Abstract
Manure-inhabiting Mesostigmata mites are important biological control agents of pest flies. However, the biodiversity of this mite community is mainly known from Europe and America, and especially from cattle manure. This study examined the diversity and abundance of Mesostigmata mites associated with various types of manure in an (intensive) agricultural region of the Middle East, i.e., the city Ahvaz and its suburbs, in southwest Iran. Mite samples were extracted from manure of cattle, buffalo, sheep, horse, poultry and quail in 30 livestock and poultry farms. In total, 40 species belonging to 24 genera and 16 families were identified. The most diverse families were Laelapidae with eight species, Macrochelidae with seven and Parasitidae with six. Macrocheles muscaedomesticae and Uroobovella marginata were the most widespread species, recorded in 28 and 27 out of 30 collection sites, respectively. Two species, M. sumbaensis and U. marginata, were found in all studied manures. Simpson’s diversity index recorded the highest diversity in buffalo and sheep manure. Real and theoretical species richness (rarefaction curves) were congruent in number of individuals. The presence of seven species of Macrochelidae in the manure confirms that these are important predators of the house fly for the region of Ahvaz and its suburbs. Members of the Parasitidae were highly prevalent, with one species known as a specialized predator of house fly eggs. This work aims to encourage further studies on the diversity of Mesostigmata in these agricultural settings, and further continue assessing the feasibility of these mites as effective biocontrol agents of filth flies in different types of manure and from different corners of the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Mesostigmata is a large, cosmopolitan taxon of parasitiform mites that comprises an extremely diverse variety of lifestyles and habitats (Lindquist et al. 2009). The greater number of species are free-living predators (Karg 1993), whilst many others are parasites or symbionts of mammals, birds, reptiles or arthropods and a few species feed on fungi, pollen, or even nectar (Walter and Proctor 1999). Free-living mesostigmatid mites are found in association with soil, litter, rotting wood, tree canopies, compost, carrion, animal manure and animal nests (Krantz 2009a; Lindquist et al. 2009). Of these varied habitats, soil – especially agricultural soils – have been so far the most investigated for mesostigmatids, although their diversity in other substrates from agricultural systems, such as manure-inhabiting mites, remains largely overlooked, despite their potential to control filth flies originated in these anthropogenic byproducts of agriculture.
The manure or dung of domestic animals including poultry are suitable media for the breeding of dipteran species from Muscidae and Fanniidae, such as Musca domestica L. or Fannia canicularis (L.) (Axtell 1964; Bohart and Gressitt 1951; Grisales and de Carvalho 2016; Hewitt 1912; Ito 1970; Kristofik 1984, 1988; Legner and Bowen 1973; Mihályi 1965; Nickolls and Disney 2001; Nuorteva 1963; Perotti 1998b, 2000a; Perotti and Bachmann 1999; Perotti and Lysyk 2003; Perotti et al. 2001; Perotti and Sardella 1998). Coprophilous Mesostigmata, mainly members of the Macrochelidae, Parasitidae, Laelapidae, Eviphididae, Pachylaelapidae and Uropodidae families are predator mites that usually feed on the eggs and larvae of Diptera, particularly muscid flies, and other micro-invertebrates from dung (Ciccolani 1979; Geden et al. 1988; Ho 1990; Perotti 1998a, 2001; Perotti et al. 2000; Rodriguez and Wade 1961; Rudzíska 1998; Schelvis 1991, 1994; Takaku et al. 1994; Wade and Rodriguez 1961). As a result, they are recognised biocontrol agents of filth flies in these habitats (Axtell 1963b, 1968, 1970a, 1970b; Azevedo et al. 2018; Ciccolani 1979; de Azevedo et al. 2015; De Jesus and Rueda 1992; Krantz 1983; Manning and Halliday 1994; Moya Borja 1981; Perotti 1996, 1998a, 1999a, 1999b, 2000b, 2001; Perotti and Brasesco 1996; Perotti et al. 2000; Rodrigueiro and Prado 2004; Rodriguez et al. 1970; Rodriguez and Wade 1961; Schelvis 1994; Wade and Rodriguez 1961). These same fly families, predated in their immature stage by mites, transport as phoretic carriers in their adult state the mites, that then found new predator-mite populations (Acs et al. 2017; Athias-Binche 1984, 1994; Axtell 1964; Bajerlein and Bloszyk 2003, 2004; Beresford and Suctcliffe 2009; Bloszyk et al. 2006; Fain 1998; Fain and Greenwood 1991; Farish and Axtell 1971; Glida et al. 2003; Greenberg and Carpenter 1960; Haloti et al. 2005; Krantz 2009b; McGarry et al. 1992; Niogret et al. 2010; Pereira and Castro 1947; Perotti 1998a; Perotti and Braig 2009; Perotti et al. 2010; Perotti and Brasesco 1996; Rodrigueiro and Prado 2004; Rudzíska 1998; Sato et al. 2018).
Studies on the potential of these mites as biocontrol agents started in the mid 1900’s with the work of Leitner (Germany), who carried out a comprehensive survey on the diversity of manure mites and their ecological roles, recording 121 species of 31 mite families (Leitner 1946a, 1946b). It was Filipponi (1955) (Italy) who first studied in detail the biology and ecology of the members of the family Macrochelidae as manure-inhabiting mites and determined the feasibility of mass production of macrochelids (Filipponi 1964). Later, Axtell (USA) conducted a series of experiments in poultry manure and found that Uropodidae was the most abundant, followed by Macrochelidae and then Parasitidae (Axtell 1961, 1963a, 1963b). He further investigated the control of house flies in poultry manure using Macrocheles muscaedomesticae (Axtell 1964, 1968, 1970a, 1970b). Moreover, Ito (1970) recorded Macrochelidae and Uropodidae as abundant mite families in livestock dungs in Japan. Krantz (1983) reported that 26 genera of mites representing nine families in the Mesostigmata order are known to be associated with dung beetles, and Halliday (2000) studied the Australian fauna of the mite genus Macrocheles (Macrochelidae) listing 49 species, most associated to ephemerous habitats such as manure. Only recently, new studies were carried out in other regions and continents. Özbek and collaborators studied the fauna of Macrochelidae in northern Turkey with the description of new species (Özbek 2017; Özbek and Bal 2013; Özbek et al. 2013, 2015a, 2015b; Özbek and Halliday 2015). Newer additions also include other disparate regions, for example South America, such as Azevedo et al. (2017) from Brazil, who described a new species of Macrocheles, and Porta et al. (2020), who reported M. subbadius from Argentina, although it was found on cattle dung pads in the Pampas region in 1994 (Perotti 1998a).
