Abstract
Ticks are globally renowned vectors for numerous zoonoses, and birds have been identified as important hosts for several species of hard ticks (Acari: Ixodidae) and tick-borne pathogens. Many European bird species overwinter in Africa and Western Asia, consequently migrating back to breeding grounds in Europe in the spring. During these spring migrations, birds may transport exotic tick species (and associated pathogens) to areas outside their typical distribution ranges. In Finland, very few studies have been conducted regarding ticks parasitizing migrating or local birds, and existing data are outdated, likely not reflecting the current situation. Consequently, in 2018, we asked volunteer bird ringers to collect ticks from migrating and local birds, to update current knowledge on ticks found parasitizing birds in Finland. In total 430 ticks were collected from 193 birds belonging to 32 species, caught for ringing between 2018 and 2020. Furthermore, four Ixodes uriae were collected from two roosting islets of sea birds in 2016 and 2020. Ticks collected on birds consisted of: Ixodes ricinus (n = 421), Ixodes arboricola (4), Ixodes lividus (2) and Hyalomma marginatum (3). Ixodes ricinus loads (nymphs and larvae) were highest on thrushes (Passeriformes: Turdidae) and European robins (Erithacus rubecula). The only clearly imported exotic tick species was H. marginatum. This study forms the second report of both I. uriae and I. arboricola from Finland, and possibly the northernmost observation of I. arboricola from Europe. The importation of exotic tick species by migrating birds seems a rare occurrence, as over 97% of all ticks collected from birds arriving in Finland during their spring migrations were I. ricinus, a species native to and abundant in Finland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hard ticks (Acari: Ixodidae) are a cosmopolitan family of ectoparasites of terrestrial vertebrates, comprised of approximately 700 species (Guglielmone et al. 2010). Some of these tick species have specific hosts or host animal groups from which they rarely deviate, such as sand martins (Riparia riparia) for the sand martin tick (Ixodes lividus) or voles for the vole tick (Ixodes trianguliceps) (Estrada-Peña et al. 2018; Hillyard 1996). Other species are generalists, feeding on a variety of possible hosts. For example, the sheep tick (Ixodes ricinus) and taiga tick (Ixodes persulcatus), two of the most common tick species in Eurasia, have both been reported from nearly 300 vertebrate species (Bowman and Nuttall 2008; Uspensky 2016). Included among these species are humans, who serve as incidental, dead-end hosts for the ticks. Unfortunately, these incidental bites frequently result in tick-borne pathogens getting transmitted to humans, who subsequently develop diseases. For example, over 200,000 cases of tick-borne Lyme borreliosis are diagnosed in Europe each year (Marques et al. 2021).
Birds, particularly species that predominately forage on the ground floor, such as thrushes (Passeriformes: Turdidae) and common pheasants (Phasianus colchicus), have been identified as important hosts for I. ricinus (Buczek et al. 2020). As evidence of frequent contact between birds and ticks, the enzootic cycles of certain frequently detected pathogens have evolved to almost exclusively include birds as reservoirs (Kurtenbach et al. 2002; Norte et al. 2013). Furthermore, several European studies have observed ticks on birds caught for ringing, most often during their spring migrations to nesting areas (Elfving et al. 2010; Hasle 2013; Hasle et al. 2011; Klitgaard et al. 2019; Movila et al. 2013; Sandelin et al. 2015; Toma et al. 2021). This preference for spring migrators as study subjects in Europe is likely due to the added interest provided by possible exotic tick species (and associated pathogens) that are transported in unknown, but likely vast quantities from Africa and Western Asia by the millions of migrating birds. For example, several exotic tick species native to Africa have been reported from migrating birds in studies conducted in southern Italy (Toma et al. 2021). However, ticks from Africa or Western Asia seldom stay attached to birds for long enough to reach the northern parts of Europe, but rather detach from their bird hosts in stopover areas in southern and central Europe. Consequently, in northern Europe, the expected and observed exotic species are mostly those inhabiting the southern and central parts of Europe, rather than those native to Africa or Western Asia (Capligina et al. 2014; Ciebiera et al. 2019; Elfving et al. 2010; Geller et al. 2013; Hasle et al. 2011; Nuorteva and Hoogstraal 1963).
