Abstract
Premature mortality in people living with a severe mental illness (SMI) is often attributed to multiple factors including the use of medicines such as antipsychotics. Second-generation antipsychotics (SGAs) are known to cause metabolic syndrome which can increase the risk of cardiovascular disease. Practice guidelines have recommended regular physical health monitoring, particularly of metabolic parameters, however, metabolic monitoring for people living with SMI using antipsychotics remains suboptimal. Therefore, highlighting the need for ongoing research. This scoping review aimed to provide an overview of current metabolic monitoring practices. We anticipate that this information will assist clinicians and policymakers and inform future research. The following databases were searched: MEDLINE (Ovid), Embase (Ovid), CINAHL (EBSCO), the Cochrane Database of Systemic Reviews (Wiley), APA PsycInfo (Ovid) and Scopus (Elsevier Science Publishers). The target group was adults (aged ≥ 18) diagnosed with SMI (including bipolar disorder, major depressive disorder and psychotic disorders) and taking SGAs. In total, 44 studies from 14 countries were retrieved. Our findings highlighted that most studies conducted in hospitals did not report on metabolic monitoring practices. Additionally, the roles and responsibilities of healthcare professionals in metabolic monitoring for SMI were infrequently described and parameters such as waist circumference and BMI were often poorly monitored. The scoping review highlights that no streamlined approach towards metabolic monitoring currently exists. There is a need to stipulate and define the roles and responsibilities of all health professionals involved in metabolic monitoring in SMI to optimise care for these individuals. Moreover, there is a need for ongoing research, particularly in the community setting, to promote increased accessibility to metabolic monitoring for SMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Reports indicate that people living with a severe mental illness (SMI) have a reduced life expectancy compared to the general population, with estimates ranging from 10 to 20 years (Liu et al., 2017). Premature mortality has been linked to various factors, including cardiovascular disease (CVD) (Lawrence et al., 2010). Research indicates that individuals with SMI are more likely to report higher rates of tobacco smoking, poorer diet and low physical activity (Wichniak et al., 2019), all of which contribute to an elevated risk of CVD (Scott & Happell, 2011). Furthermore, commonly prescribed medications such as antipsychotics can amplify this risk by increasing the likelihood of developing metabolic syndrome (MetSyn) (Dekker et al., 2005), which is characterised by elevated blood glucose, lipids and blood pressure, as well as central obesity (Penninx & Lange, 2018). Antipsychotics are categorised as either first-generation antipsychotics (FGAs) or second-generation antipsychotics (SGAs) based on their pharmacological properties. While SGAs offer improved tolerance due to a lower risk of extrapyramidal side effects than FGAs (D’Souza & Hooten, 2021), they introduce a heightened risk of metabolic disturbances, including MetSyn (Bernardo et al., 2021; Hasan et al., 2013; Dekker et al., 2005).
Global consensus or treatment guidelines (American Diabetes Association, 2004; Castle et al., 2017) stipulate the need for regular physical health monitoring for patients with SMIs taking antipsychotics. The American guidelines recommend monitoring metabolic parameters (including weight, waist circumference, blood pressure, plasma glucose and lipid profile) at baseline and at 4, 8 and 12 weeks and annually thereafter as part of routine care (American Diabetes Association, 2004). Similarly, Australian guidelines recommend regular monitoring of metabolic parameters, with an additional review at 24 weeks after antipsychotic initiation (Castle et al., 2017).
Previous research has shown suboptimal metabolic monitoring rates in patients with SMI (Chee et al., 2017; Cohn & Sernyak, 2006; Michael & MacDonald, 2020), highlighting a disparity between guidelines and existing practices (Cunningham et al., 2018; Mackin et al., 2007; Mead et al., 2021). A multi-country systematic review and meta-analysis of 218,940 patients (inpatient and community-dwelling) reported that only blood pressure and triglycerides were routinely monitored for at least 50% of participants, while weight (47.9%), blood glucose (44.3%) and cholesterol (41.5%) were measured in fewer than half of the study cohort (Mitchell et al., 2012). Suboptimal monitoring rates were also identified in one Australian inpatient ward (n = 61), where height and weight were measured in less than half (46%) of the patients, while lipid levels were measured 23% of the time (Michael & MacDonald, 2020). However, this single-site study had a small sample size and may not reflect procedures in other practice settings.
Several studies have explored the role of allied health professionals, such as nurses (Chee et al., 2017) and pharmacists (Al AdAwi et al., 2020; Sud et al., 2021) in the management of cardiometabolic risk, metabolic syndrome (MetSyn) and related diseases in the SMI. A systematic review revealed that interventional studies to improve metabolic monitoring rates in patients with SMI generated relatively positive results (Melamed et al., 2019). These aforementioned studies focused on quantifying and improving metabolic monitoring rates but did not provide details on practice implementation. For example, a systematic review by Mitchell and colleagues quantified and compared the rates of metabolic monitoring before and after guideline implementation (Mitchell et al., 2012) but did not describe the processes of metabolic monitoring. Reviews to date have not reported on the context of interventions; for example, who are the health professionals involved in metabolic monitoring, where metabolic monitoring commonly occurs (for example, primary compared to tertiary settings) and how (such as procedures and systems) is this monitoring integrated and active in practice (Melamed et al., 2019; Poojari et al., 2022)? Addressing these questions will inform future implementations of more targeted and streamlined interventional approaches.
It is important to understand current metabolic monitoring patterns in routine clinical practice to improve the management and care of SMI. This scoping review provides an overview of current metabolic monitoring practices by utilising published studies’ descriptions of existing baseline monitoring rates and procedures (that is, without the influence of study interventions) as proxy measures. This review summarised and mapped existing metabolic monitoring practices, highlighted current gaps in practice and provided directions for future research initiatives. We anticipate that this information will assist clinicians and policymakers and inform future research.
Methods
The scoping review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist (Tricco et al., 2018) and the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis updated methodological guidance for the conduct of scoping reviews (Peters et al., 2021). An a priori protocol was developed and registered on the Open Science Framework (https://doi.org/https://doi.org/10.17605/OSF.IO/YMR5C).
An initial limited search of MEDLINE (Ovid) and APA PsycInfo (Ovid) was performed. Keywords in the titles and abstracts of relevant articles were used to develop the full search strategy (Supplementary Information). The search strategies, inclusion/exclusion criteria and the data extraction tool were piloted by two members of the research team on a small sample (n = 8) of papers. The following databases were searched: MEDLINE (Ovid), Embase (Ovid), CINAHL (EBSCO), the Cochrane Database of Systematic Reviews (Wiley), APA PsycInfo (Ovid) and Scopus (Elsevier Science Publishers). The search was adapted for each database and information source. Regular input from an academic librarian further refined the search strategy and translation across different databases. A search for grey literature was undertaken via Google Scholar and ProQuest Dissertation and Theses. The scoping review was informed by the Population, Concept, Context framework (Pollock et al., 2023). The search was conducted on the 8th of September 2022 and updated on the 13th of October 2023.