For the Middle East, particularly Iran, Zakeri et al. (2012) collected eight coprophilous species of Macrochelidae from the North (Golestan Province). Sobhani et al. (2017) recorded 11 species of Macrochelidae from manure in southern Iran (Fars Province). While Babaeian (2011) studied the fauna of Macrochelidae and Laelapidae in central Iran (Chaharmahal-va-Bakhtiari Province) and reported eight Macrochelidae species as well as seven Laelapidae species from manure. Kamali et al. (2001) provided a catalogue of mites and ticks (Acari) of Iran, listing 16 species of Mesostigmata which had been collected from manure. Ahangaran et al. (2012) surveyed the fauna of the edaphic and dung dweller mites of the superfamily Eviphidoidea (Acari: Mesostigmata) in northern Iran (western Mazandaran Province) and collected 14 species from manure. In terms of mite abundance it was found higher in Macrochelidae, followed by Pachylaelapidae and then Parholaspididae. Arjomandi et al. (2013) studied the fauna and diversity of the manure-inhabiting Mesostigmata in southeastern Iran (Kerman Province). They recorded 36 species in 14 families from a variety of manure, resulting in 31 species from cattle, which was more diverse than poultry and sheep manure which carried 14 and 13 species, respectively. Kazemi and Rajaei (2013) also studied cattle, sheep, chicken, poultry and camel manure and listed 72 species of manure-inhabiting Mesostigmata from Mazandaran, Guilan, Golestan, North Khorasan, Semnan, Tehran and Fars Provinces of Iran. Nemati et al. (2018) added 50 more manure species in their updated catalogue of the Iranian Mesostigmata (Acari). The most recent studies come from southwestern Iran (Farahi et al. 2020, 2019) including the new findings of this work.
Despite the importance of manure-inhabiting mesostigmatid mites as reliable biocontrol agents of dipteran pests, the topic remains understudied, and little is known of the biodiversity, and the biology of many of these species in the Middle East. The main objective of this work was to review and revive the interest for this topic, with the initial aim of studying the Mesostigmata diversity associated with six frequently generated manure types, as the most common farming product in the agricultural lands of southwest Iran.
Materials and methods
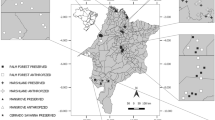
The study was conducted using manure from 30 livestock and poultry farms of Ahvaz and its suburbs, Khuzestan Province, southwest Iran. The area is a semi-desert lowland part of the province, which is excessively warm and dry in the summer and the annual average rainfall does not exceed 230 mm.
Field sampling and material preparation
Sampling was randomly done from the outermost layer of the manure (maximum depth of 20 cm) of five locations in each site, using a small trowel (Southwood and Henderson 2000). As not all types of manure were available in each site simultaneusly, the number of samples collected from different types of manure was deemed to be different in each case. Using Tullgren-Berlese funnels for up to 48 h (Tullgren 1918), mites were extracted from a total of 100 samples of six domestic animal manure types: cattle (33 samples), sheep (20), poultry (10), buffalo (27), horse (6) and quail (4), during 2015–2017. Quail manure was rarely found, which explains that only four quail manure samples were collected. The number of hours of funnel use depended on the relative humidity at the moment of extraction, if the humidity fell rapidly we used the funnels shorter, between 24 and 48 h.
The extracted specimens were preserved in 75% ethanol and placed in Nesbitt’s solution and lactophenol for clearing and then mounted in Hoyer’s medium on permanent microslides. The specimens were mounted under a stereo microscope (Olympus SZX12) and identifications were carried out with a Phase Contrast Olympus BX51 using up to 1000x magnification.
For analysis purposes, the data of the five locations in each site (by manure type) were pooled. Mesostigmata mites were identified, if possible, to the species level. Identification of species was done by the first and third authors (acarologists) using available literature including the original descriptions of the species (Berlese 1887, 1904a, 1904b, 1906, 1918; Bregetova et al. 1973; Costa 1966, 1967, 1968; Evans and Browning 1956; Evans and Hyatt 1963; Evans et al. 1961; Evans and Till 1979; Farrier and Hennessey 1993; Filipponi and Pegazzano 1962, 1963; Furman 1972; Halliday 2000; Hirschmann and Wiśniewski 1982; Hirschmann et al. 1991; Hughes 1976; Hyatt 1980; Hyatt and Emberson 1988; Karg 1962, 1989; Kazemi et al. 2014; Krantz 1962; Ma and Wang 1996; Niogret et al. 2007; Plumari and Kazemi 2012; Samšiňák 1964; Skorupski and Witaliński 1997; Witaliński 2017; Yao et al. 2019). The collected specimens (adults and identifiable nymphs, of which mainly deutonymphs were collected) were deposited in the Insect and Mite Collection of Ahvaz (IMCA), Department of plant protection, Shahid Chamran University of Ahvaz, except Parasitus sp. and some type material of Trachygamasus karuni which were deposited earlier in the Zoological Museum of the Jagiellonian University, Poland (Farahi et al. 2019). Two specimens belonging to Ascidae and Rhodacaridae were damaged and could not be identified.
Data analyses
The number and relative frequency of each species were recorded for each manure type. Boxplots were generated with STATA (2014). The Mesostigmata species richness (using rarefaction), diversity (applying Simpson’s index) and Simpson’s measure of evenness of the studied manure mites were calculated for all samples, utilizing the software Ecological Methodology v.7.2 (Krebs 2011). Simpson’s index was compared between manure types, using a randomisation test with 10,000 re-samples in the SDR software v.4.1.2 (Seaby and Henderson 2006). The taxonomic distinctness index (Δ*) of Clarke and Warwick (1998) was also calculated by PAST v.4.06 (Hammer et al. 2001).
Results
In total, 1892 mites belonging to 40 species, 24 genera and 16 families were sampled from the five domestic animal manure types. The highest number of mites was found in manure of cattle, followed by buffalo, sheep, horse, poultry and finally quail, with just a handful (Fig. 1).
Overall, Macrochelidae was the predominant family (abundance) followed by Urodinychidae and Parasitidae (Fig. 2). In terms of species diversity, Laelapidae with up to eight species, Macrochelidae with seven and Parasitidae with six contained the highest species diversity among the 16 recorded families.
According to relative frequency of species found (Table 1), M. muscaedomesticae, Parasitus beta, Uroobovella marginata, Halolaelaps sexclavatus, Macrocheles merdarius and U. marginata were the dominant species within cattle (Table S1), buffalo (Table S2), sheep (Table S3), horse (Table S4), poultry (Table S5) and quail manure (Table S6), respectively. Proctolaelaps ventrianalis was recorded for the first time from Khuzestan. Macrocheles muscaedomesticae and U. marginata were the most widespread species which were recorded in 28 and 27 out of 30 collection sites, respectively. On the other hand, Uroobovella varians and Gaeolaelaps minor were collected only in one site. Kleemannia parplumosa, Onchodellus karawaiewi and Dermanyssus gallinae were recorded for the first time associated with manure in southwest Iran.
The number of shared species between two manure types ranged from a maximum of 24 species, such as for cattle and sheep or cattle and buffalo, to a minimum of two species, for quail and all other manure types. Interestingly, only two species, Macrocheles sumbaensis and U. marginata, were found in all studied manure types.
The rarefaction curves of manure-inhabiting mesostigmatid mites in southwestern Iran estimated the number of expected species for the 95 collection sites of the studied manure types as 22.47 species for buffalo, 21.51 species for sheep, 20.95 species for cattle, 18.50 species for horse and 10 species for poultry manure (Fig. 3). Species richness of cattle dung would be higher than buffalo when number of individuals goes beyond 300. The lowest richness was observed in quail manure.