In Finland, only a few studies focusing on ticks found on migrating or local birds have been conducted (Laakkonen et al. 2009; Nuorteva and Hoogstraal 1963; Saikku et al. 1971; Ulmanen et al. 1977). A total of four tick species have been reported in these studies: I. ricinus, I. arboricola, I. lividus and Hyalomma marginatum. A paper reporting the first detection of I. frontalis from a migrating bird in Finland was published in 2009 (Laakkonen et al. 2009), but the species identification was later rebutted in a commentary to the paper (Heylen et al. 2012). In addition to the four verified species, Jaenson et al. (1994) mention that I. uriae has been reported from Finland. Likewise, Estrada-Pena et al. (2018) display a record of the species from the Vaasa region in Finland in their distribution map for the species.
Most of the records concerning ticks found parasitizing birds in Finland date back approximately half a century. Consequently, they are likely no longer representative of the current situation. Due to climate change and the degradation of natural environments by humans, relatively rapid changes are occurring in the geographical distributions of tick species and the availability of habitats suitable for tick survival (Alkishe et al. 2017; Medlock et al. 2013). For example, the survival and development to the adult stage of imported H. marginatum was recently reported for the first time in Sweden and England, following the heat wave of the summer of 2018 (Grandi et al. 2020; McGinley et al. 2021). Hyalomma marginatum is one of the tick species of interest regarding increasing range size, as it is the main vector for Crimean-Congo Hemorrhagic Fever virus (CCHFV) in Europe (Maltezou et al. 2010). The species is commonly transported to Central and Northern Europe by migrating birds from Africa, Western Asia, and Southern Europe, where the species is endemic (Capek et al. 2014; Nuorteva and Hoogstraal 1963). In addition to the surveillance needed to assess these potential threats from imported ticks and tick-borne pathogens, studies focusing specifically on local birds are needed to produce updated data on the occurrence of bird-associated endophilic tick species. With the data presently available from Finland, there is no way to assess the current distributions or occurrence of such species—let alone their potential impact in, for example, pathogen maintenance.
In 2018, we contacted Finnish bird ringers in order to conduct a study on ticks being imported to Finland by birds during their spring migration. Likewise, we sought to study the diversity of tick species and mean tick loads in migrating and local birds. Here, we present data on the occurrence and species diversity of ticks parasitizing birds in Finland. Likewise, we assess differences between migrating and local birds in mean tick loads and the probabilities of parasitized birds carrying larvae.
Materials and methods
In February 2018, kits containing sample tubes filled with RNAlater (Thermo-Fisher Scientific, AM7020), tweezers to remove ticks, an information leaflet, and a spreadsheet for recording data were distributed to volunteer bird ringers at the annual national Ringer’s Meeting (Rengastajakokous) held in Seinäjoki, central Finland. In addition, similar kits were distributed to volunteer bird ringers working at, or otherwise affiliated with, the University of Turku. Included in the kits was also a prepaid envelope for sending the samples to the Zoological Museum at the University of Turku (ZMUT). Ticks sent to ZMUT were identified under stereo microscope to species and life stage using morphological keys (Estrada-Peña et al. 2018; Hillyard 1996; Morel and Perez 1973; Nosek and Sixl 1972). Following identification, all samples were placed individually in Eppendorf tubes and stored in a deep freezer at −80 °C to await further analysis.
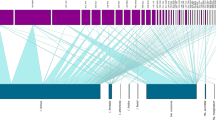
Ringed birds that were parasitized by ticks were divided into three migration categories: spring migrators, residents and autumn migrators. Members of bird species known not to overwinter in Finland that were captured for ringing in March, April or May were classified as spring migrators. Likewise, members of partially migratory species that were freshly ringed between March and early June at two bird observatories on remote islands in the Baltic Sea (Jurmo and Aspskär; Fig. 1) were classified as spring migrators. Birds known to nest in Finland that were ringed between June and September were considered local residents. Finally, individuals ringed in September or October outside the species’ nesting range or on the islands of Jurmo or Aspskär were considered likely autumn migrators.
