Abstract
The predatory mite Typhlodromalus aripo and the entomopathogenic fungus Neozygites tanajoae, both introduced from Brazil for control of the cassava green mite (CGM) Mononychellus tanajoa, now co-occur in cassava fields in Benin. However, studies on interactions between these two natural enemies and how they might affect CGM biological control are lacking. We determined in screenhouse experiments the effects of single and combined releases of N. tanajoae and T. aripo on CGM suppression. In the single natural enemy treatment, both T. aripo and N. tanajoae significantly reduced CGM densities, but the results of the predator (T. aripo) are more quickly measurable than those of the pathogen (N. tanajoae) in our short-term experiment. The level of CGM suppression in the combined natural enemy treatment was reduced considerably compared with T. aripo-alone, but only slightly when compared with N. tanajoae alone, with a simultaneous reduction in T. aripo and N. tanajoae abundance or prevalence. In a laboratory experiment, T. aripo fed more on N. tanajoae-infected CGM than on healthy CGM and its oviposition and survival were reduced when fed on the former compared with the latter, which can help in explaining the reduction in numbers of T. aripo and consequently the considerable loss in suppression of CGM in the combined natural enemy treatment in the screenhouse experiment. Together, the screenhouse and the laboratory experiments predicted negative interactions between the two natural enemies with negative consequences for CGM biological control. Long-term field observations and rigorous field experiments that simultaneously manipulate T. aripo and N. tanajoae abundance and prevalence are needed to validate the prediction of this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relative benefits of introducing single or multiple natural enemy species for classical biological control has long been debated by ecologists and biological control practitioners (Polis et al. 1989; Sih et al. 1998; Losey and Denno 1999; Gnanvossou et al. 2003; Onzo et al. 2004; Sabelis et al. 2009). Several authors have argued for screening natural enemies and releasing only the one most effective species (Briggs 1993; Ehler and Hall 1982). This strategy has been challenged, however, by others who found little evidence that one natural enemy species provides higher suppression than two or more natural enemy species applied together (Croft et al. 1992; Huffaker et al. 1971; Lang 2003; Riechert and Lawrence 1997). Interactions among natural enemy species can have two opposite effects on target pest population (Losey and Denno 1999; Wekesa et al. 2007). Two predator species may act in a complementary fashion, thereby increasing predation risk to the prey (Heinz and Nelson 1996; Losey and Denno 1998; Onzo et al. 2004, 2005; Riechert and Lawrence 1997); or two predators may interfere with each other through intra-guild predation or some other forms of interspecific interactions, and their less-than-additive effects would reduce predation risk to the prey (Rosenheim 2001; Spiller 1986).
Interactions between natural enemies are particularly relevant to classical biological control of the cassava green mite (CGM), Mononychellus tanajoa (Bondar) (Acari: Tetranychidae), in Africa, because this pest is attacked by several natural enemies. Two of these, Typhlodromalus aripo De Leon and Amblydromalus (=Typhlodromalus) manihoti Moraes (Acari: Phytoseiidae), were introduced from Brazil and have established and spread widely in African cassava fields (Hanna and Toko 2003; Yaninek and Hanna 2003). Neither of these two predatory mites, however, has consistently established in the arid, semi-arid, and some subtropical mid-altitude areas (Hanna and Toko 2003; Zundel et al. 2009). Furthermore, biological control of CGM, particularly on cassava grown in the savannas and mid-altitutdes, depends on the suitability of cassava varieties for T. aripo, as this predator prefers to colonize cassava varieties with hairy apices, leaving those with glabrous apices vulnerable to CGM attacks (Hanna et al. 2000; Zundel et al. 2009). Together, the lack of predator persistence in some agroecologies and lack of predator colonization of glabrous varieties necessitated the search for a complementary approach to enhance CGM biocontrol in areas where it has not been fully achieved by exotic phytoseiid predators. In this regard, Brazilian isolates of the entomopathogenic fungus Neozygites tanajoae (Entomophthorales: Neozygitaceae), known to be virulent to CGM (Delalibera et al. 1992) and to be specific to this pest (Hountondji et al. 2002a; Agboton et al. 2009b), were introduced into Africa where they were evaluated in the laboratory and later tested in cassava fields in southern Benin (Hountondji et al. 2002b). Particularly important was the finding that N. tanajoae did not infect T. aripo, which has become the most effective exotic predator for control of CGM in Africa (Hanna and Toko 2003; Yaninek and Hanna 2003; Hanna et al. 2005). Those two exotic natural enemies of GCM on cassava in Africa, T. aripo and N. tanajoae, therefore constitute a suitable system for assessing the impact of predator–pathogen interactions on suppression of pest populations. In some regions (e.g., Benin in West Africa), T. aripo and N. tanajoae share the same cassava fields but presently data on the combined effects of this T. aripo and fungus on control of CGM is lacking.