The highest species richness was recorded in cattle (34 species) and buffalo (28 species) manure, followed by sheep, horse, poultry and quail (Table 2). However, the mite community in buffalo and sheep manure were significantly more diverse than cattle manure, according to Simpson’s diversity index. Quail manure was found to be the most uninhabited, the least diverse in our results. Simpson’s evenness in quail and poultry were higher than other manure types. Cattle manure showed the lowest evenness value (Table 2).
The taxonomic distinctness index (Δ*) for cattle, buffalo, sheep, horse, poultry and quail manure was 3.58, 3.65, 3.75, 3.83, 3.63 and 4.00, respectively. Horse and sheep manure showed the most taxonomic diversity among manure types. Quail should be considered cautiously due to the estimations being based on four samples and two species only.
Discussion
The great species diversity found in the families Laelapidae, Macrochelidae and Parasitidae that inhabit manure was originally discovered by Leitner (1946a), who listed 121 manure mites from Eastern Alps. Arjomandi et al. (2013) also reported similar results on diversity of the manure-inhabiting Mesostigmata in Kerman County, southeastern Iran. Our results indicated the highest abundance of mites in cattle manure, with 34 species. The humidity content of cattle manure is kept higher for longer than in sheep and poultry, and this holding capacity seems to have a positive effect on the community of coprophilous gamasid mites (Kamaruzaman et al. 2018; Perotti 2001). In addition, the ratio of carbon/nitrogen (C/N) in cattle manure (19/1) is higher than in sheep (16/1) and poultry manure (4/1) which may represent another factor affecting the abundance of mites in dung in both studies (Augustin and Rahman 2010).
According to the rarefaction analysis, with a higher sampling size the number of expected species would increase, although for quail manure mites the results are inconclusive due to lack of data. For quail manure it seems that sample size was very small, due to the size of the quail manure areas themselves, therefore, more data will be needed to enable a comparison of quail manure mites with other manure types. Real (Table 2) and theoretical species richness (Fig. 3) were congruent especially in higher number of individuals. Low abundance of some species may be due to sampling area swifts, or size (e.g., like the case for quail manure). Taxonomic distinctness quantifies diversity as the relatedness of the species within a sample, based on the distances between species in a classification tree (Magurran 2004). Communities may be identical in terms of richness and evenness but differ in taxonomic diversity of taxa, species. Taxonomic distinctness has been used as a tool to examine ecological degradation in marine environments and sampling methods of invertebrates (Baños-Picón et al. 2009; Tolimieri and Anderson 2010). Horse and poultry manure showed the highest values of taxonomic distinctness, despite cattle, buffalo and sheep holding the highest number of mites. This might be due to the phoretic arrival onto the manure types, as different insect carriers will visit horse and poultry manure. Most phoretic mites associated to manure are carrier-species or family specific (Axtell 1963a; Krantz 1983, 1998). This study did not sample carriers of mites, therefore no assumptions can be made on the potential insect carriers of mites for the various manure types.
In our current study Parasitidae mite species are more prevalent in cattle, buffalo and sheep than in horse and poultry droppings. A study in the Philippines showed that parasitids were more abundant in caraboa (water buffalo), dairy cattle, and swine manure than in poultry droppings (De Jesus and Rueda 1992). The parasitid mite Poecilochirus monospinosus was reported to prey on house fly juveniles in poultry manure (Wise et al. 1988). This predator is found mostly in late spring and early summer, and was considered to be only a minor, short-term factor in suppressing fly populations (Geden et al. 1988).
Several species of the Urodinychidae, specially U. marginata were found abundant in all types of manure investigated. This species is a slow-moving mite that has been well studied in poultry and cattle manure (Gerson et al. 2003). It seems to be broadly adapted to the manure and soil habitats, as suggested by Anderson (1977). It survives on many live and dead organic diets, including fly larvae, nematodes and fungi (Faash 1967). Reproduction is sexual and eggs are oviposited only by fertilized females. The females had a long, 7-month preoviposition period (Jalil and Rodriguez 1970). Perotti (2001) compared the predatory strategies of macrochelids such as M. muscaedomesticae and uropodids, and proposed that U. marginata occurs in the same substrate or manure, feeding on immature fly stages; however, both differ in their prey location and preying strategies. Whereas M. muscaedomesticae will go after fly eggs and first instars located on the surface, U. marginata will group in ‘gangs’ that hunt first and second instars trying to hide into the manure. The uropodid strategy was also proposed by Willis and Axtell (1968). Other macrochelids, such as Glyptholaspis confusa, shelter deeper inside the manure where they prey on hidden eggs (Perotti 2001).
Macrochelidae species were also highly numerous. Macrocheles muscaedomesticae and M. merdarius were found abundantly in five of the six tested manure types (except quail manure). Similarly, it has been reported that approximately 450 species of mites representing 18 families and 48 genera in three orders are known to be associated with animal dungs in the world. Over 60% of these mites, or 280 species, are macrochelids (Krantz 1983). These mites reduce pest flies. For instance, M. muscaedomesticae is a well-known predator of the house fly M. domestica, the face fly Musca automnalis De Geer, the stable fly Stomoxys calcitrans (L.), and the horn fly Haematobia irritants (L.) (Axtell 1963b; De Jesus and Rueda 1992; Filipponi 1964; Geden et al. 1988; Perotti 1999b, 2000b, 2001). In this regard, Axtell (1963b) reported that Macrocheles species can reduce the pest flies more efficiently than any pesticides. Similarly, Rodriguez et al. (1970) showed that when machrochelid species were added to manure, they could decrease the density of the pest fly population by approximately 90%.
The only two species present in all types of manure were M. sumbaensis and U. marginata. Two of the most frequent species were M. muscaedomesticae and U. marginata; and this is in line with early studies. Anderson (1983) reported that in the USA both species are common predators of pest fly eggs, larvae, or both, in agricultural systems characterized by confined, high-density concentrations of livestock and poultry. The role and importance of M. muscaedomesticae and U. marginata in integrated pest management (IPM) programs has been already confirmed (Anderson 1983; Axtell 1968, 1970b; Geden et al. 1990; Rodriguez et al. 1970; Wicht and Rodriguez 1970). The main reason why M. muscadomestica and U. marginata currently offer the highest potential as effective predators is that they occur together in the same manure, complementing each other. Both prey on key pest fly species associated with confined livestock and poultry productions worldwide, and both species can be mass-reared for inundative or inoculative releases. Furthermore, to a large degree these species exhibit the four essential characteristics itemized by Doutt and Debach (1964) of an effective natural enemy: (1) high searching/finding ability; (2) high degree of host (prey) specificity; (3) high rate of increase in relation to the pest population; and (4) capability of dwell in all of the host- (or prey-)inhabited microhabitats. Parasitidae are almost as ubiquitous as Macrochelidae in manure, therefore they should also be included in pest control plans.
Although the mite species K. parplumosa, O. karawaiewi and D. gallinae were not know from manure in the studied region, they have been recorded previously from soil (Farahi et al. 2018).