Population
The target group was adults (aged ≥ 18) diagnosed with SMI (including bipolar disorder, major depressive disorder and psychotic disorders) and taking SGAs.
Concept
This scoping review examined the combination of the following concepts:
-
1.
SMI was diagnosed in adults with the following conditions: bipolar disorder, major depressive disorder and psychotic disorders (including schizophrenia and schizoaffective disorder)
-
2.
Currently taking SGAs
-
3.
The metabolic syndrome incidence, cardiometabolic risk and metabolic parameters included the following:
-
a.
Weight
-
b.
Waist circumference
-
c.
Blood pressure (BP)
-
d.
Plasma glucose levels, such as blood glucose levels (BGLs) and haemoglobin A1c (HbA1c) levels
-
e.
Lipid levels included total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride (TG) levels.
-
a.
-
4.
Processes of metabolic monitoring (such as onsite or referral for laboratory tests)
Context
The scoping review included studies conducted in healthcare facilities such as community settings, medical centres, hospitals, and specialised care facilities. There were no attempts to limit the search to specific countries.
Inclusion and Exclusion Criteria
This review considered a variety of study designs, including qualitative, experimental, quasi-experimental, “before and after”, analytical observational, retrospective, cross-sectional and descriptive observational studies. Studies that monitored at least three metabolic parameters (weight, blood pressure, waist circumference, plasma glucose and lipid levels) and were published after 2004 (aligning with the publication date of the American guideline (American Diabetes Association, 2004)) were included.
Studies that were deemed not to reflect real-life practice, such as randomised controlled trials were excluded. Prospective studies were also excluded as baseline monitoring was often conducted as part of the research methodology and therefore may not reflect the usual metabolic monitoring rates in the particular setting. Additionally, case–control reports, case studies or case series were not considered because they cannot be generalised to the broader cohort of SMIs. Finally, studies that were not published in English or for which the full text could not be retrieved (for example, through interlibrary loans) were excluded.
Data Screening and Extraction
The articles retrieved by the search were managed by EndNote X9 (Clarivate Analytics, PA, USA), and Covidence (Veritas Health Innovation, Melbourne, Australia) was used to screen the studies. The titles and abstracts of the identified studies were screened by two independent reviewers, and conflicts were resolved by a third reviewer. An overly inclusive approach was employed, with full-text articles obtained for any abstracts in doubt. All studies meeting the inclusion criteria were retrieved in full and underwent the same screening process as the first phase (described above). Data extraction was conducted by two researchers, who extracted data from half of the studies and cross-verified the other half. The extracted information included author, year of publication, country, sample size, study design, role and responsibilities of health care professionals, setting, monitoring process and outcomes measured. The reference lists of relevant systematic reviews were screened independently by two authors for potentially relevant studies. The findings of the review are reported using descriptive analysis.
Data Analysis
Relevant data from the retrieved studies were charted and organised into the data extraction table. Additional information was collated and summarised (Arksey & O’Malley, 2005) then presented as appropriate tables or figures. The type and frequency of metabolic monitoring for the relevant metabolic parameters, including HDL and TG when reported, was included in a graph to illustrate the range of metabolic monitoring rates across the included studies.
Results
Study Selection
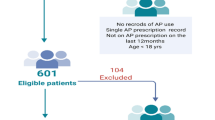
Of the 20,387 studies identified from the initial search, 7579 duplicates were removed using Endnote and Covidence. The first screening phase identified 608 studies for full-text review (Fig. 1). Systematic and literature reviews (n = 3) were assessed, and an additional two studies were identified. A study that included a small cohort of children was also included, as the majority of the participants were adults (Cotes et al., 2015).
Study Characteristics
In total, 45 studies were included (Fig. 1), and two manuscripts (a thesis and a peer review) published by the same authors covering the same content were considered single inclusions. The 44 included studies were conducted in the following countries (Table 1): the UK (n = 13) (Ali et al., 2023; Barnes et al., 2007, 2008, 2015, 2020; Gumber et al., 2010; Harrison et al., 2012; Holt et al., 2010; Lau et al., 2019; Mwebe et al., 2020; Najim & Islam, 2013; Pearsall et al., 2019; Ross et al., 2018), the US (n = 9) (Batscha et al., 2010; Butler et al., 2013; Coakley et al., 2012; Cotes et al., 2015; Kilbourne et al., 2007; Kioko et al., 2016; Mittal et al., 2014; Pereira et al., 2019; Tatreau et al., 2016), Ireland (n = 4) (Feeney & Mooney, 2005; Kelly et al., 2022; Lydon et al., 2021; O’Callaghan et al., 2011), Australia (n = 3) (Nguyen et al., 2009; Thompson et al., 2011; Viglione & Short, 2021), New Zealand (n = 2) (Keenan et al., 2020; O’Brien & Abraham, 2021), Canada (n = 2) (Fontaine et al., 2022; Stephenson et al., 2023), Denmark (n = 2) (Kjeldsen et al., 2013; Knudsen et al., 2022), South Africa (n = 2) (Marsay & Szabo, 2010; Shamima Saloojee et al., 2014a, 2014b) and one each from France (Verdoux et al., 2008), Germany (Deuschle et al., 2013), Malaysia (Hor et al., 2016), Singapore (Tan et al., 2022), South India (Poojari et al., 2020), and Spain (Bobes et al., 2011). One did not specify the country in which the study was conducted (Bomboy et al., 2021). More than half of the studies were conducted within the last 10 years (n = 29) (Ali et al., 2023; Barnes et al., 2015; Barnes et al., 2020; Bomboy et al., 2021; Butler et al., 2013; Cotes et al., 2015; Deuschle et al., 2013; Fontaine et al., 2022; Hor et al., 2016; Keenan et al., 2020; Kelly et al., 2022; Kioko et al., 2016; Kjeldsen et al., 2013; Knudsen et al., 2022; Lau et al., 2019; Lydon et al., 2021; Mittal et al., 2014; Mwebe et al., 2020; Najim & Islam, 2013; O’Brien & Abraham, 2021; Pearsall et al., 2019; Pereira et al., 2019; Poojari et al., 2020; Ross et al., 2018; Shamima Saloojee et al., 2014a, 2014b; Stephenson et al., 2023; Tan et al., 2022; Tatreau et al., 2016; Viglione & Short, 2021).