The broad objective of this paper is to determine the nature of interactions between T. aripo and N. tanajoae and their consequences for CGM biological control. Specifically, we sought to determine in screenhouse experiments the relative effect of the two natural enemies, alone or together, on CGM populations over the course of at least two CGM generations. In laboratory experiments we determined the level of attack (or feeding) by T. aripo on N. tanajoae-infected or healthy CGM and consequences of such feeding on T. aripo survival and reproduction and how that might influence CGM biological control.
Materials and methods
Source of mites and fungus
Adult CGM females were obtained from a laboratory culture initiated with a cohort of eggs from CGM females collected from a cassava field free of N. tanajoae in southwestern Benin. Nymphs from the cultures were transferred to 2- to 3-week-old potted cassava plants in small, fine-mesh cages in a screenhouse for mass rearing. Every week, 60 live mites were mounted in 0.1 % cotton blue dissolved in lactophenol and examined with a compound microscope for the presence of fungal infections; no infection was detected in our screenhouse-reared CGM.
Adult female T. aripo were collected from cassava fields near the town of Sè, Department of Mono, southwestern Benin, and maintained in a laboratory at the IITA-Benin Station on a diet consisting of all stages of CGM on potted cassava plants in a screenhouse before their use in the experiments.
Isolates of N. tanajoae were preserved as fungus-infected, mummified CGM—referred to as “mummies”. Mummies were originally collected by R. Hanna from Alto Alegre in the state of Bahia, Brazil, in 2007 and imported to IITA-Benin facilities, where they were maintained in the laboratory at 4 °C. They were then propagated by sub-culturing them in vivo on live CGM following methods described by Hountondji et al. (2002b). For each experiment, a new batch of mummies was produced (see the next section for details) and used within 2 weeks to minimize loss of viability.
Screenhouse experiment
An experiment was conducted on potted cassava plants in cages in a screenhouse at the Biological Control Centre for Africa, International Institute of Tropical Agriculture, Cotonou, Benin, from April to September 2007. The screenhouse (length x width x height = 24 × 8.5 × 5 m) had a canopy of Teflon plastic and sides covered with an amber screen of 32 × 32 μm mesh. To allow air circulation and to prevent excessive heat build-up, screened windows at opposite ends of the screenhouse were opened during the day. Temperatures inside the screenhouse ranged from 25 to 38 °C, and relative humidity ranged from 44 to 94 %. The experiments used 12 cages (length × width × height = 100 × 100 × 120 cm), each of which was covered with a fine, transparent mesh.
Cassava plants were obtained from farmers’ fields around Lokossa in southwestern Benin. Six cassava cuttings (20 cm long) of the variety “Agric” were planted in each of 12 plastic pots containing about 12 kg of topsoil collected from a field that had been under fallow for more than 4 years and that had not been treated with fertilizers and pesticides for at least 14 years. Pots with cassava plants were placed in the cages (one pot per cage) in the screenhouse within 48 h after planting. The cages were arranged in three groups (four cages per group with cages and pots placed on iron benches). Each group represented one replicate or block. Four treatments were randomly assigned to cages in each group. Before the experiment began, the cages were disinfested with 70 % alcohol and rinsed with distilled water.
The trial was designed as a 2 × 2 factorial experiment with the factors being predator addition or fungus addition. At the start of the experiment, each pot of plants (six plants per pot and one pot per cage) was infested with CGM by pinning two cassava leaf discs (2 cm diameter) with 25 uninfected adult female CGM on the oldest leaves of each of the six plants, for a total of 50 and 300 adult female CGM per plant and per pot respectively. Ten days later, CGM-infested plants were assigned to one of the following treatments: (1) Addition of 25 T. aripo adult females, (2) addition of 25 live N. tanajoae-exposed CGM, (3) addition of 25 T. aripo adult females plus 25 live N. tanajoae-exposed CGM, and (4) a control that remained free of predator and the fungus. The predators were placed directly on the first fully expanded leaf of each cassava plant using a camel-hair brush. The fungus was added to plants by pinning a cassava leaf disc with 25 N. tanajoae-infected CGM to the first fully-expanded leaf of each plant in the pot. CGM infected with N. tanajoae were obtained by exposing 30 healthy female CGM from the screenhouse colonies to fresh capilliconidia produced by a sporulating mummy on a 2-cm diameter leaf disc as described previously (Hountondji et al. 2002b; Agboton et al. 2009a).
