Abstract
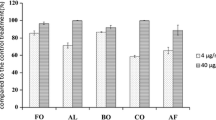
Understanding the role of chemotaxis in ecological interactions between plants and microbes in the rhizosphere is necessary to optimize biocontrol strategies targeting plant soil-borne diseases. Therefore, we examined and profiled the antagonistic endophytic bacteria (AEB) population with chemotaxis potential in the medicinal plant Panax notoginseng using a cheA gene-based approach coupled with 16S rRNA sequencing. Phylogenetic analysis of the chemotactic AEB (CAEB) community in P. notoginseng enabled the identification of 56 CAEB strains affiliated with 30 species of Actinobacteria, Firmicutes, and Proteobacteria; Firmicutes, especially Bacillus, were predominant. We then systematically quantified the chemotactic response profiles of CAEB toward five organic acid (OA) attractants: citric acid, fumaric acid (FA), malic acid, oxalic acid, and succinic acid. Further hierarchical cluster analysis revealed that the chemotaxis of CAEB to the same attractant exhibited different patterns among not only genera but also species and even strains of the same species. Following chemotaxis and hierarchical analysis, we selected the strongest chemoattractant, fumaric acid (FA), as the target for evaluating the effects of OAs on the representative CAEB strain Bacillus amyloliquefaciens subsp. plantarum YP1. Application of FA significantly stimulated the chemotaxis ability and growth of YP1, and increased the transcript levels of cheA and biocontrol-related genes in YP1. This is the first study to characterise the diversity of chemotaxis profiles toward OAs in natural bacterial assemblages of P. notoginseng and to highlight how FA promotes the biocontrol-related traits of P. notoginseng-associated CAEB.







Similar content being viewed by others
Data availability
The 16S rRNA gene sequences that support the findings of this study have been deposited in GenBank with the accession numbers listed in Table 1 shown in the manuscript.
References
Abdallah DB, Frikhagargouri O, Tounsi S (2018) Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol Control 124:61–67
Adler J (1973) A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. Microbiol 74:77–91
Allardmassicotte R, Tessier L, Lecuyer F, Lakshmanan V, Lucier J, Garneau D, Caudwell I, Vlamakis H, Bais HP, Beauregard PB (2016) Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. Mbio 7:e01664-e1716
Baermann G (1917) ine eifache methode zur auffindung von anklyostomum (Nematoden) larven in erdproben. Geneeskd Tijdschr Voor Ned-Indië 57:131–137
Bais HP, Fall R, Vivanco JM (2004) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringaeis facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Barret M, Morrissey JP, O’Gara F (2011) Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol Fertil Soils 47:729–743
Bi S, Jin F, Sourjik V (2018) Inverted signaling by bacterial chemotaxis receptors. Nat Commun 9:2927
Buchan A, Crombie B, Alexandre G (2010) Temporal dynamics and genetic diversity of chemotactic-competent microbial populations in the rhizosphere. Environ Microbiol 12:3171–3184
Chen YJ, Wang YY, Feng GQ, Li ZY (2001) Relationship between root rot of Panax notoginseng and ecological conditions. Yunnan Agric Sci Technol 6:33–35
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo J (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15:848–864
Compant S, Clement C, Sessitsc A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization. Soil Biol Biochem 42:669–678
De Silva NI, Brooks S, Lumyong S, Hyde KD (2019) Use of endophytes as biocontrol agents. Fungal Biol Rev 33:133–148
Deng ZS, Zhao LF, Kong ZY, Yang WQ, Lindstrom K, Wang ET, Wei G (2011) Diversity of endophytic bacteria within nodules of the Sphaerophysa salsula in different regions of Loess Plateau in China. FEMS Microbiol Ecol 76:463–475