This work reports the occurrence of up to seven species of Macrochelidae in the manure of livestock and poultry productions in southwest Iran (Ahvaz and suburbs), two of them in high numbers, M. muscaedomesticae and M. sumbaensis, the latter with a 100% prevalence. They co-inhabit the manure together with U. marginata and key Parasitidae species. The findings suggest that the most relevant predators of filthy flies are in place and adapted, bringing the possibility to use or consider them in future pest control plans. However, the biology and predatory traits of many of these species, e.g., of the abundant M. sumbaensis, should be studied or revised to further assess their feasibility as biocontrol agents of filth flies.
Data availability
All numerical data is disclosed in the Supplementary Tables file (Tables S1 to S6).
References
Acs A, Sutak A, Kontschan J (2017) New records of macrochelid mites and description of a new phoretic species (Acari: Mesostigmata: Macrochelidae) from Greece. Acarologia 56:63–71. https://doi.org/10.1051/acarologia/20162188
Ahangaran Y, Afshari A, Saboori A, Kazemi S (2012) Edaphic and coprophil fauna of Eviphidoidea (Acari: Mesostigmata) in Nowshahr. Taxon Biosyst 12:1–16
Anderson JR (1977) The organization of soil animal communities. Ecol Bull 25:15–23
Anderson JR (1983) Mites as biological control agents of dung-breeding pests: practical considerations and selection for pesticide resistance. In: Hoy MA, Cunningham GL, Knutson L (eds) Biological control of pests by mites. University of California, LA, pp 99–102
Anderson JR (1965) A preliminary study of integrated fly control on northern California poultry ranches. In: 33rd annual conference of the California Mosquito Control Association. vol 33. California Mosquito Control Association, pp 42–44
Arjomandi E, Kazemi S, Afshari A (2013) Fauna and diversity of the manure-inhabiting Mesostigmata (Acari) in Kerman county, south eastern Iran. Persian J Acarol 2:253–263. https://doi.org/10.22073/pja.v2i2.9958
Athias-Binche F (1984) La phorésie chez les acariens uropodides (Anactinotriches), une stratégie écologique originale [Phoresy in uropodid mites (Anactinotrichida), an interesting ecological strategy]. Acta Oecol Oecol Gen 5:119–133
Athias-Binche F (1994) La phorésie chez les acariens – aspects adaptatifs et evolutifs [Phoresy in acarina – adaptive and evolutionary aspects]. Perpignan, Editions du Castillet
Augustin C, Rahman S (2010) Composting Animal Manures: a guide to the process and management of animal manure compost. NDSU Extension Service. vol May 2010. North Dakota University, Fargo, pp 1–8
Axtell RC (1961) New records of North American Macrochelidae (Acarina: Mesostigmata) and their predation rates on the house fly. Ann Entomol Soc Am 54:748. https://doi.org/10.1093/aesa/54.5.748
Axtell RC (1963a) Acarina occurring in domestic animal manure. Ann Entomol Soc Am 56:628–633. https://doi.org/10.1093/aesa/56.5.628
Axtell RC (1963b) Effect of Macrochelidae (Acarina: Mesostigmata) on house fly production from dairy cattle manure. J Econ Entomol 56:317–321. https://doi.org/10.1093/jee/56.3.317
Axtell RC (1964) Phoretic relationship of some common manure-inhabiting Macrochelidae (Acarina: Mesostigmata) to the house fly. Ann Entomol Soc Am 57:584–587. https://doi.org/10.1093/aesa/57.5.584
Axtell RC (1968) Integrated house fly control: populations of fly larvae and predaceous mites, Macrocheles muscaedomesticae, in poultry manure after larvicide treatment. J Econ Entomol 61:245–249. https://doi.org/10.1093/jee/61.1.245
Axtell RC (1970a) Fly control in caged-poultry houses: comparison of larviciding and integrated control programs. J Econ Entomol 63:1734–1737. https://doi.org/10.1093/jee/63.6.1734
Axtell RC (1970b) Integrated fly control program for caged-poultry houses. J Econ Entomol 63:400–405. https://doi.org/10.1093/jee/63.2.400
Azevedo LH, Castilho RC, Berto MM, de Moraes GJ (2017) Macrochelid mites (Mesostigmata: Macrochelidae) from Sao Paulo state, Brazil, with description of a new species of Macrocheles. Zootaxa 4269:413–426. https://doi.org/10.11646/ZOOTAXA.4269.3.5
Azevedo LH, Ferreira PM, de Campos Castilho R, Duarte PH, Cançadoc GJ, de Moraes (2018) Potential of Macrocheles species (Acari: Mesostigmata: Macrochelidae) as control agents of harmful flies (Diptera) and biology of Macrocheles embersoni Azevedo, Castilho and Berto on Stomoxys calcitrans (L.) and Musca domestica L. (Diptera: Muscidae). Biol Control 123, 1–8. https://doi.org/10.1016/j.biocontrol.2018.04.013
Babaeian E (2011) Faunistic survey of mite of the family Laelapidae and Macrochelidae (Acari: Mesostigmata) in Shahrekord city. Shahid Chamran University of Ahvaz, Iran
Bajerlein D, Błoszyk J (2003) Two cases of hyperphoresy in mesostigmatic mites (Acari: Gamasida: Uropodidae, Macrochelidae). Biol Lett 40:135–136
Bajerlein D, Bloszyk J (2004) Phoresy of Uropoda orbicularis (Acari: Mesostigmata) by beetles (Coleoptera) associated with cattle dung in Poland. Eur J Entomol 101:185–188. https://doi.org/10.14411/eje.2004.022
Baños-Picón L, Asís JD, Gayubo SF, Tormos J (2009) Analyzing insect community structure through the application of Taxonomic Distinctness measures. Zool Stud 48:298–314
Beresford DV, Suctcliffe JF (2009) The effect of Macrocheles muscaedomesticae and M. subbadius (Acarina: Mac- rochelidae) phoresy on the dispersal of Stomoxys calcitrans (Diptera: Muscidae). Syst Appl Acarol 23:1–30. https://doi.org/10.11158/saasp.23.1.1
Berlese A (1887) Acari, Myriapoda et Scorpiones hucusque in Italia reperta. Portici et Padova 39:4
Berlese A (1904a) Acari nuovi. Manipulus II Redia 1:258–280
Berlese A (1904b) Acari nuovi. Manipulus I Redia 1:235–252
Berlese A (1906) Monografia del genere Gamasus Latr. Redia 3:65–304