Immediately prior to predator and fungus addition, one plant from each cage was removed for estimating CGM densities. This sampling was repeated on days 8, 16 and 24 after natural enemy addition. The stem of the plant was removed from the cutting, placed in a plastic bag and brought to the laboratory for counting. Eggs and active stages (larvae, nymphs, and adults) of CGM, active stages of T. aripo in the apex and on all the leaves, and all N. tanajoae-infected CGM (infected and mummified CGM) from each plant were counted in the laboratory with a binocular microscope.
Laboratory experiment
In a laboratory experiment we determined the effect of two diets—healthy or N. tanajoae-infected CGM—on T. aripo oviposition and survival. Two cohorts of 50 gravid T. aripo females (6–7 days after egg hatching, 24 h-starved) were reared for use in the experiments. The experimental unit consisted of a cassava leaf disc (2 cm diameter with the abaxial surface placed up) resting on water-saturated cotton wool in an open Petri dish (13 cm diameter). One young, gravid T. aripo female was placed on each leaf disc and fed with 20 healthy mites or 20 infected mites per day. Each treatment was replicated 10 times, with each replicate containing both treatments. The dishes were kept in a growth chamber at 25–27 °C and at 70–90 % RH. Just before new CGM individuals were added each day, T. aripo eggs and CGM active stages (seemingly healthy and visibly infected) and eggs were counted on each disc and then removed before a new batch of CGM was added—to top-up the numbers at 20. The experiment continued until all predators had died; the last predator died on day 16. Daily consumption of healthy and infected mites by T. aripo was calculated. Because of the considerable attention needed to set-up and evaluate the treatments, all 10 replicates of each treatment were established at the same time.
Statistical analyses
For the screenhouse experiment, the numbers of live CGM, live T. aripo, and N. tanajoae-infected and mummified mites on the leaves and in apices were summed for each plant. Means and standard errors were calculated from the sums per plant and plotted against sampling dates. We used univariate repeated measures analysis of variance with blocking to determine the effect of main treatments (i.e., T. aripo addition or N. tanajoae addition), and their interaction, and the effects of period of sampling and its interaction with the main treatments, on CGM and T. aripo numbers and N. tanajoae prevalence. Means of CGM (actives), T. aripo (actives), and percent N. tanajoae infected CGM (infected and mummified mites) were used in statistical analyses in SAS (SAS Institute 2007). For the laboratory experiment, a t test was used to compare the effect of healthy vs. fungus-infect CGM on T. aripo oviposition. Life table analysis (SAS Institute 2007) was used to determine whether food type affected development of T. aripo. The effect of food type (healthy vs. fungus-infect CGM) on survival of T. aripo was tested using the Wilcoxon signed-rank test.
Results
Relative effect of Typhlodromalus aripo and Neozygites tanajoae on CGM densities in the screenhouse experiment
CGM densities at the time of the addition of T. aripo and N. tanajoae were similar in all treatments (2-factor ANOVA with blocking with Tukey HSD; P > 0.05). The addition of T. aripo—predator main effect—had the greatest impact on CGM numbers. In cages where this predator was added (averaged over cages with the predator alone and together with N. tanajoae), CGM densities were reduced by 35 % (Fig. 1a; Table 1) during the 24 days of the experiment. In contrast, the addition of N. tanajoae—main pathogen effect—had little effect on overall CGM densities (Fig. 1a; Table 1). This may be explained by the time take by the pathogen to develop infection and contaminate healthy mites. This is supported by the strong interaction between T. aripo addition and N. tanajoae addition. (Table 1), which necessitated the examination of simple effects for partitioning direct and indirect effects of N. tanajoa or T. aripo addition on CGM densities. We used single degrees of freedom contrasts with Bonferroni adjustment (Milliken and Johnson 1984, p. 454) to compare treatment means to the control.
