Abstract
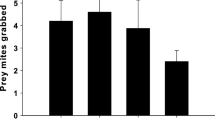
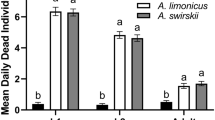
Adult female Phytoseiulus persimilis Athias-Henriot (Acari, Phytoseiidae) of one of our laboratory populations (=NR-population), show the following set of symptoms: predators shrink several days after mating, cease egg production and die several days after shrinking, show a lower degree of attraction to herbivore-induced plant volatiles and a shorter choice time in olfactometer tests, have the tendency to leave a prey patch with ample food, may carry excretory crystals in the legs, may cease prey consumption, and have a lower excretion rate. We hypothesized earlier that this characteristic syndrome, called non-responding (=NR-) syndrome, is caused by a pathogen infecting P. persimilis. To further support this hypothesis we here study several transmission modes of the factor causing the NR-syndrome. In all tests we measured size, short-term fecundity, mortality, predator position, response to plant odors and crystal location, thus including 6 of the 9 symptoms known yet. No evidence was found for vertical transmission from parent to offspring. Eggs from symptomatic females of the NR-population mated by males of the NR-population gave rise to normal-sized, well performing predators, when they had been surface sterilized or transferred to a new leaf. However, such eggs gave rise to shrunken females (17%) when left on the leaf where they had been laid. In the latter case transmission via products deposited on the leaf by the mothers was possible. We therefore tested several modes of horizontal transmission by exposing females of a commercial population that never showed the NR-syndrome (=R1-population) to products related to the symptomatic NR-population. No evidence was found for transmission via food or via squashed adult females. However, symptoms were induced in adult females of the R1-population after a 3-day exposure to a live adult female of the NR-population (incubation period=3–7 days, fraction shrunken females=53%) and after a 1-day exposure to feces and debris collected from such females (incubation period=2–4 days, fraction shrunken females=65%). Contact with live females and feces of the R1-population did not induce the syndrome. These results clearly indicate that the NR-syndrome is a contagious phenomenon and that the factor inducing the syndrome is transmitted horizontally among and between generations via feces and debris deposited by symptomatic females. The results are discussed in the context of mite pathology and biological control.
Similar content being viewed by others
References
Amano H, Chant DA (1978) Some factors affecting reproduction and sex ratios in two species of predacious mites, Phytoseiulus persimilis Athias-Henriot and Amblyseius andersoni Chant (Acarina, Phytoseiidae). Can J Zool 56:1593–1607
Andreadis TG (1987) Transmission. In: Fuxa JR, Tanada Y (eds) Epizootiology of insect diseases. John Wiley & Sons, New York, pp 159–176
Bjørnson S (1998) Morphology and Pathology of the Predatory Mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae), PhD Thesis, University of Alberta, Edmonton, Canada, p. 232
Bjørnson S, Keddie BA (1999) Effects of Microsporidium phytoseiuli (Microsporidia) on the performance of the predatory mite, Phytoseiulus persimilis (Acari: Phytoseiidae). Biol Contr 15:153–161
Bjørnson S, Keddie BA (2000) Development and pathology of two undescribed species of Microsporidia infecting the predatory mite, Phytoseiulus persimilis Athias-Henriot. J Invertebr Pathol 76:293–300
Bjørnson S, Keddie BA (2001) Disease prevalence and transmission of Microsporidium phytoseiuli infecting the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). J Invertebr Pathol 77:114–119
Bjørnson S, Steiner MY, Keddie BA (1996) Ultrastructure and pathology of Microsporidium phytoseiuli n. sp. infecting the predatory mite, Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). J Invertebr Pathol 68:223–230
Bjørnson S, Schütte C (2003) Pathogens of mass-produced natural enemies and pollinators. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CABI Publishing, Wallingford UK, pp 133–165
Breeuwer JAJ, Jacobs G (1996) Wolbachia: intracellular manipulators of mite reproduction. Exp Appl Acarol 20:421–434
Carlson DA, Alzogaray RA, Hogsette JA (2000) Behavioral response of Stomoxys calcitrans (Diptera: Muscidae) to conspecific feces and feces extracts. J Med Entomol 37:957–961
Dicke M, Sabelis MW, Bogaers RJF, Alers MPT, van Halder I (1991) Kairomone perception by a predatory mite: behavioural analysis of chemoreceptor-carrying extremities. Proc Exp Appl Entomol N.E.V. Amsterdam 2:179–184
Dicke M, Takabayashi J, Posthumus MA, Schütte C, Krips OE (1998) Plant-phytoseiid interactions mediated by herbivore-induced plant volatiles: variation in production of cues and in responses of predatory mites. Exp Appl Acarol 22:311–333
Dicke M, Schütte C, Dijkman H (2000) Change in behavioral response to herbivore-induced plant volatiles in a predatory mite population. J Chem Ecol 26:1497–1514
Gaede K (1992) On the water balance of Phytoseiulus persimilis A.-H. and its ecological significance. Exp Appl Acarol 15:181–198
Garthwaite D (2000) Changes in biological control usage in Great Britain between 1968 and 1995 with particular reference to biological control on tomato crops. Biocontr Sci Techn 10:451–457