el Zahar HF, Santaella C, Heuli AW (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80
Greer-Phillips SE, Alexandre G, Taylor BL, Zhulin IB (2003) Aer and Tsr guide Escherichia coli in spatial gradients of oxidizable substrates. Microbiol 49:2661–2667
Guo HB, Cui XM, An N, Cai GP (2010) Sanchi ginseng (Panax notoginseng (Burkill) F.H. Chen) in China: distribution, cultivation and variations. Genet Resour Crop Evolut 57:453–460
Jones DL (1998) Organic acids in the rhizosphere: a critical review. Plant Soil 205:25–44
Joshi R, Gardener BB (2006) Identification and characterization of novel genetic markers associated with biological control activities in Bacillus subtilis. Phytopathol 96:145–154
Khare E, Mishra J, Arora NK (2018) Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 15:2732
Kim OS, Cho YJ, Lee K, Yoon SH, Kim MNaH, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
King EO, Ward M, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Li P, Ma L, Feng YL, Mo MH, Yang FX, Dai HF, Zhao YX (2012) Diversity and chemotaxis of soil bacteria with antifungal activity against Fusarium wilt of banana. J Ind Microbiol Biot 39:1495–1505
Li Z, Min Q, Sun JJ, Zu Y (2015) Effect of as stress on the growth, the root as contents and root exudates in 2-year-old Panax notoginseng. J Beijing Univ Agric 30:86–91
Ling N, Raza W, Ma J, Huang Q, Shen Q (2011) Identification and role of organic acids in watermelon root exudates for recruiting Paenibacillus polymyxa SQR-21 in the rhizosphere. Eur J Soil Biol 47:374–379
Liu F, Wen XS (2006) Progress in relationship between root exudates and rhizospheric microorganism. Food Drug 8:37–40
Liu X, Zhang K, Liu Y, Xie Z, Zhang C (2019) Oxalic acid from sesbania rostrata seed exudates mediates the chemotactic response of Azorhizobium caulinodans ORS571 using multiple strategies. Front Microbiol 10:2727
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Luo WF, Yu SF, He CF, Li ZY, Wang CL, Cui XM (1997) On the combined infection of root rot pathogens on Panax notoginseng. Acta Phytopathol Sin 27:85–91
Ma L, Cao YH, Cheng MH, Huang Y, Mo MH, WangYang YJZ, Yang FX (2013) Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie Van Leeuwenhoek 103:299–312
Ma L, Zheng SC, Zhang TK, Liu ZY, Wang XJ, Zhou XK, Yang CG, Duo JL, Mo MH (2018) Effect of nicotine from tobacco root exudates on chemotaxis, growth, biocontrol efficiency, and colonization by Pseudomonas aeruginosa NXHG29. Antonie Van Leeuwenhoek 111:237–1257
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2017) Cluster: cluster analysis basics and extensions. R package version 2.0. 6. https://www.r-project.org/.
Maidak BL, Olsen GJ, Larsen N, Overbeek R, Mc-Caughey MJ, Woese CR (1997) The RDP (ribosomal database project). Nucleic Acids Res 25:109–110
Mao ZS, Long YJ, Zhu SS, Chen ZJ, Wei FG, Zhu YY (2013) Advances in root rot pathogen of Panax notoginseng research. J Chin Med Mater 36:2051–2054
Miao ZQ, Li SD, Liu XZ, Hen YJ, Li YH, Wang Y, Guo RJ, Xia ZY, Zhang KQ (2006) The causal microorganisms of Panax notoginseng root-rot disease. Sci Agric Sin 39:1371–1378
Miller LD, Russell MH, Alexandre G (2009) Diversity in bacterial chemotactic responses and niche adaptation. Adv Appl Microbiol 66:53–75
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Raina J, Fernandez V, Lambert BS, Stocker R, Seymour JR (2019) The role of microbial motility and chemotaxis in symbiosis. Nat Rev Microbiol 17:284–294
Repik A, Rebbapragada A, Johnson MS, Haznedar JO, Zhulin IB, Taylor BL (2000) PAS domain residues involved in signal transduction by the Aer redox sensor of Escherichia coli. Mol Microbiol 36:806–816