Abundance of pest mites (Cassava Green Mite: CGM), predatory mites (Typhlodromalus aripo), and the entomopathogenic fungus (Neozygites tanajoae) in the different treatments in the screenhouse experiment: a CGM (actives), b T. aripo (actives), and c N. tanajoae (infected and mummified mites). Numbers of CGM and T. aripo were log-transformed; percentages of N. tanajoae-infected CGM were arcsine-transformed (for normalizing the data) and averaged per plant. Day 0 is the day on which natural enemies were added
The addition of T. aripo alone (simple effect of T. aripo) reduced CGM densities by 64 % (P < 0.05), whereas the addition of N. tanajoae (simple effect of N. tanajoae) reduced CGM densities by 30.5 % (P < 0.05), but the addition of both T. aripo and N. tanajoae reduced CGM densities by 27.7 % which is 36.3 % less than with T. aripo alone. This is a strong indication that the impact of T. aripo on CGM densities was considerably reduced in the presence of N. tanajoae, but this effect on CGM was asymmetrical as N. tanajaoe effect was only slightly less (9 %) when together with T. aripo compared with cages when it was present alone (P > 0.05).
In addition to main treatment and their interaction’s effects on CGM densities, there were considerable differences in the rate at which the natural enemies affected their prey/host populations over the course of the experiment. The level of effect of T. aripo and the interaction effect of T. aripo addition and N. tanajoae addition depended on date of sampling (P < 0.05 for both effects; Table 1). In contrast, the effect of N. tanajoae addition on CGM densities was not affected by time period during the experiment (Table 1), but this again must be interpreted in the context of the three-way interactions of the two main effects with date as this three-way interaction provides “greater sensitivity of testing the hypothesis of treatment effects and allows subplots to act as their own control to a greater extent than in only main effect analysis” (Hanna et al. 1997). Of interest are the comparisons of the effect of T. aripo and N. tanajoae on CGM densities—when the natural enemies are present alone or together—with control (i.e., absence of natural enemies).
Trends in CGM densities over the course of the experiment were different from control regardless of T. aripo and N. tanajoae being present alone or together (F 3,16 = 3.24–6.65; P < 0.05). Stratification of the analysis by sampling date showed that in cages with T. aripo alone CGM densities were lower than in control cages at all three sampling dates after the start of the experiment (1-way ANOVA with blocking: F 1,6 = 12.05–30.47; P < 0.05). CGM densities in N. tanajoae cages followed similar trends to those in control cages during the first 8 days (F 1,6 = 0.288; P > 0.05) but quickly declined compared with control on the 18th day and remained at the same level on the 24th day (F 1,6 = 13.83 and 6.67; P < 0.05). The presence of T. aripo and N. tanajoae together produced considerably different dynamics than when the two natural enemies were present alone. CGM densities quickly decreased after the addition of the two natural enemies (F 1,6 = 12.59; P < 0.05), as in T. aripo cages, which indicates that this decline was largely due to T. aripo predation since CGM densities in N. tanajoae cages were similar to control. Unexpectedly, CGM densities in cages with the two natural enemies leveled-off but remained less than in the control on the 18th day (F 1,6 = 5.51 and P < 0.05); they however increased to similar level as in the control cages by the 24th day of the experiment (F 1,6 = 0.802; P < 0.05).
Numbers of Typhlodromalus aripo and Neozygites tanajoae in the screenhouse experiment
We succeeded in establishing T. aripo in cages where this predator was released while cages that did not receive the predators remained free of them throughout the experiment (Fig. 1b; Table 1). Average T. aripo densities in cages where it was present alone was statistically similar to that where it was present together with N. tanajoae (F 1,6 = 3.39 and P = 0.115), despite a numerical difference of 25.4 % (T. aripo alone: 26.41 ± 6.19 mean ± SE; T. aripo + N. tanajoae: 17.08 ± 1.45). In both cages where it was added, T. aripo increased rapidly and reached slightly higher densities where it was present alone compared with its presence with N. tanajoae. Its densities then quickly declined and reached numerically similar levels in the two treatments with this predator, but T. aripo densities where it was present alone remained slightly higher than where it was present with the fungus.
As in the case of T. aripo, cages that did not receive N. tanajoae remained free of this fungus while in those that received it the fungus was able to establish and cause infections in CGM populations (Fig. 1c; Table 1). The infected CGM by N. tanajoae was 30 % higher where this fungus was present alone (18.22 ± 1.15 with a peak of 32.77 ± 6.21 on day 18) compared with its presence with T. aripo (12.88 ± 1.15 with a peak of 19.47 ± 1.79 on day 24) (F 1,6 = 29.13; P = 0.002; Fig. 1c). Fungal infections were first detected at 8 days after the start of the experiment and were similar in the two fungus treatments (F 1,6 = 0.017; P < 0.05; Fig. 1c). Percentage of infected CGM in cages with the fungus alone continued to increase through day 16 with a slight decline on day 24, but leveled-off during the last two sampling dates in cages where the two natural enemies were present together. The prevalence of infected CGM was higher in cages where the fungus was present alone compared with cages where it was present with T. aripo (day 16: F 1,6 = 12.18, P < 0.01; day 24: F 1,6 = 13.69; P < 0.01).