Bird ringing locations. Numbers in parentheses represent the number of birds carrying ticks found from corresponding locations. Tick species found from birds are reported in abbreviated italics (IRI Ixodes ricinus, IAR I. arboricola, ILI I. lividus, HMA Hyalomma marginatum). Yellow triangles represent locations from where I. uriae were found. (Color figure online)
Statistical analysis
Differences in mean I. ricinus loads between spring migrators and local residents for European robins (Erithacus rubecula) and thrushes (T. merula, T. philomelos, T. pilaris and T. iliacus collectively) were modelled by generalized linear models (GLM), with negative binomial distribution and log link function. Likewise, differences in the probabilities of spring migrators and local residents of these species/groups carrying larvae were modelled by generalized linear models (GLM) with binary error distribution and logit link function. As nearly all birds carried nymphs and none carried adults, we only analyzed the probability of birds carrying larvae. Too few individuals from these species/groups classified as autumn migrators were available to include them in the analyses. All the GLMs were run with the GLIMMIX procedure of SAS v.9.4. using maximum likelihood estimation.
As we had no data regarding the numbers of birds ringed without ticks, analyses requiring such information were not pursued (such as percentage of birds carrying ticks). Likewise, we refrained from statistical analyses regarding tick load and probability of larval infestation for specific bird species apart from E. rubecula and thrushes (collectively), as too few samples, that were also unevenly divided into different migration categories, existed.
Results
Sample tubes containing ticks collected from a total of 193 birds belonging to 32 species were sent to ZMUT between 2018 and 2020. In addition, four I. uriae collected from rocky islets in 2016 and 2020 were delivered to ZMUT and included in the data set. In total, these samples contained 434 ticks, with the following species composition: I. ricinus (n = 421), I. arboricola (4), I. uriae (4), H. marginatum (3), and I. lividus (2). Out of the birds carrying ticks, 72 were classified as spring migrators, carrying 127 I. ricinus and 3 H. marginatum. In total 101 birds were classified as local residents, carrying 248 I. ricinus, 2 I. arboricola and 2 I. lividus. Finally, 20 birds were classified as autumn migrators, carrying 46 I. ricinus and 2 I. arboricola. Species diversity of parasitized birds was highest in local birds (23 species), followed by spring migrators (21) and autumn migrators (7).
Altogether 29 bird species carried I. ricinus (Table 1, Tables S1–S3). No adult I. ricinus were observed on birds. Table 1 displays data for bird species for which at least four individuals were available regarding total, nymph and/or larvae loads, whereas Tables S1–S3 display the full data by bird species, divided by migration category. Blackbirds (T. merula) and European robins (E. rubecula) were the species most frequently parasitized by I. ricinus (Table 1). Tick loads (total, nymphs, and larvae) were highest on thrushes (T. merula, T. philomelos and T. pilaris; Table 1). Simultaneous detections of both life stages were more common in local birds (22.5% of birds) than in spring or autumn migrators (5.8 and 10.5%, respectively).
In addition to the bird species parasitized by I. ricinus, two sand martins (R. riparia) were found with one adult I. lividus each. Furthermore, one barred warbler (Sylvia nisoria) was parasitized by two H. marginatum nymphs, and a wood warbler (Phylloscopus sibilatrix) by one. Both birds carrying H. marginatum were spring migrators. Finally, two great tits (Parus major) carried in total three nymphs and one larva of I. arboricola.
No statistically significant differences in mean tick loads (nymphs, larvae or total) were observed between (spring) migrating and local E. rubecula or thrushes (Table S4). However, for thrushes, local birds seemed to a have higher probability of carrying larvae than spring migrators [model-derived estimated marginal means and 95% confidence limits: 0.47 (0.31–0.64) vs. 0.14 (0.03–0.44)]. A similar trend (0.63 vs. 0.25) was observed for E. rubecula, although due to low sample size, confidence intervals remained wide (Table S4).