The studies covered a variety of settings, including hospitals (n = 14) (Coakley et al., 2012; Deuschle et al., 2013; Fontaine et al., 2022; Harrison et al., 2012; Holt et al., 2010; Hor et al., 2016; Kelly et al., 2022; Kjeldsen et al., 2013; Lydon et al., 2021; Najim & Islam, 2013; Nguyen et al., 2009; Shamima Saloojee et al., 2014a, 2014b; Tan et al., 2022; Verdoux et al., 2008), outpatient clinics (n = 5) (Kioko et al., 2016; Knudsen et al., 2022; Marsay & Szabo, 2010; O’Callaghan et al., 2011; Pereira et al., 2019), inpatients (n = 4) (Butler et al., 2013; Mwebe et al., 2020; Tatreau et al., 2016; Viglione & Short, 2021), secondary care settings (n = 2) (Barnes et al., 2008; Ross et al., 2018), primary care (n = 3) (Ali et al., 2023; Cotes et al., 2015; Stephenson et al., 2023), rural mental health services (n = 2) (Bomboy et al., 2021; Feeney & Mooney, 2005), general practices (n = 2) (Keenan et al., 2020; Lau et al., 2019), Veteran Administration Medical Centres (n = 2) (Kilbourne et al., 2007; Mittal et al., 2014), and one each from a tertiary care institution (Poojari et al., 2020), UK member trusts and healthcare services (Barnes et al., 2020), a metabolic clinic (Gumber et al., 2010) and a youth mental health service (Thompson et al., 2011). Six studies were conducted in multiple settings (Barnes et al., 2007, 2015; Batscha et al., 2010; Bobes et al., 2011; O’Brien & Abraham, 2021; Pearsall et al., 2019).
Roles and Responsibilities
The healthcare professionals involved in metabolic monitoring (such as ordering blood tests, screening or documenting) included prescribers (Bomboy et al., 2021), junior medical officers (Gumber et al., 2010; Viglione & Short, 2021), family physicians (Ross et al., 2018; Stephenson et al., 2023; Tatreau et al., 2016), attending physicians (Ross et al., 2018) and psychiatrists (Deuschle et al., 2013; Tatreau et al., 2016; Verdoux et al., 2008). Others included nursing staff (Lydon et al., 2021), clinic staff (Bomboy et al., 2021), physiotherapists (Kjeldsen et al., 2013), healthcare assistants (Hor et al., 2016) and clinical pharmacists (Kjeldsen et al., 2013). Medical doctors (such as junior medical officers, physicians and psychiatrists) were often described as being involved in patient assessments, ordering blood tests and providing clinical interventions. Other health professionals, such as nurses, were often involved in conducting screening and/or physical assessments (Bomboy et al., 2021; Fontaine et al., 2022; Hor et al., 2016; Kjeldsen et al., 2013; Mwebe et al., 2020; O’Callaghan et al., 2011; Viglione & Short, 2021), while pharmacists had limited involvement in patient metabolic monitoring. One study described the role of clinical pharmacists in providing education and medication reviews during weekly outreach visits (Kjeldsen et al., 2013).
Most studies did not stipulate the specific roles and responsibilities of healthcare professionals involved in the metabolic monitoring process. For instance, a multiyear audit mentioned the role of the mental health team (specific health professionals not specified) in the assessment and recording of metabolic parameters for one year (2012) but not for subsequent years (Barnes et al., 2015). Another study alluded to psychiatric clinical staff (including resident physicians) having a role in metabolic monitoring in relation to education for participants (Ross et al., 2018). Although the study described patient care provided by consultants, psychiatric residents, nurses, occupational therapists and social workers, there was no description of the role and responsibilities of the clinical team.
Procedure and Reported Metabolic Monitoring Practice
Seven studies reported on metabolic monitoring processes (Table 1) (Bomboy et al., 2021; Deuschle et al., 2013; Gumber et al., 2010; Kjeldsen et al., 2013; Lydon et al., 2021; Ross et al., 2018; Tatreau et al., 2016). Doctors, including psychiatrists and junior speciality trainees, were reported to be involved in the monitoring and documenting of metabolic parameters (Deuschle et al., 2013; Ross et al., 2018). Gumber and colleagues reported on a metabolic clinic managed by junior speciality trainees involving the monitoring of metabolic parameters at baseline and at 3, 6 and 12 months (Gumber et al., 2010). Any abnormal results were communicated to the GPs for their attention and appropriate intervention for the first year only. Thereafter, the responsibility for annual monitoring was transitioned to the GPs (Gumber et al., 2010). Similarly, several studies had described a screening process carried out by clinic staff, with participants assessed and reviewed by the prescriber (Bomboy et al., 2021; Tatreau et al., 2016). Blood samples were drawn onsite and subsequently transported to an offsite laboratory for processing, while the clinic staff were responsible for recording the results on the metabolic form (Bomboy et al., 2021). In all, three studies reported that blood test results were collected onsite and sent to a laboratory for assessment (Bomboy et al., 2021; Kjeldsen et al., 2013; Lydon et al., 2021). None of the studies stipulated whether metabolic monitoring was a mandated part of patient care, although three studies reported this as part of routine care (Batscha et al., 2010; Butler et al., 2013; Fontaine et al., 2022).
Monitoring of Metabolic Parameters
Several studies had measured various parameters (Table 1). Blood glucose levels varied between studies and were often reported as fasting glucose, HbA1c, and postprandial and random blood sugar levels. Figure 2 illustrates both the types and frequency of metabolic parameters measured in the included studies.
The type and frequency of metabolic monitoring for different metabolic parameters reported by the 29 included studies.*Studies that reported the frequency of metabolic monitoring over multiple years or where the prevalence was not reported were not included in this figure. Areas of overlap indicate that two or more studies reported similar monitoring rates
A total of 29 studies reported the frequency of metabolic monitoring for specific parameters, with some studies reporting these data for multiple cohorts (Ali et al., 2023; Barnes et al., 2020; Barnes et al., 2007; Barnes et al., 2008; Batscha et al., 2010; Bobes et al., 2011; Butler et al., 2013; Coakley et al., 2012; Cotes et al., 2015; Deuschle et al., 2013; Fontaine et al., 2022; Gumber et al., 2010; Harrison et al., 2012; Holt et al., 2010; Keenan et al., 2020; Kelly et al., 2022; Knudsen et al., 2022; Lau et al., 2019; Lydon et al., 2021; Nguyen et al., 2009; O’Brien & Abraham, 2021; O’Callaghan et al., 2011; Ross et al., 2018; Shamima Saloojee et al., 2014a, 2014b; Stephenson et al., 2023; Tan et al., 2022; Tatreau et al., 2016; Verdoux et al., 2008; Viglione & Short, 2021). Monitoring rates varied considerably across the 29 studies. Waist circumference (46%) and BMI (46%) were often poorly monitored, with nearly half of the studies reporting monitoring rates less than 20%. Lipids were also infrequently monitored, with many studies (54%) reporting rates of 40% or less. Just over half (55%) of the studies reported BP monitoring rates exceeding 70%, but blood glucose monitoring exhibited significant variation across studies, lacking a discernible pattern or cluster (Fig. 2).