Geest LPS, van der Elliot SL, Breeuwer JAJ, Beerling EAM (2000) Diseases of mites. Exp Appl Acarol 24:497–560
Grenacher S, Kröber T, Guerin PM, Vlimant M (2001) Behavioural and chemoreceptor cell responses of the tick, Ixodes ricinus, to its own faeces and faecal constituents. Exp Appl Acarol 25:641–660
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427
Hoy MA, Jeyaprakash A (2005) Microbial diversity in the predatory mite Metaseiulus occidentalis (Acari: Phytoseiidae) and its prey, Tetranychus urticae (Acari: Tetranychidae). Biol Contr 32:427–441
Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66:223–232
Lacey LA (ed) (1997) Manual of techniques in insect pathology. Academic Press, San Diego, p 409
Lenteren JC van Roskam MM, Timmer R (1997) Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol Contr 10:143–149
Myers JH, Rothman LE (1995) Virulence and transmission of infectious diseases in humans and insects: evolutionary and demographic patterns. TREE 10:194–198
Nagelkerke K (1993) Evolution of sex allocation strategies of pseudo-arrhenotokous predatory mites (Acari: Phytoseiidae). PhD Thesis University of Amsterdam, Amsterdam, The Netherlands, p 227
Pfennig DW, Ho SG, Hoffman EA (1998) Pathogen transmission as a selective force against cannibalism. Anim Behav 55:1255–1261
Pukall R, Schumann P, Schütte C, Gols R, Dicke M (2006) Acaricomes phytoseiuli gen. nov., sp. nov., isolated from the predatory mite Phytoseiulus persimilis. Int J Syst Evol Microbiol 56:465–469
Reed DK, Tashiro H and Beavers JB (1975) Determination of mode of transmission of the citrus red mite virus. J Invertebr Pathol 26:239–246
Rollo CD, Borden JH, Casey IB (1995) Endogenously produced repellent from american cockroach (Blattaria: Blattidae): function in death recognition. Environ Entomol 24:116–124
Sabelis MW, Dicke M (1985). Long-range dispersal and searching behaviour. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control. World Crop Pests 1B, Elsevier, Amsterdam, pp 141–160
Sabelis MW, Janssen A (1994) Evolution of life-history patterns in the Phytoseiidae. In: Houck MA (ed) Mites: ecological and evolutionary analyses of life-history patterns. Chapman and Hall, New York, pp 70–98
Sabelis MW, Janssen A, Pallini A, Venzon M, Bruin J, Drukker B, Scutareanu P (1999) Behavioral responses of predatory and herbivorous arthropods to induced plant volatiles: from evolutionary ecology to agricultural applications. In: Agrawal AA, Tuzun S, Bent E (eds) Induced plant defences against pathogens and herbivores. Biochemistry ecology and agriculture. APS Press, St. Paul, pp 269–296
Schausberger P (2003) Cannibalism among phytoseiid mites: a review. Exp Appl Acarol 29:173–191
Schausberger P, Croft BA (1999a) Predation on and discrimination between con- and heterospecific eggs among specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Environ Entomol 28:523–528
Schausberger P, Croft BA (1999b) Activity, feeding and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Environ Entomol 28:322–329
Schausberger P, Croft BA (2001) Kin recognition and larval cannibalism by adult females in specialist predaceous mites. Anim Behav 61:459–464
Schulten GGM (1985) Mating. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1B. World Crop Pests Elsevier, Amsterdam, pp 55–65
Schütte C (2006) A novel bacterial disease of the predatory mite Phytoseiulus persimilis: disease syndrome, disease transmission and pathogen isolation. PhD Thesis, Wageningen University, Wageningen, The Netherlands, p 208
Schütte C, Hulshof J, Dijkman H, Dicke M (1995) Change in foraging behavior of the predatory mite Phytoseiulus persimilis: some characteristics of a mite population that does not respond to herbivore-induced synomones. Proc Exp Appl Entomol N.E.V. Amsterdam 6:133–139
Schütte C, Baarlen P, van Dijkman H, Dicke M (1998) Change in foraging behaviour of the predatory mite Phytoseiulus persimilis after exposure to dead conspecifics and their products. Entomol Exp Appl 88:295–300
Schütte C, Bjørnson S, Kleespies RG, Huger AM (2005) Natural enemies applied in biological pest control: pathogens in field and mass-reared populations. Bull IOBC/WPRS 28:185–188
Schütte C, Kleijn PW, Dicke M (2006) A novel disease affecting the predatory mite Phytoseiulus persimilis (Acari, Phytoseiidae): symptoms in adult females. Exp Appl Acarol 38:275–297
Steiner MY, Bjørnson S (1996) Performance of Phytoseiulus persimilis and other biological control agents—on what are we basing our standards? Bull IOBC/WPRS 19:163–166
Šut’áková G (1991) Rickettsiella phytoseiuli and its relation to mites and ticks. In: Dusbábek F, Bukva V (eds) Modern Acarology, Academia Prague, pp 45–48
Yao DS, Chant DA (1990) Changes in body weight of two species of predatory mites (Acarina: Phytoseiidae) as a consequence of feeding in an interactive system. Exp Appl Acarol 8:195–220
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schütte, C., Poitevin, O., Negash, T. et al. A Novel Disease Affecting the Predatory Mite Phytoseiulus persimilis (Acari, Phytoseiidae): 2. Disease Transmission by Adult Females. Exp Appl Acarol 39, 85–103 (2006). https://doi.org/10.1007/s10493-006-0020-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-006-0020-4