Discussion
The current study reveals that most ticks found from both migrating and resident birds in Finland are I. ricinus. This finding conforms to observations of I. ricinus being by far the most common tick species detected on migrating birds in northern Europe, with proportions upwards of 90% commonly observed (Capligina et al. 2014; Ciebiera et al. 2019; Geller et al. 2013; Heylen et al. 2017; Kjelland et al. 2010). As an interesting sidenote, no I. persulcatus were found from birds, despite them being relatively common in Finland (Laaksonen et al. 2017). However, this may be explained by two points. Firstly, spring migrators arriving from the south have very few locations from which to acquire I. persulcatus on the way to Finland (namely Estonia and Latvia) (Capligina et al. 2020; Katargina et al. 2015). Secondly, regarding local birds, ringing was mostly conducted in the southern parts of Finland, where established populations of I. persulcatus have not been reported (Laaksonen et al. 2017)—see, however, Zakham et al. (2021) regarding a recent detection of I. persulcatus near Helsinki in southern Finland. As this species is known to parasitize birds, it likely can be found on birds during its local activity period in April-June in areas where established populations are present (Pakanen et al. 2020; Sormunen et al. 2020).
Regarding I. ricinus, both the species of birds parasitized as well as their tick loads were comparable to those reported in other similar studies from Northern Europe (Capligina et al. 2014; Elfving et al. 2010; Hasle et al. 2011; Kazarina et al. 2015; Kjelland et al. 2010). Thrushes and E. rubecula generally had the highest mean I. ricinus loads in both spring migrators and local residents, as was also observed in the above listed research papers from the Nordic and Baltic countries. This is generally thought to be due to the ground-foraging behavior of these species, which commonly brings them into contact with juvenile ticks residing in ground floor vegetation and litter (Buczek et al. 2020). Altogether, I. ricinus nymphs and larvae were found from 29 different bird species. No I. ricinus adults were found from the sampled birds, which is also in line with other similar studies (Buczek et al. 2020).
No obvious differences were observed between migrating and local E. rubecula or thrushes in mean tick loads. Consequently, this would suggest that the birds encounter similar numbers of nymphs and larvae in stopover areas during migration as they do at nesting sites in Finland. However, local thrushes appeared to have a higher probability of being parasitized by larvae than spring migrators, indicating a higher frequency of contacts with tick larvae. A similar trend was observed for E. rubecula, but the statistical analysis was hampered by the limited sample size (wide confidence intervals). There could be several explanations for this phenomenon. For example, many bird species make their migratory stopovers on their way to Northern Europe in early-mid spring, which may mean that larvae activity at the stopover areas (or source areas for short-distance migrators) is still low. Likewise, foraging habitats may be different in stopover and nesting areas, leading to different rates of contact with tick larvae. It is also possible that the migration flights themselves increase larvae mortality on the host, them being the life stage most susceptible to environmental stresses. However, an equally likely possibility is that this observation is just due to the fact that migrating and local birds have foraged in different locations. The distribution of I. ricinus larvae in the nature is known to be very clustered (Nilsson and Lundqvist 1978), so differences in the frequency of larvae parasitization may just signify differences in larvae occurrence between areas.
Imported exotic tick species
The only clearly imported ticks detected were three H. marginatum nymphs. They were removed from a wood warbler (P. sibilatrix) and a barred warbler (S. nisoria) ringed in May 2018. Hyalomma marginatum is a species known to be imported from Southern and Central Europe annually to Northern Europe, and has previously been reported from migrating birds in Finland (Nuorteva and Hoogstraal 1963). The species is particularly prone to travelling vast distances on birds due to its two-host life cycle, wherein the larval and nymphal stages acquire their blood meals from the same host (Hillyard 1996). As such, they spend longer on the host than three-host tick species, which in turn allows for longer travel distances during migration, should the host be a bird. However, the colder summers in the north typically do not allow for the development of immigrant nymphs to the adult stage [however, see Grandi et al. (2020) for an exception], consequently leading to no established populations of the species having been discovered in the Nordic or Baltic countries. Nevertheless, it is possible that the warming climate will, in the future, allow the establishment of populations further north as well (Grandi et al. 2020). In addition, H. marginatum serve as vectors for CCHFV, which they can transport to novel areas (Estrada‐Peña et al. 2011; Palomar et al. 2016). The virus has also been reported from I. ricinus, whose vector competence remains unclear (Albayrak et al. 2010; Gergova et al. 2012). Consequently, even if the tick species cannot survive at these northern latitudes, it is possible that the virus transported by the tick may establish itself in the local host and/or tick population, highlighting the importance of proper surveillance of migrating birds.