Even though the types of metabolic parameters monitored varied between the included studies, most of the studies did not report on all five metabolic parameters. Waist circumference, HDL, BMI and TG levels were not as frequently monitored in these studies. Najim and colleagues suggested that there was inadequate metabolic monitoring and reported that less than half (48.25%) had a baseline physical health examination (Najim & Islam, 2013). Similarly, Kioko and colleagues reported that only 22% of patients had appropriate laboratory tests available (Kioko et al., 2016). In contrast, a cross-sectional retrospective review of records for people with SMIs revealed that most (83.1%) had evidence of routine blood monitoring (including glucose, cholesterol, HbA1c and TG) within the preceding two years (Pearsall et al., 2019).
Five studies explored the frequency of metabolic monitoring (retrospectively) at different time points (Feeney & Mooney, 2005; Marsay & Szabo, 2010; Mwebe et al., 2020; Najim & Islam, 2013; Poojari et al., 2020). Of these, four that examined the frequency of routine metabolic monitoring in the same cohort reported a reduction in monitoring rates for all metabolic parameters over time (Table 2) (Feeney & Mooney, 2005; Marsay & Szabo, 2010; Najim & Islam, 2013; Poojari et al., 2020). One study reported higher metabolic monitoring rates for all parameters (BMI, BP, glucose, lipid and electrocardiogram monitoring) except waist circumference for patients at three months after admission than at baseline (Mwebe et al., 2020).
Batscha and colleagues compared the frequency of metabolic monitoring across three different settings: inpatient, outpatient and metabolic clinic (Batscha et al., 2010). The authors reported that weight (p < 0.001) and BP (p < 0.001) were most frequently measured in an inpatient setting, while glucose (p < 0.001) and waist circumference (p < 0.001) were more regularly measured in the outpatient setting. Furthermore, waist circumference was more likely to be monitored in the metabolic clinic than in the inpatient unit (p < 0.001)(Batscha et al., 2010). Similarly, Marsay and colleagues reported that the metabolic parameters of the inpatient cohort were monitored more often than those of their outpatient counterparts (Marsay & Szabo, 2010).
Discussion
Metabolic monitoring for SMI appears suboptimal, and metabolic conditions such as CVD are often undiagnosed and untreated (Heiberg et al., 2019). Research to date has explored barriers to metabolic monitoring in the SMI, highlighting barriers at the individual, organisational, and systems levels (Ali et al., 2020; Cunningham et al., 2018). However, additional research is needed to address this gap (Solmi et al., 2021). This review explored the nuances of metabolic monitoring, offering an overview of current metabolic monitoring practices for SMI. Specifically, regarding the roles and responsibilities of healthcare professionals, specific metabolic parameters were monitored, as were the types of approaches and methodologies used in routine metabolic monitoring. Our findings have highlighted several gaps in current practice, including a lack of standardised metabolic monitoring procedures and processes and suboptimal metabolic monitoring rates for SMIs taking antipsychotics (Ali et al., 2021, 2023).
The uncertainty surrounding the specific roles and responsibilities of health professionals involved in care for SMIs can impede routine physical health monitoring (Mitchell et al., 2012; Poojari et al., 2023; Roughead et al., 2017). Clarity around who is involved in particular aspects of care is pivotal, particularly for SMI, as care is often delivered by a multidisciplinary team (Ali et al., 2020; Aouira et al., 2022; Roughead et al., 2017). The majority of studies identified in this review did not describe the specific roles and responsibilities of various healthcare professionals on clinical care teams. Although studies have listed the specific healthcare professional(s) involved in the care of SMI, often, their roles and responsibilities have been poorly defined (Barnes et al., 2007, 2015, 2020; Batscha et al., 2010; Cotes et al., 2015; Fontaine et al., 2022; Gumber et al., 2010; Harrison et al., 2012; Hor et al., 2016; Mwebe et al., 2020; O’Callaghan et al., 2011; Ross et al., 2018; Stephenson et al., 2023; Thompson et al., 2011). Furthermore, none of the studies reported whether metabolic monitoring was mandated as part of routine care in the specific study setting. Most of the studies identified in this review involved interventional methods and therefore may not necessarily involve reporting on metabolic monitoring practices in a specific setting. In addition, word count limits imposed by journals could deter authors from reporting detailed metabolic screening and/or monitoring practices.
Only seven of the included studies reported the process involved in metabolic monitoring for SMI in a particular setting (Bomboy et al., 2021; Deuschle et al., 2013; Gumber et al., 2010; Kjeldsen et al., 2013; Lydon et al., 2021; Ross et al., 2018; Tatreau et al., 2016). Specific procedures and processes, such as whether blood samples were analysed onsite or off-site, were infrequently reported. Presumably, studies conducted within a hospital setting would have access to onsite pathology facilities, while primary care settings, such as general practices, would rely on offsite laboratories for analysis. However, this may not always be the case, such as in rural areas (Blattner et al., 2019). The absence of an onsite laboratory facility and services would reduce the accessibility and convenience of conducting and obtaining timely blood test results, therefore affecting metabolic monitoring rates. In addition, doctors had previously suggested that the lack of a notification system that alerts patients when blood test results are ready and/or when further action is required is a barrier to optimal metabolic monitoring (Aouira et al., 2022). Further attention is warranted to support healthcare delivery for vulnerable populations, including SMIs.
Aligned with the literature, our findings highlighted suboptimal metabolic monitoring for SMI (Ali et al., 2021, 2023; Mitchell et al., 2012). Among the studies included in this review, Lydon and colleagues recorded the highest rates of metabolic monitoring for patients with SMI who were taking clozapine (Lydon et al., 2021). This was not unexpected given the serious side effects and potential fatalities related to clozapine use (Kar et al., 2016). The prescribing and dispensing of clozapine are also associated with mandatory monitoring requirements in most countries, including Australia and the UK (Medicinewise, 2022; NHS Trust, 2023). However, monitoring of other SGAs requires further attention; for example, Keenan and colleagues reported that none of their study participants (n = 117) were fully monitored according to the RANCZP monitoring guidelines (Keenan et al., 2020). Previous reports had identified a number of barriers to routine metabolic monitoring, including patient-related barriers such as the perception of laboratory testing as aversive and intrusive (O’Brien & Abraham, 2021) and low levels of health literacy or awareness (S. Saloojee et al., 2014a, 2014b). The clinician-related factors identified included insufficient time (Kioko et al., 2016) and/or lack of reimbursement (Batscha et al., 2010). Future research initiatives to improve metabolic monitoring for SMI should consider previously identified, in addition to any other barriers that may be relevant to the particular setting or demographic. It is worth noting that given the variability across the studies reported (e.g. settings and countries), the perceived suboptimal monitoring in some contexts may be considered acceptable in others.
