated.
Both tick species are of economic importance due to the direct damage both can cause to their hosts. This includes lowered meat and milk production, host anaemia, and damage to hides and teats inflicted by its blood-feeding nature (Jongejan and Uilenberg 2004). The ability of these ticks to act as disease vectors causing babesiosis in its hosts (De Vos 1979; De Vos and Jorgensen 1992) is, however, the main cause of concern that makes information on the distribution of the two species of great importance. Rhipicephalus (B.) decoloratus is the vector for transmission of the pathogen Babesia bigemina to cattle during feeding, but R. (B.) microplus acts as a vector for both B. bigemina and Babesia bovis (Walker et al. 2003). Babesiosis presents in the host with anemia, fever, haemoglobinuria, and splenomegaly. Babesia bovis infection, however, seems to be more severe and fast working, as well as being associated with severe nervous system disorders. Animals infected with this pathogen can die soon after symptoms are visible, mostly before treatment can be administered (Bock et al. 2004).
A further factor influencing the economic impact of these two tick species is the development of resistance to chemical control measures (Rajput et al. 2006). Information on the global resistance development of R. (B.) microplus to most classes of acaricides is well-documented (De la Fuente et al. 2000; Rajput et al. 2006; Li et al. 2007; Rodríguez-Vivas et al. 2011; Abbas et al. 2014). The extent and comparison of the expression of phenotypic resistance of these two species to acaricides in South Africa is outdated and more recent information is needed to plan for future control.
Phenotypic resistance of R. (B.) decoloratus to the acaricides initially used for tick control in South Africa has been reported for arsenic (1937), DDT (1954), cyclodiene and toxaphene (1948) organophosphorus and carbamate (1966), pyrethroids (1987), and formamidines (1997) (George et al. 2004). Organophosphate-resistant R. (B.) decoloratus populations (Baker et al.1978), as well as populations resistant to pyrethroids, including cypermethrin (Coetzee et al. 1987), were later found on cattle from communal areas located in the Eastern Cape Province. The number of populations tested was, however small and only an indication of the presence and not the extent of tick resistance in the province. A NTRS carried out in South Africa at the turn of the century, showed 35.5% of the R. (B.) decoloratus populations tested to be resistant to cypermethrin, 36.1% to chlorfenvinphos, and 6.6% to amitraz (Van Dalen and Jansen van Rensburg 2023a).
Field data and validation of phenotypic resistance development of R. (B.) microplus in Africa and South Africa are relatively limited. A list with an overview of species displaying acaricide resistance (George et al. 2004) showed reports of resistance of R. (B.) microplus to DDT, cyclodienes, and toxaphene and the organophosphate (OP)—carbamate group in South Africa in 1979. Resistance of Rhipicephalus (Boophilus) species to formamidines was reported in 1997 and although the resistance of R. (B.) decoloratus to pyrethroids was reported in 1987, no mention of resistance of R. (B.) microplus to this acaricide was noted (George et al. 2004). Ntondini et al. (2008) reported R. (B.) microplus populations resistant to amitraz at three of 45 different dip tanks, resistant to cypermethrin at one of 45 dip tanks, and resistant to chlorfenvinphos at eight of 36 dip tanks tested for resistance during their survey. These dip tanks were all located on communal grounds in the eastern region of the Eastern Cape Province, where herds from different owners were dipped according to a set schedule (Ntondini et al. 2008). A few years later, Lovis et al. (2013) reported that both R. (B.) microplus populations obtained from the eastern parts of the Western Cape Province—one from a commercial and one from a communal farming system—were both susceptible to all the compounds tested. One population obtained from communal grounds in the north-eastern part of Mpumalanga Province was resistant to pyrethroids (Lovis et al. 2013).
Therefore, the present study aimed to provide field data results on the extent of displacement of R. (B.) decoloratus by R. (B.) microplus on commercial farms in South Africa. The phenotypic resistance profiles of R. (B.) microplus and R. (B.) decoloratus was compared and the possible influence of resistance on the invasion of R. (B.) microplus on the farms where they co-existed was investigated. The evolution of phenotypic resistance of R. (B.) decoloratus to different acaricides tested has been described in detail in Van Dalen and Jansen van Rensburg (2023b).
Materials and methods
Study area
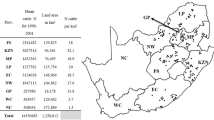
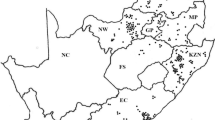
Cattle producers and pharmaceutical companies in South Africa randomly submitted Rhipicephalus (Boophilus) collections from all over South Africa to the Pesticide Resistance Testing Facility (PRTF) situated at the Department of Zoology and Entomology at the University of the Free State in Bloemfontein, South Africa. These populations were only obtained from commercial farms, representing closed farming systems as limited information on this practice is available. Communal farming practices, also found in South Africa, were excluded from this study due to the open cattle grazing practices followed and existing information available in these areas. Populations received from 2006 to 2017 were evaluated as reflected in Fig. 1.