New records of rarely observed resident species
Regarding rarely documented species that likely have established populations in Finland, three species were encountered during this study: the sand martin tick (I. lividus), the sea bird tick (I. uriae), and the tree-hole tick (I. arboricola).
Ixodes lividus is an endophilic tick species that mainly parasitizes sand martins (R. riparia) (Hillyard 1996). Previous records of this species from Finland are from two research papers published roughly 50 years previously (Nuorteva and Hoogstraal 1963; Ulmanen et al. 1977). Although two individuals are too few to determine whether an established population exists in Huittinen, they were collected from juvenile birds that were ringed in July, implying that the ticks had attached to the birds in their nests at Huittinen. The role this species might have in the maintenance of pathogens is uncertain (Hillyard 1996). Although they have been reported from some bird species other than sand martins as well (Hillyard 1996), their relatively strict adherence to this particular host and its nests likely renders them insignificant regarding the maintenance of pathogens of medical or veterinary interest.
Another seldom reported species detected is the sea bird tick, I. uriae. The only previous report of this species in Finland is from near Vaasa in western Finland (Estrada-Peña et al. 2018). Between 2016 and 2020, a total of four specimens were delivered to ZMUT, three in 2016 from Kirkkonummi (leg. E. Helve) and one in 2020 from near Aspskär, forming the second and third records of the species from the country, respectively (Fig. 1). These specimens were not found attached to birds. Rather, the three I. uriae from Kirkkonummi were found from a funneling sample of nest material collected from a great cormorant (Phalacrocorax carbo) roost. The tick from Aspskär was found on a bird ringer during a visit to a common murre (Uria aalge) roosting islet. Furthermore, in 2018 at the national Ringer’s Meeting, a bird ringer reported brushing off a tick from his arm after a painful bite while ringing seagulls on a rocky islet. This would comply with previous reports of uncharacteristically painful tick bites by I. uriae (Hillyard 1996). As the habitats of this species are rarely visited by people other than bird ringers, populations may remain undetected and unreported for untold years. Consequently, specific studies are required to determine the distribution and abundance of I. uriae in Finland, as well as to assess their potential role in the local circulation of Lyme borreliosis spirochetes, which the species is known to be able to vector (Duneau et al. 2008). This is particularly current now, as the first report of a successful roosting colony of P. carbo in an inland lake in Finland was reported in 2020 (YLE 2020), potentially enabling the spread of the tick to inland freshwater areas.
Finally, I. arboricola was detected from great tits (P. major) ringed in October 2019. Due to the late date of these detections, it seems unlikely that they could be exported ticks from more southern parts of Europe. Rather, these appear to be individuals that attached themselves to the birds in Finland. Consequently, the finding of two ticks from a great tit from Ruissalo possibly forms the northernmost observation of I. arboricola from Europe. However, this status remains somewhat tentative, as the species has been reported on a municipality level from Uppsala, Sweden, the northernmost parts of which reach latitudes further north than Ruissalo (Jaenson et al. 1994). In either case, this study forms the second report of the species from Finland (Saikku et al. 1971). Ixodes arboricola is an endophilic tick species mainly parasitizing passerines and some other tree-hole dwelling bird species (Hillyard 1996). As is evident from several reports, including the current study, generalist tick species such as I. ricinus are also frequently in contact with passerines. Consequently, I. arboricola may have a role in the enzootic cycles of tick-borne pathogens of medical interest. As with I. lividus and I. uriae, studies specifically designed to assess the distribution and abundance of I. arboricola are required to determine the current extent of their occurrence in Finland.
Conclusions
According to the current study, the importation of exotic tick species to Finland by migrating birds is a rare occurrence. In fact, > 97% of all ticks collected from birds migrating northwards in the spring were I. ricinus, a species native to and abundant in Finland. However, despite the low rate of exotic ticks in the migrating birds, the high number of migrating bird individuals may mean that transportation still occurs relatively commonly. It has been estimated that there are about 200–300 million birds in Finland during the summer, of which 90% are short- or long-distance migrants. Furthermore, the importation of foreign members of a native species may also be of consequence, as, in addition to gene flow, novel pathogens may be transported and introduced, or already present pathogens transported to novel areas. More extensive tick collections and analysis of tick-borne pathogens are required to form a more comprehensive picture of the importation of ticks and tick-borne pathogens to Finland. Finally, this study highlights the need for studies specifically targeted at native endophilic tick species parasitizing birds in Finland, for which data are nearly non-existent and outdated.