Our review revealed that parameters such as waist circumference and BMI are often poorly measured (Fig. 2). Barriers to frequent monitoring of these parameters, particularly waist circumference, have been previously explored in the literature (Hor et al., 2016; Mitchell et al., 2013; Verdoux et al., 2010). One study attributed low waist circumference monitoring rates to patients’ preferences for healthcare professionals of the same sex, who may not always be readily available (Hor et al., 2016). Verdoux and colleagues also cited sex differences as a potential barrier, noting that psychiatrists might be reluctant to perform physical examinations, especially for female patients, as such examinations can be “construed as invasive (such as waist measurement) (Verdoux et al., 2010).” There is a need to improve waist circumference monitoring rates in the SMI, particularly because it has been shown to be a useful predictor of MetSyn, with high rates of sensitivity and specificity (Mitchell et al., 2013).
The observed variation in metabolic monitoring rates between settings should be noted by clinicians and policymakers. At a practice level, clinicians should recognise that patients may not receive regular metabolic monitoring as suggested by existing guidelines. Consideration for the need and potential value of metabolic monitoring should be considered at patient interaction and a review should be initiated if deemed appropriate. Policymakers (e.g.in hospitals) should also facilitate frequent metabolic monitoring within their local settings. This can be achieved through local quality improvement efforts and implementation of mandatory clinical guidelines and/or policies at an organisational level, which can influence clinical practice. Clinical guidelines and/or policies must be tailored towards the needs of the particular site and consider other factors including staffing and resourcing requirements.
Strengths and limitations
A strength of this review was the methodologically rigorous search of published and grey literature in line with JBI recommendations. The search strategy, including its translation across databases, was guided by an academic librarian, and reviewed by the research team to ensure that a comprehensive and relevant search was conducted. However, this review included only English-language studies and was mostly based in the UK and US and from tertiary settings (hospital, inpatient and outpatient services). This skewed representation may reduce the generalisability of the present findings to other countries and settings.
Future Research
Despite the publication of practice guidelines, these findings suggest that metabolic monitoring in practice remains suboptimal, with procedures and processes varying between settings. As most of the studies included in this review did not report on the metabolic monitoring processes at the local site, we were unable to review and identify factors that influenced metabolic monitoring rates. Future research should consider the need to explore local metabolic monitoring practices, particularly to identify the barriers and facilitators relevant to the specific setting.
While this review echoes findings from previous research (Mitchell et al., 2012), it also highlighted the observed variation in metabolic monitoring. Future studies should also consider exploring the temporal trends to compare the prevalence of metabolic monitoring over the years.
Future practice guidelines should consider recommending and specifying the roles and responsibilities of healthcare professionals in metabolic monitoring for SMI. Researchers should clearly describe the local metabolic monitoring practices and/or procedures where possible to allow their findings to be appropriately interpreted and to facilitate the implementation of interventions in other settings where practices and/or procedures may differ. There is a need for ongoing research, particularly in the community setting, to promote increased accessibility to metabolic monitoring for SMI.
Conclusion
This scoping review mapped out the nuances of metabolic monitoring in practice. The most common settings, types of parameters measured, and health professionals involved in metabolic monitoring were summarised. The scoping review indicates that no streamlined approach toward metabolic monitoring currently exists, with variations observed between different settings and countries. This information highlights the need for a more systematic approach to metabolic monitoring for SGA patients with SMI. Our findings also highlight the need to clearly stipulate and define the roles and responsibilities of all health professionals involved in metabolic monitoring for SMI. Clinicians should be aware of the existing variations in metabolic monitoring and policymakers should consider the need to facilitate metabolic monitoring at an organisational level, taking into consideration existing resources and the specific needs of the organisation.
References
Al AdAwi, R. M., Stewart, D., Ryan, C., & Tonna, A. P. (2020). A systematic review of pharmacist input to metabolic syndrome screening, management and prevention. International Journal of Clinical Pharmacyd, 42, 995–1015.
Ali, R. S. A., Jalal, Z., Johal, J., & Paudyal, V. (2023). Guideline adherence for cardiometabolic monitoring of patients prescribed antipsychotic medications in UK primary care: A retrospective observational study. International Journal of Clinical Pharmacy. https://doi.org/10.1007/s11096-023-01642-5
Ali, R. A., Jalal, Z., & Paudyal, V. (2020). Barriers to monitoring and management of cardiovascular and metabolic health of patients prescribed antipsychotic drugs: A systematic review. BMC Psychiatry. https://doi.org/10.1186/s12888-020-02990-6
Ali, R. S. A., Jalal, Z., & Paudyal, V. (2021). Guidelines versus practice in screening and monitoring of cardiometabolic risks in patients taking antipsychotic medications: Where do we stand? General Psychiatry, 34(4), e100561.
American Diabetes Association. (2004). Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care, 27(2), 596–601.
Aouira, N., Khan, S., Heussler, H., Haywood, A., Karaksha, A., & Bor, W. (2022). Practitioners’ perspective on metabolic monitoring of second-generation antipsychotics: Existing gaps in knowledge, barriers to monitoring, and strategies. Journal of Child and Adolescent Psychopharmacology, 32(5), 296–303.
Arksey, H., & O’Malley, L. (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32.
Barnes, T. R. E., Bhatti, S. F., Adroer, R., & Paton, C. (2015). Screening for the metabolic side effects of antipsychotic medication: Findings of a 6-year quality improvement programme in the UK. British Medical Journal Open, 5(10), e007633.
Barnes, T. R. E., MacCabe, J. H., Kane, J. M., Delgado, O., & Paton, C. (2020). The physical health and side-effect monitoring of patients prescribed clozapine: data from a clinical audit conducted in UK mental health services. Therapeutic Advances in Psychopharmacology, 10, 204512532093790.
Barnes, T. R. E., Paton, C., Cavanagh, M.-R., Hancock, E., & Taylor, D. M. (2007). A UK audit of screening for the metabolic side effects of antipsychotics in community patients. Schizophrenia Bulletin, 33(6), 1397–1403.
Barnes, T. R. E., Paton, C., Hancock, E., Cavanagh, M. R., Taylor, D., & Lelliott, P. (2008). Screening for the metabolic syndrome in community psychiatric patients prescribed antipsychotics: A quality improvement programme. Acta Psychiatrica Scandinavica, 118(1), 26–33.
Batscha, C., Schneiderhan, M. E., Kataria, Y., Rosen, C., & Marvin, R. W. (2010). Treatment settings and metabolic monitoring for people experiencing first-episode psychosis. Journal of Psychosocial Nursing and Mental Health Services, 48(9), 44–49.