Map of collection points of field populations of Rhipicephalus (Boophilus) decoloratus (black dots), R. (B.) microplus (red dots) and localities where both species were found (orange dots) in the various South African provinces from 2006 to 2017. Provinces: FS, Free State; KZN, KwaZulu-Natal; MP, Mpumalanga; LP, Limpopo; EC, Eastern Cape; NW, Northwest; GP, Gauteng; WC, Western Cape; NC, Northern Cape
Experimental procedure
Fully engorged female ticks were collected from the body and dewlap areas of at least 10 cattle on each farm. Individuals from Rhipicephalus (Boophilus) populations received were identified as either R. (B.) decoloratus or R. (B.) microplus for each population. The distinction between the species was made microscopically by using the dentition differences between the two species (Walker et al. 2003). Rhipicephalus decoloratus has a dental organization in rows found in 3 × 3 columns on the hypostome, whereas R. (B.) microplus has a 4 × 4 column dental organization. When present in the same collection, the two species were incubated in two separate Erlenmeyer flasks at a temperature ranging between 25 and 28 °C and > 75% relative humidity to allow for oviposition and hatching of the larvae.
The Larval Immersion Test (LIT), developed by Shaw (1966) and most consistently used in South Africa to evaluate tick resistance (Coetzee et al. 1987; Mekonnen et al. 2002; Ntondini et al. 2008), was employed to test for resistance to amitraz, cypermethrin, and chlorfenvinphos. The full description of the methodology used, as adapted by the PRTF, can be found in Van Dalen and Jansen van Rensburg (2023b). Commercially available cypermethrin found in Curatik (15% m/v, 2006–2008) and Pro-dip (20% m/v, 2008–2017); amitraz found in Triatix 125 (12.5% m/v, 2006–2017) and chlorfenvinphos found in Disnis NF dip (9% m/v, 2006–2008) and Coopers Supadip (30% m/v, 2009–2017) were used for acaricide exposure of the larvae of the different populations. Acaricide concentrations were assumed to be correct as indicated on the packaging.
Field concentrations recommended for each acaricide were prepared from an initial 1% m/v, commercial acaricide stock solution to obtain exposure concentrations of 0.025% m/v for amidines, 0.015% m/v for pyrethroids, and 0.05% m/v for organophosphates. These field concentrations were considered to be sufficient to kill > 80% of the individuals in the population as it was assumed to be at least equal to the discriminating concentration to which efficacy needs to be determined. This supposition originated from the conjecture that the correct application of any acaricide remedy at field concentration, registered at the pharmaceutical regulatory body in South Africa, should be effective with at least an 80–100% kill rate. Tick populations exposed to field concentrations of these acaricides should, therefore, fall into this category to be considered susceptible. Populations were further categorized as resistant when the mortality was between 0 and 49%, and emerging resistant between 50 and 79%. Fifty % efficacy was considered to be the tipping point between emerging resistance and resistance as 50% of the individuals in the population were still considered to be susceptible. Producers were, however, warned that urgent action should be taken when the population falls into the emerging resistance category. This evaluation system was devised to accommodate a more economic testing method where a reference strain and a resistance ratio was not taken into consideration to save time, labor and money.
Seventy-two h after exposure of the larvae to a specific acaricide, the live vs. dead larvae were counted to calculate the efficacy of the acaricide for control of the specific population. Larvae from each population were exposed to all three acaricides to also determine multi-resistance.
Data analysis
Corrections due to incidental mortalities were calculated by using Abbott’s formula where the mean of the duplicate tests was compared to the mean of the control samples to account for control mortalities (Abbott 1987). Only assays with control values of < 10% mortality were included in the results. A population was considered resistant when the mortality was between 0 and 49%, emerging resistant between 50 and 79%, and susceptible between 80 and 100%. The Microsoft Excel 2010 data analysis package was used for statistical analyses and the difference in species frequency was tested with a two-sample t-test assuming unequal variance (α = 0.05).
Safety measurements
All ticks and larvae were contained and tested in a Section 20 accredited laboratory that assured the safe collection, transport, and handling of ticks to control animal disease. Ethical clearance (No 25/2011A) was obtained from the Interfaculty Animal Ethics Committee of the University of the Free State on a meeting held on 23 February 2012.
Results
Distribution of the Rhipicephalus (Boophilus) species collected
Rhipicephalus (Boophilus) populations were submitted from commercial farms located along the south-eastern to eastern coastal regions and then inland towards the north and the north-west of South Africa (Fig. 1). Rhipicephalus (B.) decoloratus populations were received from all provinces in South Africa except the Northern Cape Province (Fig. 1, Table 1). Rhipicephalus (B.) microplus collections were submitted from all provinces except Northern Cape, Gauteng, and Limpopo provinces. A significantly higher frequency of R. (B.) decoloratus than R. (B.) microplus populations was submitted to the facility over a 12-year period as tested with a two-sample t-test assuming unequal variance (t = 7.08, d.f. = 10, P < 0.05). The total number of collections received over 12 years (389), contained R. (B.) decoloratus populations on 369 (94.9%), R. (B.) microplus on 14 (3.7%), and both species co-existed on six farms (1.6%) (Table 1).