References
Albayrak H, Ozan E, Kurt M (2010) Molecular Detection of Crimean-Congo Haemorrhagic Fever Virus (CCHFV) but not West Nile Virus (WNV) in Hard Ticks from Provinces in Northern Turkey. Zoonoses Public Health 57:e156–e160
Alkishe AA, Peterson AT, Samy AM (2017) Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS ONE 12:e0189092
Bowman AS, Nuttall PA (2008) Ticks: biology, disease and control. Cambridge University Press, Cambridge
Buczek AM, Buczek W, Buczek A, Bartosik K (2020) The potential role of migratory birds in the rapid spread of ticks and tick-borne pathogens in the changing climatic and environmental conditions in Europe. Int J Environ Res Public Health 17:2117
Capek M, Literak I, Kocianova E, Sychra O, Najer T, Trnka A, Kverek P (2014) Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick Borne Dis 5:489–493
Capligina V, Salmane I, Keišs O, Vilks K, Japina K, Baumanis V, Ranka R (2014) Prevalence of tick-borne pathogens in ticks collected from migratory birds in Latvia. Ticks Tick Borne Dis 5:75–81
Capligina V, Seleznova M, Akopjana S, Freimane L, Lazovska M, Krumins R, Kivrane A, Namina A, Aleinikova D, Kimsis J (2020) Large-scale countrywide screening for tick-borne pathogens in field-collected ticks in Latvia during 2017–2019. Parasit Vectors 13:1–12
Ciebiera O, Jerzak L, Nowak-Chmura M, Bocheński M (2019) Ticks (Acari: Ixodida) on birds (Aves) migrating through the Polish Baltic coast. Exp Appl Acarol 77:241–251
Duneau D, Boulinier T, Gómez-Díaz E, Petersen A, Tveraa T, Barrett RT, McCoy KD (2008) Prevalence and diversity of Lyme borreliosis bacteria in marine birds. Infect Genet Evol 8(3):352–359
Elfving K, Olsen B, Bergström S, Waldenström J, Lundkvist Å, Sjöstedt A, Mejlon H, Nilsson K (2010) Dissemination of spotted fever rickettsia agents in Europe by migrating birds. PLoS ONE 5:e8572
Estrada-Peña A, Martínez Avilés M, Muñoz Reoyo MJ (2011) A population model to describe the distribution and seasonal dynamics of the tick Hyalomma marginatum in the Mediterranean Basin. Transbound Emerg Dis 58:213–223
Estrada-Peña A, Mihalca AD, Petney TN (2018) Ticks of Europe and North Africa: a guide to species identification. Springer, New York
Geller J, Nazarova L, Katargina O, Leivits A, Järvekülg L, Golovljova I (2013) Tick-borne pathogens in ticks feeding on migratory passerines in western part of Estonia. Vector Borne Zoonotic Dis 13:443–448
Gergova I, Kunchev M, Kamarinchev B (2012) Crimean-Congo hemorrhagic fever virus—tick survey in endemic areas in Bulgaria. J Med Virol 84:608–614
Grandi G, Chitimia-Dobler L, Choklikitumnuey P, Strube C, Springer A, Albihn A, Jaenson TGT, Omazic A (2020) First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick Borne Dis 11:101403
Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, Shao R, Barker SC (2010) The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2528:1–28
Hasle G (2013) Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front Cell Infect Microbiol 3:48
Hasle G, Bjune GA, Midthjell L, Røed KH, Leinaas HP (2011) Transport of Ixodes ricinus infected with Borrelia species to Norway by northward-migrating passerine birds. Ticks Tick Borne Dis 2:37–43
Heylen DJA, Jacobs I, Matthysen E, Madder M (2012) Comments on article ”First report of Ixodes frontalis (Acari: Ixodidae) in Finland, an example of foreign tick species transported by a migratory bird” (Memoranda Soc. Fauna Flora Fennica 85: 16–19. 2009). Memoranda Soc Fauna Flora Fennica