Bernardo, M., Rico-Villademoros, F., García-Rizo, C., Rojo, R., & Gómez-Huelgas, R. (2021). Real-world data on the adverse metabolic effects of second-generation antipsychotics and their potential determinants in adult patients: A systematic review of population-based studies. Advances in Therapy, 38(5), 2491–2512.
Blattner, K., Beazley, C. J., Nixon, G., Herd, G., Wigglesworth, J., & Rogers-Koroheke, M. G. (2019). The impact of the introduction of a point-of-care haematology analyser in a New Zealand rural hospital with no onsite laboratory. Rural and Remote Health, 19(2), 1–8.
Bobes, J., Alegria, A. A., Saiz-Gonzalez, M. D., Barber, I., Perez, J. L., & Saiz-Ruiz, J. (2011). Change in psychiatrists’ attitudes towards the physical health care of patients with schizophrenia coinciding with the dissemination of the consensus on physical health in patients with schizophrenia. European Psychiatry, 26(5), 305–312.
Bomboy, K. T., Graber, J. S., & Wallis, E. P. (2021). Improved prescriber adherence to guidelines on antipsychotic medication management through increased access to metabolic monitoring forms. Journal of the American Psychiatric Nurses Association, 27(2), 162–168.
Butler, P., Simonson, C., Goldie, C., Kennedy, A., & Goldstone, L. W. (2013). Baseline metabolic monitoring of atypical antipsychotics in an inpatient setting [Article]. Mental Health Clinician, 3(3), 122–128. https://doi.org/10.9740/mhc.n166819
Castle, D. J., Galletly, C. A., Dark, F., Humberstone, V., Morgan, V. A., Killackey, E., Kulkarni, J., McGorry, P., Nielssen, O., & Tran, N. T. (2017). The 2016 Royal Australian and New Zealand College of psychiatrists guidelines for the management of schizophrenia and related disorders. The Medical Journal of Australia, 206(11), 501–505.
Chee, G.-L., Wynaden, D., & Heslop, K. (2017). Improving metabolic monitoring rate for young people aged 35 and younger taking antipsychotic medications to treat a psychosis: A literature review. Archives of Psychiatric Nursing, 31(6), 624–633.
Coakley, C., Bolton, P., Flaherty, L., Kopeski, L. M., Slifka, K., & Sutherland, M. A. (2012). The incidence of metabolic risk factors in an inpatient psychiatric setting. Journal of Psychosocial Nursing and Mental Health Services, 50(3), 24–30.
Cohn, T. A., & Sernyak, M. J. (2006). Metabolic Monitoring for patients treated with antipsychotic medications. The Canadian Journal of Psychiatry / La Revue Canadienne De Psychiatrie, 51(8), 492–501.
Cotes, R. O., de Nesnera, A., Kelly, M., Orsini, K., Xie, H., McHugo, G., Bartels, S., & Brunette, M. F. (2015). Antipsychotic cardiometabolic side effect monitoring in a state community mental health system. Community Mental Health Journal, 51(6), 685–694.
Cunningham, C., Riano, N. S., & Mangurian, C. (2018). Screening for metabolic syndrome in people with severe mental illness. Journal of Clinical Outcomes Management, 25(1).
D’Souza, R. S., & Hooten, W. M. (2021). Extrapyramidal symptoms. StatPearls.
Dekker, J. M., Girman, C., Rhodes, T., Nijpels, G., Stehouwer, C. D. A., Bouter, L. M., & Heine, R. J. (2005). Metabolic syndrome and 10-year cardiovascular disease risk in the hoorn study. Circulation, 112(5), 666–673.
Deuschle, M., Paul, F., Brosz, M., Bergemann, N., Franz, M., Kammerer-Ciernioch, J., Lautenschlager, M., Lederbogen, F., Roesch-Ely, D., Weisbrod, M., Kahl, K. G., Reichmann, J., Gross, J., & Umbreit, J. (2013). Assessment of cardiovascular disease risk in patients with schizophrenia spectrum disorders in German psychiatric hospitals: Results of the pharmacoepidemiologic CATS study. Social Psychiatry and Psychiatric Epidemiology, 48(8), 1283–1288.
Feeney, L., & Mooney, M. (2005). Atypical antipsychotic monitoring in the kilkenny mental health services [Article]. Irish Journal of Psychological Medicine, 22(3), 101–102. https://doi.org/10.1017/S0790966700009113
Fontaine, J., Chin, E., Provencher, J. F., Rainone, A., Wazzan, D., Roy, C., Rej, S., Lordkipanidze, M., & Dagenais-Beaule, V. (2022). Assessing cardiometabolic parameter monitoring in inpatients taking a second-generation antipsychotic: The CAMI-SGA study-a cross-sectional study. British Medical Journal Open, 12(4), e055454.
Gumber, R., Abbas, M., & Minajagi, M. (2010). Monitoring the metabolic side-effects of atypical antipsychotics. The Psychiatrist, 34(9), 390–395.
Harrison, M. R., McMillan, C. F., & Dickinson, T. (2012). Service innovation: A comparison of two approaches for physical screening of psychiatric inpatients. International Journal of Psychiatry in Clinical Practice, 16(2), 157–160.
Hasan, A., Falkai, P., Wobrock, T., Lieberman, J., Glenthoj, B., Gattaz, W. F., Thibaut, F., & Möller, H.-J. (2013). World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. The World Journal of Biological Psychiatry, 14(1), 2–44.
Heiberg, I. H., Jacobsen, B. K., Balteskard, L., Bramness, J. G., Næss, Ø., Ystrom, E., Reichborn-Kjennerud, T., Hultman, C. M., Nesvåg, R., & Høye, A. (2019). Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatrica Scandinavica, 139(6), 558–571.
Holt, R. I. G., Abdelrahman, T., Hirsch, M., Dhesi, Z., George, T., Blincoe, T., & Peveler, R. C. (2010). The prevalence of undiagnosed metabolic abnormalities in people with serious mental illness. Journal of Psychopharmacology, 24(6), 867–873.
Hor, E. S. L., Subramaniam, S., Koay, J. M., Bharathy, A., Vasudevan, U., Panickulam, J. J., Ng, I. T., Arif, N. H., & Russell, V. (2016). Improving metabolic monitoring in patients maintained on antipsychotics in Penang. Malaysia. Australasian Psychiatry, 24(1), 67–71.
Kar, N., Barreto, S., & Chandavarkar, R. (2016). Clozapine monitoring in clinical practice: Beyond the mandatory requirement [Review]. Clinical Psychopharmacology and Neuroscience, 14(4), 323–329.