Seven of the 20 R. (B.) microplus collections were submitted from commercial farms in the Eastern Cape Province located near Cathcart (1), Alexandria (1), Nqanqarhu (previously Maclear) (1), East London (1), Ntabozuko (previously Berlin) (2), and Gqeberha (previously Port Elizabeth) (1). The two collections from farms near Ntabozuko and one from Gqeberha each had co-existing R. (B.) decoloratus populations (Table 2). Five R. (B.) microplus collections from KwaZulu-Natal were submitted from farms close to Bergville, Glencoe, Ixopo, New Castle, and Mooi River (Table 2). Three R. (B.) microplus populations received from the Western Cape Province were submitted from locations near George, Mosselbay, and Swellendam. The R. (B.) microplus population submitted from the Free State Province was found near Vrede and one from the Northwest Province, near Lichtenburg (Table 2). All three collections received from the Mpumalanga Province consisted of R. (B.) decoloratus and R. (B.) microplus and were found on three farms in the Piet Retief area (Table 2).
Acaricide resistance profile
Of the 20 R. (B.) microplus populations tested, 70% were susceptible to all three acaricides compared to the 9.8% of 369 R. (B.) decoloratus populations tested (Table 1). Three of the 20 R. (B.) microplus populations were tested as resistant, and a further three as emerging resistant to cypermethrin (Table 2). One of these populations, received from a farm near Alexandria in the Eastern Cape Province, was resistant to all three acaricides tested. This was the only population where resistance of R. (B.) microplus to amitraz and chlorfenvinphos was found (Table 2).
A comparison of the level of resistant populations to the acaricides between the two species tested showed that the mean percentage phenotypic resistance of R. (B.) decoloratus to cypermethrin (66.2%) and amitraz (26.5%) was significantly higher than for R. (B.) microplus (cypermethrin 23.0%, amitraz 4.1% and chlorfenvinphos 3.6%) (Fig. 2). This was found when the percentage resistance of all the R. (B.) decoloratus populations tested (369) was compared to the 20 R. (B.) microplus populations received (Fig. 2a), as well as when the two species from the six co-existing localities were compared (Fig. 2b). Mean percentage resistance to chlorfenvinphos was significantly higher for R. (B.) decoloratus (13.2%) when the 369 populations of this species were compared to the 20 R. (B.) microplus (3.6%) populations received. However, when the six co-existing populations were compared, no significant difference was found (Fig. 2b), and both species had a mean percentage resistance of < 1%.
Comparison of the mean (± SE) resistance (%) calculated for (a) all the Rhipicephalus (Boophilus) decoloratus vs. R. (B.) microplus populations received from 2006 to 2017 in South Africa, and (b) the six populations where both R. (B.) decoloratus and R. (B.) microplus were present in the same blue tick collection obtained from a specific farm in South Africa
Discussion
Worldwide resistance of R. (B.) microplus to the major chemical classes of acaricides currently in use has been reported (De la Fuente et al. 2000; Li et al. 2007; Rajput et al. 2006; Rodríguez-Vivas et al. 2011). Historically R. (B.) microplus parasitized bovid hosts in India and Indonesia (Barré and Uilenberg 2010; Labruna et al. 2009) and was introduced into South Africa, with imported cattle, via Madagascar after the rinderpest epidemic in 1896 (Theiler 1962).
Many factors can influence the distribution and possible invasion of alien species into an area. The movement of hosts from one area to another, linked with favorable environmental conditions for the invasive species, could possibly be some of the key factors (Tønnesen et al. 2004). One important aspect must however also be considered. Once introduced into an area, tick control practices and selection for resistance to acaricides can play an important role in the rate of invasion of R. (B.) microplus on commercial farms. Although phenotypic resistance was found for R. (B.) microplus in a communal grazing area, Mnisi, located in the Mpumalanga Province (Malan 2015), acaricide resistance of this species had not been extensively tested for commercial farms in South Africa. Rhipicephalus (B.) decoloratus, however, showed a high prevalence of resistance to pyrethroid-based acaricides as well as amidines on commercial farms in South Africa (Van Dalen and Jansen van Rensburg 2023b).