Berlese A (1918) Centuria quarta di Acari nuovi. Redia 13:113–190
Bloszyk J, Klimczak J, Lesniewska M (2006) Phoretic relationships between Uropodina (Acari: Mesostigmata) and centipedes (Chilopoda) as an example of evolutionary adaptation of mites to temporary microhabitats. Eur J Entomol 103:699–707
Bohart GE, Gressitt JL (1951) Filth-inhabiting flies of Guam. Bull Bish Mus 204:1–152
Bregetova NG, Vainshtein BA, Kadite BA, Koroleva EV, Petrovs AD, Tikhomirov SI, Shcherbat GI (1973) A key to the Soil-inhabiting Mites of the Mesostigmata translate [A key to the Soil-inhabiting Mites of the Mesostigmata] (trans: Lindquist, E.E.). Leningrad., Zoologicheskogo Institute Academi Nauk SSSR
Ciccolani B (1979) The intrinsic rate of natural increase in dung macrochelid mites, predators of Musca domestica eggs. Boll Zool 46:171–178. https://doi.org/10.1080/11250007909440296
Clarke KR, Warwick RM (1998) A taxonomic distinctness index and its statistical properties. J Appl Ecol 35:523–531. https://doi.org/10.1046/j.1365-2664.1998.3540523.x
Costa M (1966) Notes on macrochelids associated with manure and coprid beetles in Israel. I. Macrocheles robustulus (Berlese, 1904), development and biology. Acarologia 8:532–548
Costa M (1967) Notes on macrochelids associated with manure and coprid beetles in Israel. II. Three new species of the Macrocheles pisentii complex, with notes on their biology. Acarologia 9:304–329
Costa M (1968) Little known and new litter-inhabiting Laelapine mites (Acari, Mesostigmata) from Israel. Isr J Zool 17:1–30. https://doi.org/10.1080/00212210.1968.10688258
de Azevedo LH, Emberson RM, Esteca FCN, de Moraes GJ (2015) Macrochelid mites (Mesostigmata: Macrochelidae) as biological control agents. In: Carrillo D, de Moraes GJ, Peña JE (eds) Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms. Progress in Biological Control, vol 19. Springer, Cham
De Jesus LAR, Rueda LM (1992) Seasonal abundance, life history and predatory activity of common mites (Acarina) attacking house fly (Musca domestica Linn.) and other filth flies (Diptera) associated with poultry and livestock manure. Philipp Entomol 8:1213–1227. https://doi.org/10.3923/ijzr.2014.30.36
Doutt RL, Debach P (1964) Some biological control concepts and questions. In: Debach P (ed) Biological control of insect pests and weeds. Chapman and Hall, London
Evans GO, Browning E (1956) British mites of the subfamily Macrochelinae Trägårdh (Gamasina, Macrochelidae). Bull Brit Mus (Nat Hist) Zool 4:1–55
Evans GO, Hyatt KH (1963) Mites of the genus Macrocheles Latr. (Mesostigmata) associated with coprid beetles in the collections of the British Museum (Natural History). Bull Brit Mus (Nat Hist) Zool 9:327–407
Evans GO, Till WM (1979) Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes): an introduction to their external morphology and classification. Trans Zoological Soc Lond 35:139–270
Evans GO, Sheals JG, MacFarlane D (1961) The terrestrial acari of the British Isles: And introduction to their morphology, Biology and Classification, vol 1. Dorking
Faash H (1967) Beitrag zur Biologie der einheimischen uropodiden Uroobovella marginata (C.L. Koch 1839) und Uropoda orbicularis (O.F. Müller 1776) und experimentelle Analyse ihres Phoresieverhaltens. Zool Jahrb 94:521–608
Fain A (1998) Description of mites (Acari) phoretic on Phoridae (Insecta: Diptera) with description of four new species of the genus Uroseius Berlese (Parasitiformes, Uropodina, Polyaspididae). Int J Acarol 24:213–220. https://doi.org/10.1080/01647959808683586
Fain A, Greenwood MT (1991) Notes on a small collection of mites Acari phoretic on Diptera mainly Phoridae from the British Isles. Bull Inst R Sci Nat Belg Entomol 61:193–197
Farahi S, Shishehbor P, Nemati A (2018) Some mesostigmatic mites (Acari: Parasitiformes) of Khuzestan Province, southwestern Iran. Persian J Acarol 7:323–344. https://doi.org/10.22073/pja.v7i4.38663
Farahi S, Shishehbor P, Nemati A (2020) Records of Parasitidae and Laelapidae (Acari: Mesostigmata) from domestic animal manure in Khuzestan province, southwestern Iran with a new record for the Asian fauna. J Insect Biod Syst 6:247–260
Farahi S, Shishehbor P, Nemati A, Witalinski W (2019) Trachygamasus karuni sp. nov., a new mite species from Iran (Parasitiformes: Parasitidae). Zootaxa 4706:439–450. https://doi.org/10.11646/zootaxa.4706.3.4
Farish DJ, Axtell RC (1971) Phoresy redefined and examined in Macrocheles muscaedomesticae (Acarina: Macrochelidae). Acarologia 13, 16–29
Farrier MH, Hennessey MK (1993) Soil-inhabiting and free-living Mesostigmata (Acari-Parasitiformes) from North America: an annotated checklist with bibliography and index. N C Agric Res Service Tech Bull 302:1–408
Filipponi A (1955) Sulla natura dell’ associazione tra Macrocheles muscaedomesticae e Musca domestica. Riv Parassitol 16:83–102
Filipponi A (1964) The feasibility of mass producing macrochelid mites for field trials against house flies. Bull World Health Organ 31:499–501
Filipponi A, Pegazzano F (1962) Specie italiane del gruppo-glaber (Acarina, Mesostigmata, Macrochelidae, Macrocheles). Redia 47:211–238
Filipponi A, Pegazzano F (1963) Specie Italiane del gruppo-subbadius (Acarina, Mesostigmata, Macrochelidae). Redia 48, 69–91
Furman DP (1972) Laelapid mites (Laelapidae: Laelapinae) of Venezuela Brigham Young University Science Bulletin (Biological Series). 17:19
Geden CJ, Stinner RE, Axtell RC (1988) Predation by predators of the house-fly in poultry manure - Effects of predator density, feeding history, interspecific interference, and field conditions. Environ Entomol 17:320–329. https://doi.org/10.1093/ee/17.2.320