Laboratory experiment
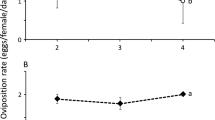
Although adult female T. aripo consumed both healthy and infected CGM, the predator consumed more (P < 0.05) infected than healthy CGM (Fig. 2a). Predator oviposition rate was also reduced (P < 0.05) when the predators were fed infected CGM compared with healthy CGM (Fig. 2b). The predators also lived longer (P < 0.01; df = 1; P = 0.0042 and 0.0048 for log-rank test and Wilcoxon test, respectively) when fed healthy CGM compared with infected CGM (Fig. 3).
Discussion
Our screenhouse experiment confirmed the beneficial effects of both T. aripo and N. tanajoae in the biological control of cassava green mite (Alvarez Afanador et al. 1993; Roy and Pell 2000; Hountondji et al. 2002b; Hanna and Toko 2003; Yaninek and Hanna 2003; Delalibera et al. 2004; Hanna et al. 2005). When present alone, T. aripo numbers and N. tanajoae infections increased rapidly, but T. aripo eventually caused 2.1 fold higher level of CGM suppression compared with N. tanajoae (64 % for T. aripo and 30.5 % for N. tanajoae). The highest performance of T. aripo in our short-term screenhouse experiment is likely due to its ability to feed immediately on CGM, while the entomopathogenic fungus N. tanajoae requires some time to penetrate, infect, sporulate, and spread (Hajek et al. 2001). Entomopathogenic fungi kill the host only after completely growing through the host’s body (Hajek et al. 2001). It follows that transmission of infective conidia from infected to healthy hosts and the development of epizootics require both time and favorable environmental conditions (Hajek et al. 2001; Delalibera et al. 2006). In our screenhouse experiment, conditions may not have been optimal for N. tanajoae, and this along with the experiment’s short duration may explain why T. aripo performed better than N. tanajoae in reducing CGM numbers.
In this study, we tested the hypothesis that the presence of both natural enemies on the same cassava plant would result in additive or greater-than-additive suppression of CGM numbers, because the two natural enemies inhabit different structures of the cassava plan and therefore should not compete directly. Whereas T. aripo inhabits the plant apex during the day and forages on upper leaves only during the night (Onzo et al. 2003), N. tanajoae is restricted largely to cassava leaves (Hountondji et al. 2002b). Unexpectedly, our screenhouse experiment showed that the co-occurrence of T. aripo and N. tanajoae on the same cassava plant had a less-than-additive suppression. When present together, combined CGM suppression by T. aripo and N. tanajoae was 27.7 %, or 2.3 fold lower than when T. aripo was alone, and only 1.1 fold lower than when N. tanajoae was alone. The two natural enemies clearly interfered with each other, which negatively impacted suppression of their prey/host. This interference was asymmetric as the impact was greater on T. aripo’s ability to suppress CGM compared with N. tanajoae. It was clear that the presence of T. aripo decreased CGM infections by N. tanajoa by 30 % (F 1,6 = 29.13; P = 0.002). Similarly, the presence of N. tanajoae reduced T. aripo numbers by 25.2 %, but this difference was not significant (F 1,6 = 3.39 and P = 0.115), probably due to large variations in T. aripo numbers on day 8 and 14 (51.0 ± 13.05 and 25.7 ± 9.49 respectively) in cages where T. aripo was alone Similarly, cages that did not receive N. tanajoae remained free of this fungus while in those that received it the fungus was able to establish and cause infections in CGM populations (Fig. 1c; Table 1). Statistical similarities or differences notwithstanding, both natural enemies reduced each other’s numbers/prevalence by nearly equal level, which evidently caused the reduction in CGM suppression in the combined presence of the two natural enemies. Undoubtedly, however, the negative impact of N. tanajoae on T. aripo is the likely cause of this substantial loss in CGM suppression, as T. aripo alone was able to reduce CGM populations by 2.1 fold more than N. tanajoe alone. Our laboratory evidence and that by Ariori and Dara (2007) provides possible explanations for the observed interactions between T. aripo and N. tanajoae and consequences for the suppression of their shared prey/host the cassava green mite.