Studies mapping the prevalence of different tick species in South Africa (Bryson et al. 2002; Horak et al. 2009, 2015; Tonetti et al. 2009; Spickett et al. 2011; Nyangiwe et al. 2017), showed that R. (B.) microplus was present in all provinces in localized areas. The rapid spread of R. (B.) microplus, when introduced to new areas, throughout Africa (Madder et al. 2007, 2011; Adakal et al. 2013), has also been reported for communal areas in South Africa by Ntondini et al. (2008), Horak et al. (2009) and Nyangiwe et al. (2013). This led to the questions whether this total invasion was also true for commercial farms in South Africa? And if not, what prevented invasion of this alien species, seen in the light that the first reports of R. (B.) microplus was already made in 1908 (Howard 1908) and the rapid spread of this species, once introduced, was seen in other African countries (Madder et al. 2007, 2011; Adakal et al. 2013). Surprisingly R. (B.) microplus populations were only received from 3.7% of the commercial farms that submitted blue tick populations during the current study.
Although represented by low percentages, the prevalence, and localities where R. (B.) microplus populations were found, agreed with localities of previous reports of its presence over the years. In the Western Cape province isolated pockets of R. (B.) microplus were previously reported by Howell et al. (1978) and more recently by Nyangiwe et al. (2017) in corresponding areas along the southern coast where R. (B.) microplus in the current study were collected on three commercial farms. In the Free State Province, only one dominant R. (B.) microplus population was found on a farm near Vrede situated in the northeast of the province where Horak et al. (2015) and Nyangiwe et al. (2017) also previously confirmed the presence of R. (B.) microplus. Baker et al. (1967) reported R. (B.) decoloratus to be one of four species that were most prevalent in KwaZulu-Natal without mentioning the presence of R. (B.) microplus in this survey. In 2014 Oberholster reported a 60% presence of R. (B.) decoloratus on farms in KwaZulu-Natal, with a discontinuous distribution of R. (B.) microplus in temperate parts of the province corresponding to the areas where five dominant R. (B.) microplus populations were collected during the current study. Both Bryson et al. (2002) and Spickett et al. (2011) reported limited distribution of R. (B.) microplus in the north-western areas of the Northwest Province. Oberholster (2014) found R. (B.) decoloratus to be more prevalent than R. (B.) microplus on commercial farms, in this province matching the results of the current study. The absence of R. (B.) microplus was ascribed to the more arid climatic conditions of this province (Oberholster 2014).
In Mpumalanga province Malan (2015) reported only the presence of R. (B.) microplus on cattle from a communal dipping system in the Mnisi community, bordering the Kruger National Park in the northeastern part of the province. The three collections of co-existing Rhipicephalus (Boophilus) species collected in the current study were, however, found on commercial farms in the southeastern part of the province which were quite a distance from Mnisi. This presence of R. (B.) microplus must have represented another introduction of this species than possible closeby introduction as was found in the previous provinces.
In the Limpopo province, Tønnesen et al. (2004) reported the presence of R. (B.) microplus at 93.4% vs. the 6.6% of R. (B.) decoloratus at dip tanks during a survey done from 1999 to 2001. They also found R. (B.) microplus to co-exist with R. (B.) decoloratus on two of the five commercial farms investigated in this province. The current study did, however, not yield any R. (B.) microplus populations on any of the 30 commercial farms investigated in both this province and the seven for Gauteng province.
In an attempt to link invasion with resistance, the results obtained from the Eastern Cape province may be of importance. Initially, R. (B.) microplus was only found in communal areas east of East London in the eastern part of this province (Ntondini et al. 2008; Horak et al. 2009). In the current study, three of the four R. (B.) microplus populations received, as part of 159 commercial farms investigated, were from the eastern part of this province. Co-existence with R. (B.) decoloratus occurred on a further two farms near Ntabozuko, situated in the more central part closer to East London. These findings can be expected due to possible introduction of R. (B.) microplus on cattle from infested areas close to these farms. If the focus is moved more to the western part of this province, Horak et al. (1999) found only R. (B.) decoloratus on a commercial farm close to Alexandria in a survey carried out from 1982 to 1983. Years later, Nyangiwe et al. (2017) reported the presence of R. (B.) microplus throughout the coastal regions of the western part of the Eastern Cape Province, but Yawa et al. (2019) on the other hand were unable to confirm its presence in the western central regions of this province. In the current study R. (B.) microplus was only present on a farm near Alexandria, indicating a possible introduction, invasion, and eventual displacement of R. (B.) decoloratus on this farm. A high occurrence of multi resistance of R. (B.) decoloratus populations was observed on farms in the Alexandria district over a period of 12 years (Van Dalen and Jansen van Rensburg 2023b).
