Abstract
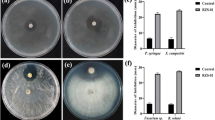
Plant endophytes play important roles in biocontrol of plant diseases. Actinomycetes are used for biocontrol of fungal diseases caused by Verticillium dahliae. Many studies have focused on the endophytic actinomycetes isolated from the roots of healthy plants, but few on those from the roots of diseased plants. In the present research, actinomycetes were isolated from the roots of diseased and healthy tomato plants, respectively. The results showed that, in total, 86 endophytic actinomycetes were isolated for screening of their antimicrobial activities, 8 of which showed antagonism to V. dahliae in vitro. Among the 8 antagonistic strains, 5 (out of 36) were from the roots of diseased plants, with inhibition diameter zones ranging from 11.2 to 18.2 mm, whereas 3 (out of 50) were from the roots of healthy plants, with inhibition diameter zones ranging from 11.5 to 15.5 mm. Endophytic strain DHV3-2 was isolated from the root of a diseased plant and demonstrated a potent effect against V. dahliae and other pathogenic fungi by showing the largest inhibition diameter zones among all the eight antagonistic strains. Thus, strain DHV3-2 was chosen to investigate its biological control efficacies in vivo. Further study showed that the disease incidence and disease severity indices of tomato Verticillium wilt decreased significantly (P < 0.05). We also found that the plant shoot fresh weight and height increased greatly (P < 0.05) upon treatment with strain DHV3-2 compared to the plants uninoculated in greenhouse conditions. Root colonization showed that strain DHV3-2 had the higher root-colonizing capacity in the roots of infected plants compared with the roots of healthy plants. This isolate was identified as Streptomyces sp. based on morphological characteristics and 16S rRNA gene analysis. In conclusion, the roots of diseased tomato plants are a potential reservoir of biological control actinomycetes, and Streptomyces sp. strain DHV3-2 is a potential biocontrol agent against V. dahliae and growth elicitor in tomato.




Similar content being viewed by others
References
Atlas RM (1993) Handbook of microbiological media. Edited by Parks LC, CRC Press, Boca Raton
Bais HP, Prithiviraj B, Jha AK, Ausubel FM, Vivanco JM (2005) Mediation of pathogen resistance by exudation of antimicrobials from roots. Nature 434:217–221
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bonaldi M, Chen X, Kunova A, Pizzatti C, Saracchi M, Cortesi P (2015) Colonization of lettuce rhizosphere and roots by tagged Streptomyces. Front Microbiol 6:25
Bull CT, Weller DM, Thomashow LS (1991) Relationship between root colonization and suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens strain 2-79. Phytopathology 81:954–959
Chai Y, Zhao L, Liao Z, Sun X, Zuo K, Zhang L, Tang K (2003) Molecular cloning of a potential Verticillium dahliae resistance gene SlVe 1 with multi-site polyadenylation from Solanum licopersicoides. DNA Seq 14:375–384
Cook RJ, Ownley BH, Zhang H, Vakoch D (2000) Influence of paired-row spacing and fertilizer placement on yield and root diseases of direct-seeded wheat. Crop Sci 40:1079–1087
Dijk KV, Nelson EB (2000) Fatty acid competition as a mechanism by which Enterobacter cloacae suppresses Pythium ultimum sporangium germination and damping-off. Appl Environ Microbiol 66:5340–5347
Faheem M, Raza W, Zhong W, Nan Z, Shen Q, Xu Y (2015) Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum, f. sp. niveum. Biol Control 81:101–110
Fang ZD (1998) Research methodology for plant diseases, 3rd edn. Chinese Agriculture Press, Beijing
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fradin EF, Zhang Z, Ayala JCJ, Castroverde CD, Nazar RN, Robb J, Liu CM, Thomma BP (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332
Fradin EF, Abd-El-Haliem A, Masini L, Berg GCvd, Joosten MH, Thomma BP (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol 156:2255–2265
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phitol 157:493–502
Fu X, Wu X, Zhou X, Liu S, Shen Y, Wu F (2015) Companion cropping with potato onion enhances the disease resistance of tomato against Verticillium dahliae. Front Plant Sci 6:726
Gao W, Long L, Zhu LF, Xu L, Gao WH, Sun LQ, Liu LL, Zhang XL (2013) Proteomic and virus-induced gene silencing (VIGS) analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol Cell Proteom 12:3690–3703
Hayakawa M, Nonomura H (1987) Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Technol 65:501–509
Hossain MM, Sultana F, Kubota M, Hyakumachi M (2008) Differential inducible defense mechanisms against bacterial speck pathogen in Arabidopsis thaliana by plant-growth-promoting-fungus Penicillium sp. GP16-2 and its cell free filtrate. Plant Soil 304:227–239