Geden CJ, Stinner RE, Kramer DA, Axtell RC (1990) Macmod: A simulation model for Macrocheles muscaedomesticae (Acari: Macrochelidae) population dynamics and rates of predation on immature house flies (Diptera: Muscidae). Environ Entomol 19:578–586
Gerson U, Smiley RL, Ochoa R (2003) Mites (Acari) for pest control. Blackwell Science, Oxford
Glida H, Bertrand M, Peyrusse V (2003) A limiting factor in the abundance of predatory phoretic mites (Acari: Macrochelidae): the seasonal abundance of their phorionts (dung beetles) in southern France. Can J Entomol 81:2066–2072
Greenberg B, Carpenter PD (1960) Factors in phoretic association of a mite and fly. Science 132:738–739
Grisales D, de Carvalho CJB (2016) Family Fanniidae Zootaxa. https://doi.org/10.11646/zootaxa.4122.1.69
Halliday RB (2000) The Australian species of Macrocheles (Acarina: Macrochelidae). Invertebr Syst 14:273–326
Haloti S, Glida H, Niogret J, Janati-Idrissi A, Bertrand M, Lumaret J-P (2005) Acariens-Macrochelidae (Acari: Mesostigmata) phoretiques d’Afrique 1: Macrochelides coprophiles du Maroc [Phoretic macrochelids (Acari: Mesostigmata) from Africa 1: Coprophilous Macrochelidae from Morocco]. Acarologia 45:155–159
Hammer Ø, Harper DAT, Ryan PD (2001) Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hewitt CG (1912) Fannia (Homalomyia) canicularis Linn. and F. scalaris Fab. An account of the bionomics and the Larvae of the flies and their relation to myiasis of the intestinal and urinary tracts. Parasitology 5:161–174. https://doi.org/10.1017/S0031182000000263
Hirschmann W, Wiśniewski J (1982) Weltweite revision der Gattungen Dendrolaelaps Halbert 1915 und Longoseius Chant 1961 (Parasitiformes). Band 1. Beschreibung der Untergattungen und Arten, Bestimmungstabellen, Chätotaxie. Porotaxie Acarologie 29:1–190
Hirschmann W, Wiśniewski J, Kaczmarek S (1991) Gangsystematik der Parasitiformes, Teil 530, Weltweite Revision der Ganggattung Sejus C. L. Koch 1836 (Trichopygidiina), Neubeschreibung von 26 Sejus-Arten, Wiederbeschreibung der Typenart. Acarologie 38:136–214
Ho TM (1990) Phoretic association between Macrocheles muscaedomesticae (Acari: Macrochelidae) and flies inhabiting poultry manure in Peninsular Malaysia. Exp Appl Acarol 10:61–68. https://doi.org/10.1007/BF01193974
Hughes AM (1976) The Mites of Stored Food and Houses. Her Majesty’s Stationary Office, London
Hyatt KH (1980) Mites of the subfamily Parasitinae (Mesostigmata: Parasitidae) in the British Isles. Bull Brit Mus (Nat Hist) Zool 38:237–378
Hyatt KH, Emberson RM (1988) A review of the Macrochelidae (Acari: Mesostigmata) of the British Isles. Bull Brit Mus (Nat Hist) Zool 54:63–125
Ito Y (1970) Preliminary surveys on macrochelid and some other mesostigmatid mites occurring in the experimentally deposited livestock dungs as predators of muscid flies. Med Entomol Zool 21:205–208. https://doi.org/10.7601/mez.21.205
Jalil M, Rodriguez JG (1970) Studies of behaviour of Macrocheles muscaedomesticae (Acarina: Macrochelidae) with emphasis on its attraction to the house fly. Ann Entomol Soc Am 63:738–744. https://doi.org/10.1093/aesa/63.3.738
Kamali K, Ostovan H, Atamehr A (2001) A Catalog of Mites and Ticks (Acari) of Iran. Azad, Islamic Azad University
Kamaruzaman NAC, Masan P, Velasquez Y, Gonzalez-Medina A, Lindstrom A, Braig HR, Perotti MA (2018) Macrocheles species (Acari: Macrochelidae) associated with human corpses in Europe. Exp Appl Acarol 76:453–471. https://doi.org/10.1007/s10493-018-0321-4
Karg W (1962) Zur Systematik und postembryonalen Entwicklung der Gamasiden (Acarina: Parasitiformes) landwirtschaftlich genutzter. Bodenbiozonose [On the systematics and post-embryonic development of the gamasids (Acarina: Parasitiformes) used in agriculture. Soil biozoonosis] Mitt Zool Museum Berlin 38:23–119. https://doi.org/10.1002/mmnz.4830380103
Karg W (1989) Acari (Acarina), Milben Unterordnung Parasitiformes (Anactinochaeta) Uropodina Kramer, Scheldkrötenmilben [Acari (Acarina), mites suborder Parasitiformes (Anactinochaeta) Uropodina Kramer, scamp mites]. VEB Gustav Fischer Verlag, München
Karg W (1993) Acari (Acarina), milben Parasitiformes (Anactinochaeta); cohors Gamasina Leach. Raubmilben. [Predatory Mites - Acari (Acarina), Mites; Parasitiformes (Anactinochaeta); cohort Gamasina Leach]. Jena, Gustav Fischer
Kazemi S, Rajaei A (2013) An annotated checklist of Iranian Mesostigmata (Acari), excluding the family Phytoseiidae. Persian J Acarol 2:63–158. https://doi.org/10.22073/pja.v2i1.9950
Kazemi S, Rajaei A, Beaulieu F (2014) Two new species of Gaeolaelaps (Acari: Mesostigmata: Laelapidae) from Iran, with a revised generic concept and notes on significant morphological characters in the genus. Zootaxa 3861:501–530. https://doi.org/10.11646/zootaxa.3861.6.1
Krantz GW (1962) A review of the genera of the family Macrochelidae Vitzthum 1930 (Acarina: Macrochelidae). Acarologia 4:143–173
Krantz GW (1983) Mites as biological control agents of dung-breeding flies, with special reference to the Macrochelidae. In: Hoy MA, Cunningham GL, Knutson T (eds) Biological control of pests by mites.. University of California, Berkeley
Krantz GW (1998) Reflections on the biology, morphology and ecology of the Macrochelidae. Exp Appl Acarol 22:125–137. https://doi.org/10.1023/a:1006097811592
Krantz GW (2009a) Habits and habitats. In: Krantz GW, Walter DE (eds) A manual of acarology. 3rd edn. Texas University Press, Lubbock
Krantz GW (2009b) A new genus of the family Macrochelidae (Acari: Mesostigmata) based on Macrocheles mycotrupetes Krantz and Mellott and M. peltotrupetes K. and M., phoretic associates of beetles (Coleoptera: Geotrupidae: Geotrupinae) in southeastern USA. Int J Acarol 35:47–51. https://doi.org/10.1080/01647950902870537
Krebs C (2011) Ecological Methodology. Version 7.2 edn.