From this information the steps of displacement can perhaps be explained as follows. The introduction of a susceptible strain of R. (B.) microplus may initially be successfully controlled by the acaricide in use to mask the presence of this tick species. A gradual resistance development of the introduced R. (B.) microplus population may then increasingly outcompete R. (B.) decoloratus individuals on the specific farm, even if they were also resistant, due to the higher level of survival of the chemical onslaught. This assumption is further strengthened by the resistance profile of R. (B.) decoloratus vs. R. (B.) microplus coexisting on another farm in the Gqeberha area close to Alexandria. The acaricide resistance profile of R. (B.) decoloratus indicated multi-resistance to cypermethrin and amitraz, whereas the coexisting R. (B.) microplus indicated resistance to only cypermethrin. This could substantiate the possible first steps of R. (B.) microplus invasion to displacement. The one R. (B.) microplus population, with resistance to all three acaricides tested, and without the presence of a coexisting R. (B.) decoloratus found, could represent the ultimate displacement occurrence where the resistance of both species to these acaricides caused R. (B.) microplus to be the winner in this war. This postulate needs to be further investigated to confirm or deny this possibility.
The ability of R. (B.) microplus to outcompete R. (B.) decoloratus when on equal footing—e.g., both susceptible or both resistant—is grounded in the life cycle advantages of R. (B.) microplus compared to R. (B.) decoloratus. These advantages include a slightly shorter life cycle (Londt and Arthur 1975), the production of a higher number of eggs, and the production of sterile eggs when cross mating between the two species takes place (Spickett and Malan 1978). Cross mating occurs due to the sex ratio favoring males (2:1) (Horak et al. 1992, 2003). Although conspecific mating is preferred (Norval and Sutherst 1986), sexually mature R. (B.) microplus males do mate with both R. (B.) microplus and R. (B.) decoloratus females in mixed populations before R. (B.) decoloratus males can do so, causing R. (B.) decoloratus females to produce sterile eggs (Horak et al. 2009). Nyangiwe et al. (2013) found cross mating of R. (B.) microplus males with R. (B.) decoloratus females but none between R. (B.) decoloratus males and R. (B.) microplus females. They furthermore noticed R. (B.) microplus males clasping engorged R. (B.) decoloratus nymphs while attached next to them and upon dissection of these nymphs discovered that nine out of 10 of these occurrences were destined to molt into females (Nyangiwe et al. 2013).
Review of Rhipicephalus (Boophilus) species populations received over 12 years in the current study indicated that the level of invasion of R. (B.) microplus and displacement of R. (B.) decoloratus by R. (B.) microplus on commercial farms were still low in comparison to the prevalence of R. (B.) decoloratus populations. These results indicated that commercial farming practices might, to a great extent help to conserve the dominant prevalence of R. (B.) decoloratus on commercial farms. Closed farming practices followed by commercial producers, compared to the greater movement of cattle in communal areas, seem to help limit the spread of R. (B.) microplus to commercial farms even when located close to a communal area. It further suggests that a susceptible strain of R. (B.) microplus may sometimes be introduced onto most commercial farms but is initially successfully controlled by the acaricide in use. This displacement of R. (B.) decoloratus will, however, also occur if the R. (B.) microplus individuals introduced are already resistant to the acaricide in use on the specific farm and might again be controlled if another acaricide is used, for which resistance was not selected. Collections made on communal grounds and tested for susceptibility to cypermethrin and amitraz in the Mnisi community located in the Mpumalanga Province showed the presence of only R. (B.) microplus with a high prevalence of resistance to these acaricides (Malan 2015) and may also confirm that the resistance profile of the invasive species in the end played the dominant role.
When both species are susceptible to acaricide control, R. (B.) microplus populations can outcompete R. (B.) decoloratus (Arthur and Londt 1973; Londt & Arthur 1975; Spickett and Malan 1978) while gradually developing resistance to the acaricide in use. This will, however, be a slow process, as initial effective acaricide control will keep the numbers of both species low. In this case, the race between the two species to obtain resistance to the acaricide in use will more likely be the determining factor. The higher tendency in South Africa to make use of pyrethroid-based acaricides in the past (Van Dalen and Jansen van Rensburg 2023a), can explain the higher occurrence of resistance of R. (B.) microplus to pyrethroids, compared to amitraz and chlorfenvinphos resistance on commercial farms investigated. Unfortunately, the treatment history of these farms was not available to come to more specific conclusions.
The problem with resistance is that it could have caused producers to indiscriminately use all available acaricides in short succession, giving R. (B.) microplus populations an advantage over R. (B.) decoloratus for resistance development once introduced on a specific farm.
The close morphological similarities between the two tick species sometimes causes producers to only become aware of the presence of R. (B.) microplus during an outbreak of babesiosis caused by B. bovis (Tønnesen et al. 2004). This disease has a more severe clinical result than babesiosis caused by B. bigemina. The economic consequences of the invasion of R. (B.) microplus, and tick resistance development to chemical control, calls for early detection and monitoring of both the presence and resistance of R. (B.) microplus on commercial farms.