Heylen D, Krawczyk A, Lopes de Carvalho I, Núncio MS, Sprong H, Norte AC (2017) Bridging of cryptic Borrelia cycles in European songbirds. Environ Microbiol 19:1857–1867
Hillyard PD (1996) Ticks of North-West Europe. The Dorset Press, Dorchester
Jaenson TGT, Tälleklint L, Lundqvist L, Olsen B, Chirico JAN, Mejlon H (1994) Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol 31:240–256
Katargina O, Geller J, Ivanova A, Värv K, Tefanova V, Vene S, Lundkvist Å, Golovljova I (2015) Detection and identification of Rickettsia species in Ixodes tick populations from Estonia. Ticks Tick Borne Dis 6:689–694. https://doi.org/10.1016/j.ttbdis.2015.06.001
Kazarina A, Japiņa K, Keišs O, Salmane I, Bandere D, Capligina V, Ranka R (2015) Detection of tick-borne encephalitis virus in I. ricinus ticks collected from autumn migratory birds in Latvia. Ticks Tick Borne Dis 6:178–180
Kjelland V, Stuen S, Skarpaas T, Slettan A (2010) Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from migratory birds in Southern Norway. Acta Vet Scand 52:1–6
Klitgaard K, Højgaard J, Isbrand A, Madsen JJ, Thorup K, Bødker R (2019) Screening for multiple tick-borne pathogens in Ixodes ricinus ticks from birds in Denmark during spring and autumn migration seasons. Ticks Tick Borne Dis 10:546–552
Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell HS, Brade V, Kraiczy P (2002) Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol 10:74–79
Laakkonen J, Terhivuo J, Huhtamo E, Vapalahti O, Uzcátegui NY (2009) First report of Ixodes frontalis (Acari: Ixodidae) in Finland, an example of foreign tick species transported by a migratory bird. Memoranda Soc Fauna Flora Fennica
Laaksonen M, Sajanti E, Sormunen JJ, Penttinen R, Hänninen J, Ruohomäki K, Sääksjärvi I, Vesterinen EJ, Vuorinen I, Hytönen J, Klemola T (2017) Crowdsourcing-based nationwide tick collection reveals the distribution of Ixodes ricinus and I. persulcatus and associated pathogens in Finland. Emerg Microbes Infec 6:e31. https://doi.org/10.1038/emi.2017.17
Maltezou HC, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, Kalvatchev N, Nichol S, Niedrig M, Platonov A (2010) Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Eurosurveillance 15:19504
Marques AR, Strle F, Wormser GP (2021) Comparison of lyme disease in the United States and Europe. Emerg Infect Dis 27(8):2017–2024. https://doi.org/10.3201/eid2708.204763
McGinley L, Hansford KM, Cull B, Gillingham EL, Carter DP, Chamberlain JF, Hernandez-Triana LM, Phipps LP, Medlock JM (2021) First report of human exposure to Hyalomma marginatum in England: Further evidence of a Hyalomma moulting event in north-western Europe? Ticks Tick Borne Dis 12:101541
Medlock JM, Hansford KM, Bormane A, Derdakova M, Estrada-Peña A, George JC, Golovljova I, Jaenson TGT, Jensen JK, Jensen PM (2013) Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 6:1–11
Morel PC, Perez C (1973) Morphologie des stades préimaginaux des Ixodidae s. str. d’Europe occidentale: 3. Les nymphes des Pholeoixodes Schulze 1942. Série Entomologie Médicale Et Parasitologie 11:285–291
Movila A, Alekseev AN, Dubinina HV, Toderas I (2013) Detection of tick-borne pathogens in ticks from migratory birds in the Baltic region of Russia. Med Vet Entomol 27:113–117
Nilsson A, Lundqvist L (1978) Host Selection and movements of Ixodes ricinus (Acari) Larvae on small mammals. Oikos 31:313–322. https://doi.org/10.2307/3543656