Kristofik J (1984) Synanthropic Flies (Diptera) of Floodplain Forests of the Danubian Lowland. Biologia 39:227–238
Kristofik J (1988) Synanthropic Flies (Diptera) of the North-South Road System in Slovakia. Biologia 43:903–917
Legner EF, Bowen WR (1973) Influence of available poultry manure breeding habitat on emergence density of synanthropic flies (Diptera). Ann Entomol Soc Am 66:533–538. https://doi.org/10.1093/aesa/66.3.533
Leitner E (1946a) Zur Kenntnis der Milbenfauna auf Düngerstätten. I. Einleitung, vol 1
Leitner E (1946b) Zur Kenntnis der Milbenfauna auf Düngerstätten. III. Die Ökologie der Düngermilben, vol 1
Lindquist EE, Krantz GW, Walter DE (2009) Order Mesostigmata. In: Krantz GW, Walter DE (eds) A manual of acarology. 3rd edn. Texas University Press, Lubbock
Ma LM, Wang S (1996) Four new species of the family Parasitidae and discovery of the genus Trachygamasus in China. Acta Ararchnol Sin 5:81–88
Magurran AE (2004) Measuring biological diversity. Blackwell, Malden
Manning MJ, Halliday RB (1994) Biology and reproduction of some Australian species of Macrochelidae (Acarina). Aus Entomol 21:89–94
McGarry JW, Gusbi AM, Baker AS, Hall MJ, Megademi K (1992) Phoretic and parasitic mites infesting the New World screwworm fly, Cochliomyia hominivorax, following sterile insect releases in Libya. Med Vet Entomol 6, 255–260. https://doi.org/10.1111/j.1365-2915.1992.tb00615.x
Mihályi F (1965) Rearing flies from faeces and meat, infected under natural condition. Acta Zool Hung 11:153–164
Moya Borja GE (1981) Effects of Macrocheles muscadomesticae (Scopoli) on the sexual behavior and longevity of Dermatobia hominis. Rev Bras Biol 41:237–241
Nemati A, Riahi E, Khalili-Moghadam A, Gwiazdowicz DJ (2018) A catalogue of the Iranian Mesostigmata (Acari): additions and updates of the previous catalogue. Persian J Acarol 7:115–191. https://doi.org/10.11646/zootaxa.3709.5.4
Nickolls P, Disney RHL (2001) Flies discovered at Casey station. Aust Antarct Mag 1:54
Niogret J, Nicot ABM (2007) New Macrocheles species from France in grazed areas (Mesostigmata: Macrochelidae). Acarologia 47:115–120
Niogret J, Lumaret JPM (2010) Bertrand Generalist and specialist strategies in macrochelid mites (Acari: Mesostigmata) phoretically associated with dung beetles (Coleoptera: Scarabaeidae). In: Sabelis MW, Bruin J (eds) 12th International Congress of Acarology, Amsterdam. Springer, Berlin. pp 343–347
Nuorteva P (1963) Synanthropy of blowflies (Dipt., Calliphoridae) in Finland. Ann Entomologici Fennici 29:1–49
Özbek HH (2017) A review of the macrochelid mites of Turkey (Acari: Macrochelidae), with new records and descriptions of three new species. Zootaxa 4317:559–572. https://doi.org/10.11646/zootaxa.4317.3.7
Özbek HH, Bal DA (2013) Three new species of the genus Nothrholaspis (Acari: Macrochelidae) from the Kelkit Valley. Turk Zootaxa 3635:40–50. https://doi.org/10.11646/zootaxa.3635.1.4
Özbek HH, Halliday B (2015) A new species and a new form of sexual dimorphism in Nothrholaspis (Acari: Macrochelidae) from Turkey, with a key to the world species. Int J Acarol 41:507–514. https://doi.org/10.1080/01647954.2015.1072246
Özbek HH, Bal DA, Doğan S (2013) Two new species of the genus Longicheles Valle, 1953 from the Kelkit Valley, Turkey, with redescription Longicheles lagrecai (Valle, 1963) (Acari: Macrochelidae). Zootaxa 3709:461–472. https://doi.org/10.11646/zootaxa.3709.5.4
Özbek HH, Bal DA, Doğan S (2015a) The genus Macrocheles Latreille (Acari: Mesostigmata: Macrochelidae) from Kelkit Valley (Turkey), with three newly recorded mite species. Turkish J Zool 39:768–780. https://doi.org/10.3906/zoo-1409-14
Özbek HH, Doğan S, Bal DA (2015b) The genus Glyptholaspis Filipponi & Pegazzano (Acari: Macrochelidae) of Kelkit Valley (Turkey), with first description of male of the species G. saprophila Mašán. Turkish J Zool 39:119–125. https://doi.org/10.3906/zoo-1401-65
Pereira C, Castro MP (1947) Forese e partenogénese arrenótoca em “Macrocheles muscaedomesticae (Scopoli)” (Acarina: Macrochelidae) e sua significacao ecológica [Phoresy and arrhenotokous parthenogenesis in Macrocheles muscaedomesticae (Scopoli) (Acarina: Macrochelidae) and its ecological importance]. Arch Inst Biol (Sao Paulo) 18:71–89
Perotti MA (1998a) Interacciones entre ácaros (depredadores y foréticos) y dípteros muscoideos (presas y forontes) en hábitats rurales y suburbanos de la pendiente atlántica bonaerense [Predatory and phoretic interactions between mites and flies in the Argentinean pampas (ecology and physiology)]. Universidad Nacional de Mar del Plata - PhD thesis
Perotti MA (1999a) Control biologico de moscas sinantrópicas [Biological control of synanthropic flies]. Mar del Plata Universidad Nacional de Mar del Plata Special Editions
Perotti MA (1996) Guano y la fauna beneficial asociada [Poultry manure and associated beneficial fauna]. Rev CAPIA Informa Tech Monograph 159:15–25
Perotti MA (1998b) Moscas sinantrópicas (Diptera: Muscidae y Fanniidae) en producciones avícolas del sud este bonaerense [Synantropic flies (Diptera: Muscidae y Fanniidae) associated with poultry farms in South-eastern Buenos Aires province]. Nat Neotrop 29:145–154. https://doi.org/10.14409/natura.v2i29.3734
Perotti MA (1999b) Localization of distant preys by Glyptholaspis confusa Foa (Acari: Macrochelidae). Rev Soc Entomol Arg 58:106–108
Perotti MA (2000a) Acaros foréticos (Acari: Pygmephoridae) de la mosca de los cuernos Haematobia irritans (L.). Aspectos ecológicos de la interacción. [Phoretic mites (Acari: Pygmephoridae) of the horn fly, Haematobia irritans (L.). Ecology of the interaction]. Rev Med Vet (Arg) 80:449–452
Perotti MA (2000b) Control biológico de la mosca de los cuernos, Haematobia irritans (L.) (Diptera: Muscidae) [Biological control of the horn fly, Haematobia irritans (L.) (Diptera: Muscidae)]. Bol Soc Entomol Argentina 16:6–10
Perotti MA (2001) Prey location and predation rates of predatory mites (Acari: Macrochelidae) on immature stages of pest flies (Diptera: Muscidae). Syst Appl Acarol 6:27–34. https://doi.org/10.11158/saa.6.1.5
Perotti MA, Brasesco MJA (1996) Especificidad forética de Macrocheles muscaedomesticae (Acari: Macrochelidae) [Phoretic specificity of Macrocheles muscaedomesticae (Acari: Macrochelidae)]. Ecol Austral 6:3–8
Perotti MA, Sardella N (1998) Sobre la presencia de la mosca hematófaga Stomoxys calcitrans (Diptera: Muscidae) en Mar del Plata: Una advertencia sanitaria. [About the occurrence of the stable fly Stomoxys calcitrans (Diptera: Muscidae) in Mar del Plata: a health hazard warning]. Rev Soc Entomol Arg 57:71–72
Perotti MA, Bachmann JA (1999) First record and population ecology of the horn fly, Haematobia irritans L (Diptera: Muscidae) on cattle from Southeast of Buenos Aires province (Argentina). Res Rev Parasitol 59:106–108
Perotti MA, Lysyk TJ (2003) Novel Growth Media for Rearing Larval Horn Flies, Haematobia irritans (Diptera: Muscidae). J Med Entomol 40:22–29. https://doi.org/10.1603/0022-2585-40.1.22