Keenan, R., Chepulis, L., Ly, J., Carter, S., Lao, C., Asim, M., Bhat, A., Deo, S., Lim, K. P., Mohammed, R., Scarlet, S., & Lawrenson, R. (2020). Metabolic screening in primary care for patients with schizophrenia or schizoaffective disorder and taking antipsychotic medication. Journal of Primary Health Care, 12(1), 29–34. https://doi.org/10.1071/HC19023
Kelly, J. R., Gounden, P., McLoughlin, A., Legris, Z., O’Carroll, T., McCafferty, R., Marques, L., Haran, M., Farrelly, R., Loughrey, K., Flynn, G., Corvin, A., & Dolan, C. (2022). Minding metabolism: Targeted interventions to improve cardio-metabolic monitoring across early and chronic psychosis. Irish Journal of Medical Science, 191(1), 337–346.
Kilbourne, A. M., Post, E. P., Bauer, M. S., Zeber, J. E., Copeland, L. A., Good, C. B., & Pincus, H. A. (2007). Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. Journal of Affective Disorders, 102(1–3), 145–151.
Kioko, E., Williams, K., & Newhouse, B. (2016). Improving metabolic syndrome screening on patients on second generation antipsychotic medication. Archives of Psychiatric Nursing, 30(6), 671–677.
Kjeldsen, L. J., Hansen, P. S., Kristensen, A. M. F., Christensen, A., Sorensen, C. H., & Nielsen, B. (2013). Outreach visits by clinical pharmacists improve screening for the metabolic syndrome among mentally ill patients. Nordic Journal of Psychiatry, 67(4), 249–257.
Knudsen, L., Hansen, D. L., Joensen, L. E., Wibaek, R., Benros, M. E., Jorgensen, M. E., & Andersen, G. S. (2022). Need for improved diabetes support among people with psychiatric disorders and diabetes treated in psychiatric outpatient clinics: Results from a danish cross-sectional study. BMJ Open Diabetes Research and Care, 10(1), e002366.
Lau, G., Murphy, J., Chaudhury, N., & Agius, M. (2019). Physical health checks in patients on antipsychotic medication. Psychiatria Danubina, 31(Suppl 3), 608–612.
Lawrence, D., Kisely, S., & Pais, J. (2010). The epidemiology of excess mortality in people with mental illness. The Canadian Journal of Psychiatry, 55(12), 752–760.
Liu, N. H., Daumit, G. L., Dua, T., Aquila, R., Charlson, F., Cuijpers, P., Druss, B., Dudek, K., Freeman, M., & Fujii, C. (2017). Excess mortality in persons with severe mental disorders: A multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry, 16(1), 30–40.
Lydon, A., Vallely, J., Tummon, A., Maher, S., Sabri, S., McLoughlin, J., Liew, A., McDonald, C., & Hallahan, B. (2021). Routine screening and rates of metabolic syndrome in patients treated with clozapine and long-acting injectable antipsychotic medications: A cross-sectional study. Irish Journal of Psychological Medicine, 38(1), 40–48.
Mackin, P., Bishop, D. R., & Watkinson, H. M. (2007). A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry, 7(1), 28.
Marsay, C., & Szabo, C. P. (2010). A retrospective review of state sector outpatients (tara hospital) prescribed olanzapine: Adherence to metabolic and cardiovascular screening and monitoring guidelines [Conference Abstract]. South African Journal of Psychiatry, 16(3), 107–108.
Mead, L., Ayres, A., Blake, J. A., & Scott, J. G. (2021). Monitoring of metabolic side-effects in children and adolescents prescribed antipsychotic medication: A systematic review. Australian and New Zealand Journal of Psychiatry, 55(8), 763–771.
Medicinewise, N. (2022). Clozaril (Clozapine) Retrieved 5 August from https://www.nps.org.au/medicine-finder/clozaril-tablets#full-pi
Melamed, O. C., Wong, E. N., LaChance, L. R., Kanji, S., & Taylor, V. H. (2019). Interventions to improve metabolic risk screening among adult patients taking antipsychotic medication: A systematic review. Psychiatric Services, 70(12), 1138–1156.
Michael, S., & MacDonald, K. (2020). Improving rates of metabolic monitoring on an inpatient psychiatric ward. BMJ Open Quality, 9(3), e000748.
Mitchell, A. J., Delaffon, V., Vancampfort, D., Correll, C. U., & De Hert, M. (2012). Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: Systematic review and meta-analysis of screening practices. Psychological Medicine, 42(1), 125–147.
Mitchell, A. J., Vancampfort, D., Sweers, K., Van Winkel, R., Yu, W., & De Hert, M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis [Review]. Schizophrenia Bulletin, 39(2), 306–318. https://doi.org/10.1093/schbul/sbr148
Mittal, D., Li, C., Viverito, K., Williams, J. S., Landes, R. D., Thapa, P. B., & Owen, R. (2014). Monitoring for metabolic side effects among outpatients with dementia receiving antipsychotics. Psychiatric Services, 65(9), 1147–1153.
Mwebe, H. P., Volante, M., & Weaver, T. (2020). Monitoring cardiovascular disease risk in individuals with severe mental illness in an inpatient mental health setting: A secondary data analysis. British Journal of Cardiac Nursing, 15(11), 1–15. https://doi.org/10.12968/bjca.2020.0157
Najim, H., & Islam, N. (2013). Checking physical care of people on risperidone long term injectable depot [Psychological Disorders 3210]. Psychiatria Danubina, 25(Suppl 2), 171–173.
Nguyen, D., Brakoulias, V., & Boyce, P. (2009). An evaluation of monitoring practices in patients on second generation antipsychotics. Australasian Psychiatry, 17(4), 295–299.
O’Brien, A. J., & Abraham, R. M. (2021). Evaluation of metabolic monitoring practices for mental health consumers in the Southern district health board region of New Zealand. Journal of Psychiatric and Mental Health Nursing, 28(6), 1005–1017.
O’Callaghan, C., Liew, A. Y. L., Yusof, M. S. D., Duffy, R., Breen, E. G., Kinsley, B., & Kelly, B. D. (2011). Screening for metabolic syndrome in long-term psychiatric illness: Audit of patients receiving depot antipsychotic medication at a psychiatry clinic. The European Journal of Psychiatry, 25(4), 213–222.
Pearsall, R., Shaw, R. J., McLean, G., Connolly, M., Hughes, K. A., Boyle, J. G., Park, J., Smith, D. J., & Mackay, D. (2019). Health screening, cardiometabolic disease and adverse health outcomes in individuals with severe mental illness. Bjpsych Open, 5(6), e97.
Penninx, B. W., & Lange, S. M. (2018). Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues in Clinical Neuroscience, 20(1), 63.
Pereira, L., Budovich, A., & Claudio-Saez, M. (2019). Monitoring of metabolic adverse effects associated with atypical antipsychotics use in an outpatient psychiatric clinic. Journal of Pharmacy Practice, 32(4), 388–393.
Peters, M. D., Marnie, C., Tricco, A. C., Pollock, D., Munn, Z., Alexander, L., McInerney, P., Godfrey, C. M., & Khalil, H. (2021). Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Implementation, 19(1), 3–10.