Regular monitoring for tick resistance development on a commercial farm, combined with the identification of the Rhipicephalus (Boophilus) species found, can be useful tools to plan control strategies. This in turn will prevent increased economic losses due to resistance development to chemical control and Asiatic babesiosis caused by the invasive R. (B.) microplus species as vector. Molecular identification for the presence of mutations causing tick resistance in a population can be useful (Robbertse et al. 2016), but to characterize acaricide resistance in an area or on a farm, conventional testing such as the LIT (Shaw 1966) is still necessary to determine the extent of the resistance development in the population.
Conclusion
Although the invasion of R. (B.) microplus on communal farms seems to be rapid and, in some cases, replaces the native species in these areas, this phenomenon does not seem to be true for commercial farms in South Africa, at least not for now. Closed commercial farming systems seem to have a preventative advantage over communal grazing systems for the invasion of R. (B.) microplus. A very low percentage of commercial farms showed the presence of R. (B.) microplus populations. This can be ascribed to low introduction of cattle from outside herds that could introduce R. (B.) microplus onto a commercial farm, as well as the susceptible status of the introduced R. (B.) microplus to acaricide control practices on the specific farm.
Once introduced on a commercial farm, the possibility of displacement of R. (B.) decoloratus does exist. The first step after introduction can be total chemical control of R. (B.) microplus with the acaricide in use, and therefore eradication, followed by the gradual resistance development and once both species become resistant to acaricide in use, R. (B.) microplus can start to outcompete R. (B.) decoloratus. Environmental conditions, acaricide control and resistant management practices may play an important role to determine the rate at which R. (B.) microplus might take over.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z (2014) Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet Parasitol 203(1–2):6–20
Abbott WS (1987) A method of computing the effectiveness of an insecticide. J Am Mosquito Contr 3(2):301–302
Adakal H, Biguezoton A, Zoungrana S, Courtin F, De Clercq EM, Madder M (2013) Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa—another three countries are affected: Burkina Faso, Mali and Tsogo. Exp Appl Acarol 61:383–386. https://doi.org/10.1007/s10493-013-9706-6
Arthur DR, Londt JGH (1973) The parasitic life cycle of Boophilus decoloratus (Koch, 1844). J Entomol Soc South Africa 36:87–116
Baker MK, Ducasse FBW (1967) Tick infestation of livestock in Natal. I. The predilection sites and seasonal variations of cattle ticks. J S Afr Vet Assoc 38:447–453
Baker JAF, Miles JO, Robertson WP, Stanford GD, Taylor RJ (1978) The current status of resistance to organophosphorus ixodicides by the blue tick, Boophilus decoloratus (Koch) in the Republic of South Africa and Transkei. J S Afr Vet Assoc 49:327–333
Barré N, Uilenberg G (2010) Spread of parasites transported with their hosts: case study of two species of cattle tick. Rev Sci Et Tech, Off Int Des Épizoot 29:149–160
Bock R, Jackson L, De Vos A, Jorgensen W (2004) Babesiosis of cattle. Parasitology 129:S247–S269
Bryson NR, Tice GA, Horak IG, Stewart CG, Du Plessis BJA (2002) Ixodid ticks on cattle belonging to small-scale farmers at 4 communal grazing areas in South Africa. J S Afr Vet Assoc 73(3):98–103
Coetzee BB, Stanford GD, Davis DAT (1987) The resistance spectrum shown by a fenvalerate-resistant strain of blue tick (Boophilus decoloratus) to a range of ixodicides. Onderstepoort J Vet Res 54:79–82
De la Fuente J, Rodriguez M, Garcia-Garcia JC (2000) Immunological control of ticks through vaccination with Boophilus microplus gut antigens. Ann N.Y Acad Sci 916:617–621
De Vos AJ (1979) Epidemiology and control of bovine babesiosis in South Africa. J S Afr Vet Assoc 50:357–362
De Vos AJ, Jorgensen WK (1992) Protection of cattle against babesiosis in tropical and subtropical countries with a live, frozen vaccine. In: Fivaz B, Petney T, Horak IG (eds) Tick vector biology: medical and veterinary aspects. Springer-Verlag, Berlin, pp 159–174
George JE, Pound JM, Davey RB (2004) Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 129:S353–S366
Horak JG (1999) Parasites of domestic and wild animals in South Africa XXXVII Ixodid ticks on cattle on Kikuyu grasspastures and in Valley Bushveld in the Eastern Cape Province. Onderstepoort J Vet Res 66(3):175–184
Horak IG, Boomker J, Spickett AM, De Vos V (1992) Parasites of domestic and wild animals in South Africa. XXX. Ectoparasites of kudus in the eastern Transvaal Lowveld and the Eastern Cape Province. Onderstepoort J Vet Res 59:259–273
Horak IG, Gallivan GJ, Braack LEO, Boomker J, De Vos V (2003) Parasites of domestic and wild animals in South Africa. XLI. Arthropod parasites of impalas (Aepyceros melampus) in the Kruger National Park. Onderstepoort J Vet Res 70:131–163
Horak IG, Nyangiwe N, De Matos C, Neves L (2009) Species composition and geographic distribution of ticks infesting cattle, goats and dogs in a temperate and a subtropical coastal region of south-eastern Africa. Onderstepoort J Vet Res 76:263–278. https://doi.org/10.4102/ojvr.v76i3.28
Horak IG, Jordaan AJ, Nel PJ, Van Heerden J, Heyne H, Van Dalen EM (2015) Distribution of endemic and introduced tick species in Free State Province, South Africa. J S Afr Vet Assoc 86(1) Art.#1255 9 pages https://doi.org/10.4102/jsava.v86i1.1255
Horak IG, Heyne H, Williams R, Gallivan GJ, Spickett AM, Bezuidenhout JD, Estrada-Peña A (2018) The Ixodid Ticks (Acari: Ixodidae) of Southern Africa, Springer, p 311, 403 https://doi.org/10.1007/978-3-319-70642-9
Howard CW (1908) A list of the ticks of South Africa, with descriptions and keys to all the forms known. Annls Transv Mus 1:73–188. https://doi.org/10.5962/bhl.title.84612
Howell CJ, Walker JB, Nevill EM (1978) Ticks, mites and insects infesting domestic animals in South Africa. Part 1. Descriptions and biology, Science Bulletin No. 393, Department of Agricultural Technical Services, Pretoria.