Perotti MA, Braig HR (2009) Phoretic mites associated with animal and human decomposition. Exp Appl Acarol 49:85–124
Perotti MA, Mariategui P, Speicys C (2000) Predator mites of dung-breeding flies (Mesostigmata: Macrochelidae, Parasitidae) on Ontherus sulcator (F.) (Coleoptera: Scarabaeidae). Rev Soc Entomol Arg 59:200–204
Perotti MA, Lysyk TJ, Kalischuk-Tymensen LD, Yanke LJ, Selinger LB (2001) Growth and survival of immature Haematobia irritans (Diptera: Muscidae) is influenced by bacteria isolated from cattle manure and conspecific. J Med Entomol. https://doi.org/10.1603/0022-2585-38.2.180
Perotti MA, Braig HR, Goff ML (2010) Phoretic mites and carcasses. In: Amendt J, Campobasso CP, Grassberger M, Goff ML (eds) Current Concepts in Forensic Entomology: Novel Arthropods, Environments and Geographical Regions. Springer, Amsterdam
Plumari M, Kazemi S (2012) Redescription and neotype designation of Lobogynium sudhiri (Datta) (Acari: Diplogyniidae), a mite associated with beetles of the genus Atholus (Thomson) (Coleoptera: Histeridae) in the Palaearctic region. Zootaxa 3352:1–16. https://doi.org/10.11646/ZOOTAXA.3352.1.1
Porta AO, Soto IM, Soto EM, Saint Esteven A (2020) First record of Macrocheles subbadius (Berlese) (Acari: Macrochelidae) in Argentina, associated with the cactophilic fly Drosophila koepferae Fontdevila & Wasserman (Diptera: Drosophilidae). Rev Soc Entomol Argent 79:47–50. https://doi.org/10.25085/rsea.790408
Rodrigueiro TSC, Prado AP (2004) Macrocheles muscaedomesticae (Acari, Macrochelidae) and a species of Uroseius (Acari, Polyaspididae) phoretic on Musca domestica (Diptera, Muscidae): effects on dispersal and colonization of poultry manure. Iheringia Sér Zool 94:181–185. https://doi.org/10.1590/S0073-47212004000200011
Rodriguez JG, Wade CF (1961) The nutrition of Macrocheles muscaedomesticae (Acarina: Macrochelidae) in relation to its predatory action on the house fly egg. Ann Entomol Soc Am 54:782–788. https://doi.org/10.1093/aesa/54.6.782
Rodriguez JG, Singh P, Taylor B (1970) Manure mites and their role in fly control. J Med Entomol 7:335–341. https://doi.org/10.1093/jmedent/7.3.335
Rudzíska M (1998) Life history of the phoretic predatory mite Arctoseius semiscissus (Acari: Ascidae) on a diet of sciarid fly eggs. Exp Appl Acarol 22:643–648. https://doi.org/10.1023/A:1006050020450
Samšiňák K (1964) Termitophile Milben aus der V. R. China. 1. Mesostigmata [Thermophilic mites from PR China. 1. Mesostigmata]. Entomol Abhandlungen 32:33–52
Sato TP, Caetano RL, Carrico C, Ferrera A, Da Silva G, Salles Gazeta Z, Teixeira, Pinto (2018) First record of phoretic and parasitic mites (Arachnida: Acari) associated with necrophagous flies in Brazil. Rev Colomb Entomol 44:48–52. https://doi.org/10.25100/socolen.v44i1.6542
Schelvis J (1991) Predatory mites (Acari: Gamasida) as specific dung indicators in archaeology. Neth Entomol Soc Exp Appl Entomol Sec Proc 2:8–13
Schelvis J (1994) Predatory mites (Acari, Gamasida) in excrements of 5 domestic animal species. Pedobiologia 38:72–80
Seaby RM, Henderson PA (2006) Species Diversity and Richness, 4 edn. Pisces Conservation Ltd., Lymington, England
Skorupski M, Witaliński W (1997) Cornigamasus ocliferius sp. n. a new gamasid mite from Poland (Acari: Parasitidae). Genus 8:145–152
Sobhani MJ, Ostoban H, Hesami S (2017) Fauna of macrochelid mites (Acari: Mesostigmata) in Ramjerd region (Fars Province). Plant Protec J 9:19–40
Southwood TRE, Henderson PA (2000) Ecological methods, 3rd edn. Blackwell, Berlin
STATA (2014) Stata/SE College Station: StataCorp LP. vol 13.1 for Mac
Takaku G, Katakura H, Yoshida N (1994) Mesostigmatic mites (Acari) associated with ground, burying, roving carrion and dung beetles (Coleoptera) in Sapporo and Tomakomai, Hokkaido, Northern Japan. Zoolog Sci 11:305–311
Tolimieri N, Anderson MJ (2010) Taxonomic distinctness of demersal fishes of the California current: moving beyond simple measures of diversity for marine ecosystem-based management. PLoS ONE 5:e10653. https://doi.org/10.1371/journal.pone.0010653
Tullgren A (1918) Ein sehr einfacher auslese apparat für terricole tier- faunen [A very simple readout device for terricole animal forms]. Z angew Entomol 4:149–150
Wade CF, Rodriguez JG (1961) Life history of Macrocheles muscaedomesticae (Acarina, Mesostigmata), a predator of the house fly. Ann Entomol Soc Am 54:776–781. https://doi.org/10.1093/aesa/54.6.776
Walter DE, Proctor HC (1999) Mites – Ecology, Evolution and behaviour. Wallingford, CABI Publishing
Wicht MC Jr, Rodriguez JG (1970) Integrated control of muscid flies in poultry houses using predator mites, selected pesticides and microbial agents. J Med Entomol 7:687–692. https://doi.org/10.1093/jmedent/7.6.687
Willis RR, Axtell RC (1968) Mite predators of the house fly: A comparison of Fuscuropoda vegetans and Macrocheles muscaedomesticae. J Econ Entomol 61:1669–1674. https://doi.org/10.1093/jee/61.6.1669
Wise GU, Henneberry MK, Axtell RC (1988) A new species of manure-inhabiting mite in the genus Poecilochirus (Acari: Mesostigmata: Parasitidae) predacious on house fly egg and larvae. Ann Entomol Soc Am 81:209–224. https://doi.org/10.1093/aesa/81.2.209
Witaliński W (2017) A new species of Trachygamasus from Poland, a new definition of the genus, and a key to the world species (Parasitiformes: Parasitidae). Zootaxa 4303:407–416. https://doi.org/10.11646/zootaxa.4303.3.6
Yao M, Yi TC, Guo JJ, Jin DC, Zhang RZ (2019) Three new species of Trachygamasus (Mesostigmata: Parasitidae) from China, with a key to world species of the genus. Syst Appl Acarol 24:1465–1489. https://doi.org/10.11158/saa.24.8.9
Zakeri V, Kamali K, Hajiqanbar HR (2012) Coprophage and edaphic mites of the families Macrochelidae and Pachylaelapidae in eastern region of Golestan Province, Iran. J Iran Plant Pests Res 1:17–23
Acknowledgements
The authors would like to thank the people in the rural districts for their company and support during sampling. We also express our appreciation to Dr. Wojciech Witaliński of the Jagiellonian University, Poland, and Dr. Esmaeil Babaeian of the University of Tehran, Iran, for help in identifications of Parasitidae (by W.W.), Oplitidae and some Uroobovella species (by E.B.).
Funding
This study was financially supported by the Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Contributions
SF, PS and AN contributed to the study conception and design. Material preparation, data collection were performed by SF, PS and AN. Data analyses and interpretation of results was performed by all authors. The first draft of the manuscript was written by SF and PS. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farahi, S., Shishehbor, P., Nemati, A. et al. Mesostigmata diversity by manure type: a reference study and new datasets from southwestern Iran. Exp Appl Acarol 86, 517–534 (2022). https://doi.org/10.1007/s10493-022-00710-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00710-1