Pollock, D., Peters, M. D. J., Khalil, H., McInerney, P., Alexander, L., Tricco, A. C., Evans, C., de Moraes, É. B., Godfrey, C. M., Pieper, D., Saran, A., Stern, C., & Munn, Z. (2023). Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth, 21(3), 520–532. https://doi.org/10.11124/JBIES-22-00123
Poojari, P. G., Khan, S. A., Shenoy, S., Acharya, L. D., Shetty, S., Bose, S., Pai, K., Kunhikatta, V., & Thunga, G. (2020). Identification of risk factors and metabolic monitoring practices in patients on antipsychotic drugs in South India. Asian Journal of Psychiatry, 53, 102186.
Poojari, P. G., Khan, S., Shenoy, S., Shetty, S., Bose, S., Pai, K., Acharya, L. D., & Thunga, G. (2022). A narrative review of metabolic monitoring of adult prescribed second-generation antipsychotics for severe mental illness. Clinical Epidemiology and Global Health, 15, 101035.
Poojari, P. G., Khan, S., Shenoy, S., Shetty, S., Pai, K., Acharya, L. D., Bose, S., & Thunga, G. (2023). Perspectives of patients and healthcare professionals on metabolic monitoring of adult prescribed second-generation antipsychotics for severe mental illness: A meta-synthesis. PLoS ONE, 18(4), e0283317.
Ross, E., Barnett, R., Tudhope, R., & Vasudev, K. (2018). Can We Improve Physical Health Monitoring for Patients Taking Antipsychotics on a Mental Health Inpatient Unit? Journal of Clinical Psychopharmacology, 38(5), 447–453.
Roughead, L., Procter, N., Westaway, K., Sluggett, J., & Alderman, C. (2017). Medication safety in mental health. ACSQHC.
Saloojee, S., Burns, J. K., & Motala, A. A. (2014). Very low rates of screening for metabolic syndrome among patients with severe mental illness in Durban, South Africa. BMC Psychiatry, 14
Saloojee, S., Burns, J. K., & Motala, A. A. (2014a). Low frequency of screening for metabolic syndrome in patients with severe mental illness at a general hospital psychiatric unit in Durban [Conference Abstract]. South African Journal of Psychiatry, 20(3), 109.
Scott, D., & Happell, B. (2011). The high prevalence of poor physical health and unhealthy lifestyle behaviours in individuals with severe mental illness. Issues in Mental Health Nursing, 32(9), 589–597.
Solmi, M., Fiedorowicz, J., Poddighe, L., Delogu, M., Miola, A., Høye, A., Heiberg, I. H., Stubbs, B., Smith, L., & Larsson, H. (2021). Disparities in screening and treatment of cardiovascular diseases in patients with mental disorders across the world: Systematic review and meta-analysis of 47 observational studies. American Journal of Psychiatry, 178(9), 793–803.
Stephenson, E., Yusuf, A., Gronsbell, J., Tu, K., Melamed, O., Mitiku, T., Selby, P., & O’Neill, B. (2023). Disruptions in primary care among people with schizophrenia in Ontario, Canada, during the COVID-19 pandemic. The Canadian Journal of Psychiatry, 68(6), 426–435.
Sud, D., Laughton, E., McAskill, R., Bradley, E., & Maidment, I. (2021). The role of pharmacy in the management of cardiometabolic risk, metabolic syndrome and related diseases in severe mental illness: A mixed-methods systematic literature review. Systematic Reviews, 10(1), 92.
Tan, X. W., Chan, C. Y. W., Lum, A. W. M., Lee, E. S., Mok, Y. M., Fung, D. S. S., & Tor, P. C. (2022). Association of cardiovascular metabolic risk factor measurements with psychiatric readmission among in-hospital patients with severe mental illness: A retrospective study. BMC Psychiatry. https://doi.org/10.1186/s12888-022-03704-w
Tatreau, J. R., Harris, S., Sheitman, B., & Steiner, B. D. (2016). Cardiometabolic assessment, diagnosis, and treatment of chronic medical illnesses during an inpatient psychiatric hospitalization: Colocated medical care versus treatment as usual. Primary Care Companion to the Journal of Clinical Psychiatry. https://doi.org/10.4088/PCC.16m02017
Thompson, A., Hetrick, S. E., Alvarez-Jimenez, M., Parker, A. G., Willet, M., Hughes, F., Gariup, M., Gomez, D. L., & McGorry, P. D. (2011). Targeted intervention to improve monitoring of antipsychotic-induced weight gain and metabolic disturbance in first episode psychosis. Australian and New Zealand Journal of Psychiatry, 45(9), 740–748.
Tricco, A. C., Lillie, E., Zarin, W., O’Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D., Horsley, T., & Weeks, L. (2018). PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine, 169(7), 467–473.
NHS Trust. (2023). Clinical Protocol for the Safe Use of Clozapine
Verdoux, H., Boulon, S., & Cougnard, A. (2008). Gender differences in metabolic monitoring of second-generation antipsychotic prescription. Human Psychopharmacology: Clinical and Experimental, 23(6), 471–474.
Verdoux, H., Tournier, M., & Begaud, B. (2010). Antipsychotic prescribing trends: A review of pharmaco-epidemiological studies. Acta Psychiatrica Scandinavica, 121(1), 4–10.
Viglione, L., & Short, B. L. (2021). Metabolic screen and intervene: Improving mental health inpatient metabolic monitoring. Australasian Psychiatry, 29(3), 289–293.
Wichniak, A., Dudek, D., Heitzman, J., Kaplon-Cieslicka, A., Mamcarz, A., Samochowiec, J., Szulc, A., & Bienkowski, P. (2019). Metabolic risk reduction in patients with schizophrenia treated with antipsychotics: Recommendations of the polish psychiatric association. Psychiatria Polska, 53(6), 1191–1218.
Acknowledgements
Author TB wishes to acknowledge the Australian Government for the Research Training Program domestic (RTPd) fee offset scholarship and the University of South Australia for the Postgraduate Award (USAPA). The authors also thank Dr Fiona Kelly for her assistance in drafting of the scoping review protocol and provision of expert advice.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Not applicable.
Author information
Authors and Affiliations
Contributions
TB was involved in the conception and design of the work; and the acquisition, analysis, or interpretation of data for the work and drafted the manuscript. RA, VS, TB and JJ were involved in the screening of the studies. TB and RA were involved in the data extraction. All authors read and review the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bui, T.N.T., Au, R.T., Janetzki, J.L. et al. Metabolic Monitoring for Adults Living with a Serious Mental Illness on a Second-Generation Antipsychotic Agent: A Scoping Review. Adm Policy Ment Health (2024). https://doi.org/10.1007/s10488-024-01408-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10488-024-01408-9