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology. https://doi.org/10.1017/S0031182004005967
Labruna M, Naranjo A, Thompson C, Estrada-Peña A, Gugliemone A, Jongejan F, La Fuente De (2009) Allopatric speciation in ticks: genetic and reproductive divergence between geographic strains of Rhipicephalus (Boophilus) microplus. BMC Evol Biol 9:46. https://doi.org/10.1186/1471-2148-9-46
Li AY, Chen AC, Miller RJ, Davey RB, George JE (2007) Acaricide resistance and synergism between permethrin and amitraz against susceptible and resistant strains of Boophilus microplus (Acari: Ixodidae). Pest Manag Sci 63:882–889. https://doi.org/10.1002/ps.1417
Londt JGH, Arthur DR (1975) The structure and parasitic life cycle of Boophilus microplus (Canestrini, 1888) in South Africa (Acarina: Ixodidae). Afr Entomol 38:321–340
Lovis L, Reggi J, Berggoetz M, Betschart B, Sager H (2013) Determination of acaricide resistance in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) field populations of Argentina, South Africa, and Australia with the larval tarsal test. J Med Entomol 50(2):326–335. https://doi.org/10.1603/ME12127
Madder M, Thys E, Geysen D, Baudoux C, Horak I (2007) Boophilus microplus ticks found in West Africa. Exp Appl Acarol 43:233–234. https://doi.org/10.1007/s10493-007-9110-1
Madder M, Thys E, Achi L, Touré A, De Deken R (2011) Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West-Africa. Exp Appl Acarol 53:139–145. https://doi.org/10.1007/s10493-010-9390-8
Malan R (2015) Acaricide resistance in Rhipicephalus (Boophilus) species at a communal dipping system in the Mnisi Community, Mpumalanga Province. Dissertation: Masters in Veterinary Science, Department of Veterinary Tropical diseases, Faculty of Veterinary Science, University of Pretoria
Mekonnen S, Bryson NR, Fourie LJ, Peter RJ, Spickett AM, Taylor RJ, Strydom T, Horak IG (2002) Acaricide resistance profiles of single and multi-host ticks from communal and commercial farming areas in the Eastern Cape and North-West provinces of South Africa. Onderstepoort J Vet Res 69(2):99–105
Norval RAI, Sutherst RW (1986) Assortative mating between Boophilus decoloratus and Boophilus microplus (Acari: Ixodidae). J Med Entomol 23:459–460
Ntondini Z, Van Dalen EMSP, Horak IG (2008) The extent of acaricide resistance in 1-, 2- and 3-host ticks on communally grazed cattle in the eastern region of the Eastern Cape Province, South Africa. J S Afr Vet Assoc 79(3):130–135
Nyangiwe N, Harrison A, Horak IG (2013) Displacement of Rhipicephalus (Boophilus) decoloratus by Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in the Eastern Cape Province, South Africa. Exp Appl Acarol 61:371–382
Nyangiwe N, Horak IG, Van der Mescht L, Matthee S (2017) Range expansion of the economically important Asiatic blue tick, Rhipicephalus microplus, in South Africa. J S Afr Vet Assoc 88:a1482. https://doi.org/10.4102/jsava.v88i0.1482
Oberholster T (2014) Characterisation of the genetic diversity of the southern cattle tick, Rhipicephalus microplus, populations from South Africa. Dissertation: masters in genetics, faculty of natural and agricultural sciences. University of Pretoria, Pretoria
Rajput ZI, Hu SH, Chen WJ, Arijo AG, Xiao CW (2006) Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci B 7:912–921. https://doi.org/10.1631/jzus.2006.B0912
Robbertse L, Baron S, van der Merwe NL, Madder M, Stoltsz WH, Maritz-Olivier C (2016) Genetic diversity, acaricide resistance status and evolutionary potential of a Rhipicephalus microplus population from a disease-controlled cattle farming area in South Africa. Ticks Tick-Borne Dis 7:595–603
Rodríguez-Vivas RI, Trees AJ, Rosado-Aguilar JA, Villegas-Perez SL, Hodgkinson JE (2011) Evolution of acaricide resistance: phenotypic and genotypic changes in field populations of Rhipicephalus (Boophilus) microplus in response to pyrethroid selection pressure. Int J Parasitol 4:895–903. https://doi.org/10.1016/j.ijpara.2011.03.012