The reduction in T. aripo abundance in the combined treatment of the screenhouse experiment was at least partially explained by the laboratory experiment that used cassava leaf discs on which T. aripo females were fed either N. tanajoae-infected CGM or healthy CGM. Those laboratory data indicated that T. aripo consumed significantly more infected than healthy CGM, perhaps because infected mites are less mobile and thus more easily captured than healthy mites. By feeding on infected mites, T. aripo not only reduced the density of fungal inoculum but also reduced its own reproduction and longevity, probably because of the poorer nutritional quality of infected compared with healthy mites, or together with only partial consumption of infected mites (particularly if the greater attack on the infected mites was due to greater prey catch efficiency of the predator rather than preference for the infected mites). It is also possible that N. tanajoae produces toxins, like some entomopathogenic fungi (Boucias and Pendland 1998) that could harm a predator that consumed the infected CGM.
Ariori and Dara (2007) demonstrated that the consumption of pathogen-infected pest mites by predatory mites can reduce the effectiveness of the pathogen. This effect may be enhanced by a ‘preference’ of the predator for feeding on infected hosts, thereby reducing the entomopathogen inoculum, which otherwise would be dispersed among living host mites. The greater consumption of infected mites, however, may not mean a preference for these mites by the predator, but rather a greater prey catch efficiency by the predator on the relatively less mobile infected mites. Nevertheless, the interactions reported by Ariori and Dara (2007) is consistent with our results in that high T. aripo densities in the combined treatments were associated with decreased N. tanajoae infections. This mechanism may explain also the failure of N. tanajoae and the predatory mite Neoseiulus idaeus to control a population of CGM in central Bahia, Brazil (Elliot et al. 2008). While predation of T. aripo on infected CGM may reduce the level of fungus inoculum, the predator could also indirectly increase fungus inoculum by spreading fungus propagules as it foraged on cassava plants. This effect could be greater in the field than in our screenhouse experiment.
Taken together, the results of our screenhouse and laboratory experiments point to interactions between T. aripo and N. tanajoae that might affect the dynamics of these natural enemies in the field and in turn their impact on the target host mite M. tanajoa. Our experimental results are not sufficient, however, to declare that the presence of the two predators together will have less effect on CGM populations compared with their presence alone. Cassava plants used in our screenhouse experiment were young (four to eight weeks old) and small relative to the 12 month typical growth cycle (from planting to harvest) and large size of cassava plants in the field in West Africa. Larger plants may facilitate the dispersal of prey to avoid predators (Magalhães et al. 2002) and pathogens (Hountondji 2005), which may result in greater coexistence of the two natural enemies. Furthermore, cassava plants in cages in the screenhouse tended to have smaller apices than equally-aged plants in the field; this together with the small plant size and shading in the screenhouse results in T. aripo being present on cassava leaves more often than under field conditions, which probably increases the intensity of the interactions between the two natural enemies. Similar results were obtained in screenhouse experiments by Onzo et al. (2004) in which significant asymmetrical negative interactions occurred between T. aripo and another leaf inhabiting mite A. manihoti. In subsequent field observations (Onzo et al. 2003; Zannou et al. 2007) and field manipulative experiments (A. Onzo, R. Hanna, and M. W. Sabelis, unpubl. data), no such negative interactions were observed. Furthermore, all field observations and experimental manipulations showed that the presence of two predators is more beneficial for biological pest control than when each predator is alone, despite the negative inter-predator interactions observed in the screenhouse. Similar field experiments are needed in which T. aripo abundance and N. tanajoae incidence are simultaneously manipulated to determine their relative effects—alone or in combination—on CGM populations.
The results of our experiments underscore the complexity and difficulty we and countless others have faced in deciding whether to release one or multiple natural enemies, particularly where natural enemies interact in a way that will either reduce or enhance the suppression of the target pest(s), which largely depends on the characteristics of the natural enemies and the ecosystem. The debate on the use of single ore multiple natural enemy species in biological control of arthropod pests will surely continue, but with every carefully planned and executed study we will move closer to a unified framework for predicting which system would benefit from a particular release strategy. Until then, there is no substitute for field experiments that simultaneously manipulate the most significant explanatory variables to determine their outcome in the suppression of the target pest.