Norte AC, Ramos JA, Gern L, Núncio MS, Lopes de Carvalho I (2013) Birds as reservoirs for Borrelia burgdorferi sl in Western Europe: circulation of B. turdi and other genospecies in bird–tick cycles in Portugal. Environ Microbiol 15:386–397
Nosek J, Sixl W (1972) Central-European ticks (Ixodoidea). Mitt Abt Zool Landesmus Joanneum 1:480
Nuorteva P, Hoogstraal H (1963) The incidence of ticks (Ixodoidea, Ixodidae) on migratory birds arriving in Finland during the spring of 1962. Ann Med Exp Biol Fenn 41
Pakanen VM, Sormunen JJ, Sippola E, Blomqvist D, Kallio ER (2020) Questing abundance of adult taiga ticks Ixodes persulcatus and their Borrelia prevalence at the north-western part of their distribution. Parasit Vectors 13:1–9
Palomar AM, Portillo A, Mazuelas D, Roncero L, Arizaga J, Crespo A, Gutiérrez Ó, Márquez FJ, Cuadrado JF, Eiros JM (2016) Molecular analysis of Crimean-Congo hemorrhagic fever virus and Rickettsia in Hyalomma marginatum ticks removed from patients (Spain) and birds (Spain and Morocco), 2009–2015. Ticks Tick Borne Dis 7:983–987
Saikku P, Ulmanen I, Brummer-Korvenkontio M (1971) Ticks (Ixodidae) on migratory birds in Finland. Acta Entomol Fennica
Sandelin LL, Tolf C, Larsson S, Wilhelmsson P, Salaneck E, Jaenson TGT, Lindgren PE, Olsen B, Waldenström J (2015) Candidatus Neoehrlichia mikurensis in ticks from migrating birds in Sweden. PLoS ONE 10:e0133250
Sormunen JJ, Andersson T, Aspi J, Bäck J, Cederberg T, Haavisto N, Halonen H, Hänninen J, Inkinen J, Kulha N, Laaksonen M, Loehr J, Mäkelä S, Mäkinen K, Norkko J, Paavola R, Pajala P, Petäjä T, Puisto A, Sippola E, Snickars M, Sundell J, Tanski N, Uotila A, Vesilahti EM, Vesterinen EJ, Vuorenmaa S, Ylönen H, Ylönen J, Klemola T (2020) Monitoring of ticks and tick-borne pathogens through a nationwide research station network in Finland. Ticks Tick Borne Dis 11:101449. https://doi.org/10.1016/j.ttbdis.2020.101449
Toma L, Mancuso E, d’Alessio SG, Menegon M, Spina F, Pascucci I, Monaco F, Goffredo M, Di Luca M (2021) Tick species from Africa by migratory birds: a 3-year study in Italy. Exp Appl Acarol 83:147–164
Ulmanen I, Saikku P, Vikberg P, Sorjonen J (1977) Ixodes lividus (Acari) in sand martin colonies in Fennoscandia. Oikos 20–26
Uspensky I (2016) The taiga tick Ixodes persulcatus (Acari: Ixodidae), the main vector of Borrelia burgdorferi sensu lato in Eurasia. Lyme Disease. SM Group, Dover
YLE—Finnish Broadcast Company (2020) Merimetsoilla Suomen ensimmäinen onnistunut pesintä sisämaassa: kiistelty lintu valloitti Enäjärven Porissa. https://yle.fi/uutiset/3-11478397
Zakham F, Jääskeläinen AJ, Castrén J, Sormunen JJ, Uusitalo R, Smura T, Von Troil G, Kuivanen S, Sironen T, Vapalahti O (2021) Molecular detection and phylogenetic analysis of Borrelia miyamotoi strains from ticks collected in the capital region of Finland. Ticks Tick Borne Dis 12:101608
Acknowledgements
We would like to thank all the volunteer bird ringers for participating in the tick collection—naturally this study could not have been conducted without their help. We would also like to thank Jane and Aatos Erkko Foundation and Maj and Tor Nessling Foundation for financial support of the study.
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sormunen, J.J., Klemola, T. & Vesterinen, E.J. Ticks (Acari: Ixodidae) parasitizing migrating and local breeding birds in Finland. Exp Appl Acarol 86, 145–156 (2022). https://doi.org/10.1007/s10493-021-00679-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00679-3