Schroder B, Reilly BK (2013) A comparison between tick species collected in a controlled and control free area on a game ranch in South Africa. J S Afr Vet Assoc 84(1):Art. #907, 5 pages https://doi.org/10.4102/jsava.v84i1.907
Shaw RD (1966) Culture of an organophosphorus-resistant strain of Boophilus microplus (Can.) and an assessment of its resistance spectrum. Bull Ent Res 56:389–405
Spickett AM, Malan JR (1978) Genetic incompatibility between Boophilus decoloratus (Koch, 1844) and Boophilus microplus (Canestrini, 1888) and hybrid sterility of Australian and South African Boophilus microplus (Acarina: Ixodidae). Onderstepoort J Vet Res 45:149–153
Spickett AM, Heyne IH, Williams R (2011) Survey of the livestock ticks of North-West Province South Africa. Onderstepoort J Vet Res 78:305
Theiler G (1962) The Ixodidae parasites of vertebrates in Africa south of the Sahara. Project S 9958. Report to the Director of Veterinary Services, Onderstepoort, South Africa 154–159
Tonetti N, Berggoetz M, Rühle C, Pretorius AM, Gern L (2009) Ticks and tick-borne pathogens from wildlife in the Free State province. South Africa J Wildl Dis 45(2):437–446
Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH (2004) Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol 32:199–208
Van Dalen EMSP, Jansen van Rensburg C (2023a) Acaricide resistance of Rhipicephalus (Boophilus) decoloratus (Acari: Ixodidae) on commercial farms in South Africa: filling a gap in historical data. Exp Appl Acarol. https://doi.org/10.1007/s10493-023-00817-z
Van Dalen EMSP, Jansen van Rensburg C (2023b) Evolution of acaricide resistance of Rhipicephalus decoloratus on commercial farms in South Africa. Exp Appl Acarol. https://doi.org/10.1007/s10493-023-00820-4
Van Wyk RD, Baron S, Maritz-Olivier C (2016) An integrative approach to understanding pyrethroid resistance in Rhipicephalus microplus and R. (B.) decoloratus ticks. Ticks Tick Borne Dis 7(4):586–594
Walker AR, Bouattour A, Camicas J-L, Estrada-Peña A, Horak IG, Lati AA, Pegram RG, Preston PM (2003) Ticks of domestic animals in Africa a guide to identification of species. Bioscience Reports, Edinburgh
Yawa M, Nyangiwe N, Kadzere CT, Muchenje V, Mpendulo TC, Marufu MC (2019) In search of the Rhipicephalus (Boophilus) microplus in the western-central regions of the Eastern Cape province. Ticks Tick Borne Dis 10:564–567. https://doi.org/10.1016/j.ttbdis.2019.01.009
Acknowledgements
The authors gratefully acknowledge the pharmaceutical companies Bayer, Virbac and Novartis Animal Health divisions for making use of the services of the Pesticide Resistance Testing Facility and for their representatives for collection and submission of tick populations to the PRTF for testing.
Funding
Open access funding provided by University of the Free State. Funding was generated through the entrepreneurial services offered by the Pesticide Resistance Testing facility within the Department of Zoology & Entomology.
Author information
Authors and Affiliations
Contributions
EMSPD was a Ph.D. student and researcher at the Department of Zoology and Entomology, UFS, Bloemfontein, SA. She conceptualized the study, conduct the bioassays and computerized and analyzed all the data in preparation of the draft manuscript. CJR was the academic supervisor for the PhD thesis and assisted in constructive criticism and editing of the manuscript. Both authors proofread the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Ethical approval
Ethical clearance by the Interfaculty Animal Ethics Committee of the University of the Free State was obtained during a meeting held on 23 February 2012 for the routine tick resistance profile testing done on tick samples sent to the PRTF. The clearance number allocated was 25/2011A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Dalen, E.M.S.P., Jansen van Rensburg, C. Competitive displacement and acaricide resistance of two Rhipicephalus (Boophilus) species collected on commercial farms in South Africa. Exp Appl Acarol 92, 135–149 (2024). https://doi.org/10.1007/s10493-023-00871-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00871-7