References
Agboton VB, Delalibera I Jr, Hanna R, von Tiedemann A (2009a) Molecular detection and differentiation of Brazilian and African strains of the entomopathogen Neozygites tanajoae (Entomophthorales: Neozygitaceae) with PCR using specific primers. Biocontrol Sci Technol 19:67–79
Agboton VB, Hanna R, Hountondji FCC, von Tiedemann A (2009b) Pathogenicity and host specificity of Brazilian and African isolates of the acaropathogenic fungus Neozygites tanajoae to mite species associated with cassava. J Appl Entomol 133:651–658
Alvarez Afanador JM, Acosta A, Bellotti AC, Braun AR (1993) Pathogenicity studies of a fungus associated to the cassava (Manihot esculenta Crantz) pest Mononychellus tanajoa (Bondar). Revista Colomb de Entomol 19:10–20
Ariori SL, Dara SK (2007) Predation of Neozygites tanajoae infected cassava green mites by the predatory mite, Typhlodromalus aripo (Acari: Phytoseiidae). Agriculturae Conspectus Scientificus 72:169–172
Boucias DG, Pendland JC (1998) Principles of insect pathology. Kluwer, Boston, p 548
Briggs CJ (1993) Competition among parasitoid species on a stage-structured host and its effect on host suppression. Am Nat 141:372–397
Croft BA, MacRae IV, Currans KG (1992) Factors affecting biological control of apple mites by mixed populations of Metaseiulus occidentalis and Typhlodromus pyri. Exp Appl Acarolo 14:343–355
Delalibera JI, Sosa-Gómez DR, de Moraes GJ, Alenca JA, Farias AW (1992) Infection of Mononychellus tanajoa (Acari: Tetranychidae) by the fungus Neozygites sp. (Entomophthorales) in northeastern Brazil. Fla Entomol 75:145–147
Delalibera JI, Hajek AE, Humber RA (2004) Neozygites tanajoae sp. nov., a pathogen of the cassava green mite. Mycologia 96:1002–1009
Delalibera JI, Demetrio CGB, Manly BFJ, Hajek AE (2006) Effect of relative humidity and origin of isolates of Neozygites tanajoae (Zygomycetes: Entomophthorales) on production of conidia from cassava green mite, Mononychellus tanajoa (Acari: Tetranychidae), cadacers. Biol Control 39:489–496
Ehler LE, Hall RW (1982) Evidence for competitive exclusion of introduced natural enemies in biological control. Environ Entomol 11:1–4
Elliot SL, de Moraes GJ, Mumford JD (2008) Failure of the mite-pathogenic fungus Neozygites tanajoae and the predatory mite Neoseiulus idaeus to control a population of the cassava green mite, Mononychellus tanajoa. Exp Appl Acarol 46:211–222
Gnanvossou D, Hanna R, Dicke M (2003) Infochemical-mediated intraguild interactions among three predatory mites on cassava plants. Oecologia 135:84–90
Hajek AE, Wraight SP, Vandenberg JD (2001) Control of arthropods using pathogenic fungi. Bio-Exploitation Filamentous Fungi, Fungal Divers Res Ser 6:309–347
Hanna R, Toko M (eds) (2003) Development and applications of a biological control program for cassava green mite in Africa. In: Proceedings of the 3rd inter-regional meeting of the Africa-wide Cassava Green Mite Biocontrol Project. International Institute of Tropical Agriculture, Biological Control Centre for Africa, Cotonou, Republic of Benin
Hanna R, Wilson LT, Zalom FG, Flaherty DL (1997) Effects of predation and competition on population dynamics of Tetranychus pacificus on grapevines. J Appl Ecol 34:878–888
Hanna R, Ojo D, Yaninek JS, Toko M, Gnanvossou D, Onzo A (2000) Effects of cassava cultivar on abundance of exotic phytoseiid mites in Africa. In: Abstracts of the XXI international congress of entomology, Iguassu Falls, Brazil, 20–26 August 2000, p 10
Hanna R, Onzo A, Lingeman R, Yaninek JS, Sabelis MW (2005) Seasonal cycles and persistence in a predator-prey system on cassava in Africa. Popul Ecol 47:107–117
Heinz KM, Nelson JM (1996) Interspecific interactions among natural enemies of Bemisia in an innundative biological control program. Biol Control 6:284–393
Hountondji FCC (2005) Classical microbial control of the cassava green mite: from individual behaviour to population dynamics. PhD Dissertation, University of Amsterdam, the Netherlands
Hountondji FCC, Yaninek JS, de Moraes GJ, Oduor GI (2002a) Host specificity of the cassava green mite pathogen Neozygites floridana. Biocontrol 47:61–66
Hountondji FCC, Lomer CJ, Hanna R, Cherry AJ, Dara SK (2002b) Field evaluation of Brazilian isolates of Neozygites floridana (Entomophthorales: Neozygitaceae) for the microbial control of cassava green mite in Benin, West Africa. Biocontrol Sci Technol 12:361–370
Huffaker CB, Messenger PS, DeBach P (1971) The natural enemy component in natural control and the theory of biological control. In: Huffaker CB (ed) Biological control. Plenum Press, New York, pp 16–67