Rosenblueth M, Martínez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19:827–837
Rudrappa T, Czymmek KJ, Paré PW, Bais HP (2008) Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol 148:1547–1556
Samanta SK, Bhushan B, Chauhan A, Jain RK (2000) Chemotaxis of a Ralstonia sp. SJ98 toward different nitroaromatic compounds and their degradation. Biochem Biophys Res Co 269:117–123
Schreiter S, Babin D, Smalla K, Grosch R (2018) Rhizosphere competence and biocontrol effect of Pseudomonas sp. RU47 independent from plant species and soil type at the field scale. Front Microbiol 9:97
Sha MC, Zhou YF, Zhang HZ, Li Y, Qin Q (2018) Study on differences of chemical components in different parts of Panax notoginseng. Mod Chin Med 20:832–836
Singh N, Kumar S, Bajpai VK, Dubey RC, Maheshwari DK, Kang SC (2010) Biological control of Macrophomina phaseolina by chemotactic fluorescent Pseudomonas aeruginosa PN1 and its plant growth promotory activity in chir-pine. Crop Prot 29:1142–1147
Tan S, Yang C, Mei X, Shen S, Raza W, Shen Q, Xu Y (2013) The effect of organic acids from tomato root exudates on rhizosphere colonization of Bacillus amyloliquefaciens T-5. Appl Soil Ecol 64:15–22
Wang CL, Cui XM, Li ZY, He CF, Yu SF, Luo WF (1998) Studies on relationship between root rot on Panax notoginseng Burk. F. H. Chen and its environmental conditions. Chin J Chin Mater Med 23:714–716
Wang Y, Lu ZX, Wu H, Lv FX (2009) Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int J Food Microbiol 136:71–74
Wu H, Wu L, Zhu Q, Wang J, Qin X, Xu J, Kong L, Chen J, Lin S, Khan MU, Amjad H, Lin W (2017) The role of organic acids on microbial deterioration in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Sci Rep 7:1–13
Wu K, Fang Z, Guo R, Pan B, Shi W, Yuan S, Guan H, Gong M, Shen B, Shen Q (2015) Pectin enhances bio-control efficacy by inducing colonization and secretion of secondary metabolites by Bacillus amyloliquefaciens SQY 162 in the rhizosphere of tobacco. PLoS Ones 10:e0127418
Xu C, Wang W, Wang B, Zhang T, Cui X, Pu Y, Li N (2019) Analytical methods and biological activities of Panax notoginseng saponins: recent trends. J Ethnopharmacol 236:443–465
Yaish MW, Antony I, Glick BR (2015) solation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 107:1519–1532
Yang W, Briegel A (2020) Diversity of bacterial chemosensory arrays. Trends Microbiol 28:68–80
Yuan J, Zhang N, Huang Q, Raza W, Li R, Vivanco JM, Shen Q (2015) Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci Rep 5:13438
Zhang N, Wang D, Liu Y, Li S, Shen Q, Zhang R (2013) Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 374:689–700
Zhang F, Meng X, Yang X, Ran W, Shen Q (2014) Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol Bioch 83:250–257
Acknowledgements
This work was supported by the NSFC (31960022, 32170131, 31870091), the Department of Science and Technology of Yunnan Province (202001BB050072, 2019ZG00901, YNWR-CYJS-2019-042).
Author information
Authors and Affiliations
Contributions
LM wrote the manuscript and funding acquisition, designed the article. W-QW analysed data, drew the figures of this manuscript. RS and X-MZ revised the manuscript, collected the literature, XL and Y-SY added references and participated in revising the manuscript. MHM contributed to the writing of the manuscript, suggested and added references, critically revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, L., Wang, WQ., Shi, R. et al. Effects of organic acids on the chemotaxis profiles and biocontrol traits of antagonistic bacterial endophytes against root-rot disease in Panax notoginseng. Antonie van Leeuwenhoek 114, 1771–1789 (2021). https://doi.org/10.1007/s10482-021-01636-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-021-01636-1