Huang J, Wei Z, Tan S, Mei X, Yin S, Shen Q, Xu Y (2013) The rhizosphere soil of diseased tomato plants as a source for novel microorganisms to control bacterial wilt. Appl Soil Ecol 72:79–84
Kaewkla O, Franco CM (2013) Rational approaches to improving the isolation of endophytic actinobacteria from Australian native trees. Microbial Ecol 65:384–393
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV (2009) Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol 47:39–62
Lemessa F, Zeller W (2007) Screening rhizobacteria for biological control of Ralstonia solanacearum in Ethiopia. Biol Control 42:336–344
Maldonado-González MM, Bakker PA, Prieto P, Mercado-Blanco J (2015) Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front Microbiol 6:266
Messiha N, Diepeningen ADV, Farag NS, Abdallah SA, Janse JD, Bruggen AHCv (2007) Stenotrophomonas maltophilia: a new potential biocontrol agent of Ralstonia solanacearum, causal agent of potato brown rot. Eur J Plant Pathol 118:211–225
Okubara PA, Kornoely JP, Landa BB (2004) Rhizosphere colonization of hexaploid wheat by Pseudomonas fluorescens strains Q8r1-96 and Q2-87 is cultivar-variable and associated with changes in gross root morphology. Biol Control 30:392–403
Olivain C, Humbert C, Nahalkova J, Fatehi J, L’Haridon F, Alabouvette C (2006) Colonization of tomato root by pathogenic and nonpathogenic Fusarium oxysporum strains inoculated together and separately into the soil. Appl Environ Microbiol 72:1523–1531
Passari AK, Mishra VK, Saikia R, Gupta VK, Singh BP (2015) Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front microbiol 6:273
Pegg GF, Brady BL (2002) Verticillium wilts. CABI, Wallingford
Rybakova D, Cernava T, Köberl M, Liebminger S, Etemadi M, Berg G (2015) Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil 2015:1–16
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schippers B, Bakker AW, Bakker P (1987) Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annu Rev Phytopathol 25:339–358
Schneider R (1984) Effects of nonpathogenic strains of Fusarium oxysporum on celery root infection by F. oxysporum f. sp. apii and a novel use of the Lineweaver-Burk double reciprocal plot technique. Phytopathology 74:646–653
Shi LY, Wang B, Wen X (1993) Study on strain of defoliating type of Verticillium wilt in cotton. Acta Gossypii Sin 5:89–92
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int Syst Bacteriol 16:313–340
Shomura T, Yoshida J, Amano S, Kojima M, Inouye S, Niida T (1979) Studies on Actinomycetales producing antibiotics only on agarculture. I. Screening, taxonomy and morphology–productivity relationship of Streptomyces halstedii, strain SF-1993. J Antibiot 32:427–435
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitiv ity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tian L, Xu J, Zhou L, Guo W (2014) VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 550:238–244
Wei Z, Yang X, Yin S, Shen Q, Ran W, Xu Y (2011) Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48:152–159
Xue QY, Chen Y, Li SM, Chen LF, Ding GC, Guo DW, Guo JH (2009) Evaluation of the strains of Acinetobacter and Enterobacter as potential biocontrol agents against Ralstonia wilt of tomato. Biol Control 48:252–258
Yang W, Xu Q, Liu HX, Wang YP, Wang YM, Yang HT, Guo JH (2012) Evaluation of biological control agents against Ralstonia wilt on ginger. Biol Control 62:144–151
Yu X, Ai C, Xin L, Zhou G (2011) The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur J Soil Biol 47:138–145
Yuan S, Wang L, Wu K, Shi J, Wang M, Yang X, Shen Q, Shen B (2014) Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol 75:86–94
Zhang BL, Yang YW, Chen TZ, Yu WG, Liu TL, Li HJ, Fan XH, Ren YZ, Shen DY, Liu L, Dou DL, Chang YH (2012) Island cotton Gbve1 gene encoding a receptor-like protein confers resistance to both defoliating and non-defoliating isolates of Verticillium dahliae. PLoS ONE 2012:7
Zhao K, Penttinen P, Guan TW, Xiao J, Chen QA, Xu J, Lindstrom K, Zhang LL, Zhang XP, Strobel GA (2011) The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi Plateau, China. Curr Microbiol 62:182–190
Acknowledgments
We gratefully acknowledge the National Nature Science Foundation of China (31471917) and Science and Technology Research Project of Heilongjiang Province Department of Education, China (12541859), for their funding of this project.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Peng Cao, Chongxi Liu and Pengyu Sun contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cao, P., Liu, C., Sun, P. et al. An endophytic Streptomyces sp. strain DHV3-2 from diseased root as a potential biocontrol agent against Verticillium dahliae and growth elicitor in tomato (Solanum lycopersicum). Antonie van Leeuwenhoek 109, 1573–1582 (2016). https://doi.org/10.1007/s10482-016-0758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0758-6