Lang A (2003) Intraguild interference and biocontrol effects of generalist predators in a winter wheat field. Oecologia 134:144–153
Losey JE, Denno RF (1998) Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology 79:2143–2152
Losey JE, Denno RF (1999) Factors facilitating synergistic predation: the central role of synchrony. Ecol Appl 9:378–386
Magalhães S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149
Milliken GA, Johnson DE (1984) Analysis of messy data: designed experiments, vol 1. Van Nostrand Reinhold Co., New York
Onzo A, Hanna R, Zannou I, Sabelis MW, Yaninek JS (2003) Dynamics of refuge use: diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos 101:59–69
Onzo A, Hanna R, Janssen A, Sabelis MW (2004) Interactions between two neotropical phytoseiid predators on cassava plants and consequences for biological control of a shared spider mite prey: a screen house evaluation. Biocontrol Sci. Technol. 14:63–76
Onzo A, Hanna R, Negloh K, Toko M, Sabelis MW (2005) Biological control of cassava green mite with exotic and indigenous phytoseiid predators—effects of intraguild predation and supplementary food. Biol Control 33:143–152
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Riechert SE, Lawrence K (1997) Test for predation effects of single versus multiple species of generalist predators: spiders and their insect prey. Entomol Exp Appl 84:147–155
Rosenheim JA (2001) Source-sink dynamics for a generalist insect predator in habitats with strong higher-order predation. Ecol Monogr 71:93–116
Roy HE, Pell JK (2000) Interaction between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci Technol 10:737–752
Sabelis MW, Hanna R, Onzo A, Palini A, Cakmak I, Janssen A (2009) Multiple predators, intraguild interactions and biological control of a single spider mite species. In: IOBC Proceedings, Florence, Italy, March 2009 (in press)
SAS Institute (2007) SAS system for windows, statistics. Release 6.12, version 9.1. SAS Institute Inc., Cary
Sih A, Englund G, Wooster D (1998) Emergent impact of multiple predators on prey. Trends Ecol Evol 13:325–329
Spiller DA (1986) Interspecific competition between spiders and its relevance to biological control by general predators. Environ Entomol 15:177–181
Wekesa VW, de Moraes GJ, Knapp M, Delalibera JI (2007) Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulus longipes (Acari: Phytoseiidae). Biol Control 41:408–414
Yaninek JS, Hanna R (2003) Cassava green mite in Africa: a unique example of successful classical biological control of a mite pest on a continental scale. In: Neuenschwander P, Borgemeister C, Langewald L (eds) Biological control in IPM systems in Africa. CABI Publishing, Wallingford, pp 61–75
Zannou ID, Hanna R, Agboton VB, de Moraes GJ, Kreiter S, Phiri G, Jone A (2007) Non-target effects of the classical biological control of a neotropical herbivorous mite pest Mononychellus tanajoa (Acari: Tetranychidae) a mite pest of cassava in Southern Africa. Biol Control 41:190–198
Zundel C, Nagel P, Hanna R, Kroner F, Scheideger U (2009) Environment and host-plant genotype effects on the seasonal dynamics of a predatory mite on cassava in sub-humid tropical Africa. Agric For Entomol. doi:10.1111/j.1461-9563.2009.00429
Acknowledgments
We are grateful to H. Dossounon and K. Yenou for their valuable help while collecting and processing samples; R. Houndafoché for his assistance in rearing the predatory mites; S. Korie for his assistance in the statistical analyses; and I. Delalibera Jr, M. Toko, F. Hountondji, D. Gnanvossou, B. Koopmann, and anonymous reviewers for providing valuable comments on an earlier version of this manuscript. This research was supported by the International Institute of Tropical Agriculture (IITA) through core support for R. Hanna and a grant from the International Fund for Agricultural Development (IFAD), and through a fellowship to the senior author by Deutscher Akademischer Austausch Dienst (DAAD) and the University of Göttingen in Germany for supporting this research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Agboton, B.V., Hanna, R., Onzo, A. et al. Interactions between the predatory mite Typhlodromalus aripo and the entomopathogenic fungus Neozygites tanajoae and consequences for the suppression of their shared prey/host Mononychellus tanajoa . Exp Appl Acarol 60, 205–217 (2013). https://doi.org/10.1007/s10493-012-9630-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9630-1