Abstract
Operating Room (OR) management has been among the mainstream of hospital management research, as ORs are commonly considered as one of the most critical and expensive resources. The complicated connection and interplay between ORs and their upstream and downstream units has recently attracted research attention to focus more on allocating medical resources efficiently for the sake of a balanced coordination. As a critical step, surgical scheduling in the presence of uncertain surgery durations is pivotal but rather challenging since a patient cannot be hospitalized if a recovery bed will not be available to accommodate the admission. To tackle the challenge, we propose an overflow strategy that allows patients to be assigned to an undesignated department if the designated one is full. It has been proved that overflow strategy can successfully alleviate the imbalance of capacity utilization. However, some studies indicate that implementation of the overflow strategy exacerbates the readmission rate as well as the length of stay (LOS). To rigorously examine the overflow strategy and explore its optimal solution, we propose a Fuzzy model for surgical scheduling by explicitly considering downstream shortage, as well as the uncertainty of surgery duration and patient LOS. To solve the Fuzzy model, a hybrid algorithm (so-called GA-P) is developed, stemming from Genetic Algorithm (GA). Extensive numerical results demonstrate the plausible efficiency of the GA-P algorithm, especially for large-scale scheduling problems (e.g., comprehensive hospitals). Additionally, it is shown that the overflow cost plays a critical role in determining the efficiency of the overflow strategy; viz., benefits from the overflow strategy can be reduced as the overflow cost increases, and eventually almost vanishes when the cost becomes sufficiently large. Finally, the Fuzzy model is tested to be effective in terms of simplicity and reliability, yet without cannibalizing the patient admission rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The healthcare of surgical patients is typically operated through a sequence of standard stages. Among them, there are two key stages, the operative stage and the postoperative stage, which characterize the transition from a high- to a low-intensity care (Liu et al., 2019; Wang et al., 2022). The transition of surgery patients flows from the upstream to the downstream, and they are eventually discharged after recovery. Given that most upstream resources are relatively expensive and scarce, especially for operating rooms (ORs), the mainstream of extant research focuses on the scheduling issues of upstream resource (e.g., ORs), while simply assuming ample sufficiency of downstream resources (e.g., inpatient beds) (Durán et al., 2017; Roshanaei et al., 2017; Shylo et al., 2013). However, in practice, if the downstream resources are exhausted, then the whole system will be disrupted accordingly. In this case, a shortage of downstream resources, especially inpatient beds, not only hinders the timely treatment of patients, but also further devastates the utilization of related resources in the operating rooms (ORs).
One of the most effective ways to address a shortage of the downstream resources is to balance the resource by “coupling” the capacity of the upstream with the one in the downstream (Bastos et al., 2019; Gao et al., 2020). For instance, Schneider et al. (2020) indicate that blocking can be alleviated by balancing the capacities at different stages, viz., by minimizing variations of bed usage. In practice, balancing capacity alone could not always iron out the wrinkles, because there are many factors in play overwhelmingly causing a shortage of inpatient beds, such as insufficient capacity (Bazzoli et al., 2003), inefficient capacity allocation (Green, 2012), and certain unexpected emergency occurrences (Gupta, 2007). In this case, many hospitals adopt the regionalized practices by allocating beds and medical resources in terms of the dedicated specialties (e.g., oncology wards, obstetric wards, etc.). Operationally, this can curtail the bed shortages (Song et al., 2020). On the flip side of the coin, such resource allocation without a centralized coordination can also cause resource idling for some wards while others are suffering from shortages. Practically, raising hospital capacity, such as increasing the number of inpatient beds or nurses, is not always feasible under such a situation (Best et al., 2015; Wang, Li, et al., 2020; Wang, Qin, et al., 2020, 2021).
Instead, hospitals are recommended to adopt an overflow strategy (or off-service placement), which admits patients to a ward with sufficient resources, even though that ward may not be the designated one (Dai & Shi, 2019; Izady & Israa, 2021; Song et al., 2020). In practice, many hospitals first classify their inpatients according to specialties (e.g., cardiology, orthopedic, general medicine, etc.) based on which patients are then assigned to corresponding wards (i.e., primary wards) for treatments (Shi et al., 2016). Take the general medicine ward for example, as depicted in Fig. 1, when its capacity is fully used, incoming patients must wait in a queue for beds; however, patient condition may deteriorate, or they may suffer from some emotional distress while waiting. To address the issue, the overflow strategy thus suggests diverting patients of over-capacity wards to other wards with available capacity for the sake of reducing waiting time. In view of Fig. 1, surgery patients of general medicine wards are transferred to cardiology wards for postoperative recoveries in accordance with the overflow strategy. In this case, those diverted patients and the diverted ward are called overflow patients and overflow wards, respectively.
Although the overflow strategy may effectively solve the resource allocation issue and reduce patient waiting time while increasing the patient admission rate simultaneously, it comes with the price of increasing postoperative costs. For instance, Izady and Israa (2019) point out that the overflow strategy may increase the length of stay (LOS) as well as the re-admission rates. Therefore, surgical scheduling needs to be systematically and judiciously examined by not only the benefits brought by the overflow strategy, but also its associated costs. To this end, this study proposes a two-stage model to holistically balance the costs and benefits pertaining to the overflow strategy.
In the real healthcare system, there are a myriad of inevitable uncertainties, which add more wrinkles to resource management. For example, a hospital manager may lack knowledge in advance on the exact surgery duration and LOS of a patient, which is the essential information for managing the bed and OR utilization. The absence of such knowledge or its unpredictability is the most cause that hinders the efficiency and effectiveness of resource allocation and scheduling (Shylo et al., 2013). Typically, for such resource allocation problem with uncertain demand, it is usually solved by stochastic programming or robust optimization (Wang et al., 2022), which radically assumes variables following a known probability distribution. However, the underlying distribution of a variable is often unknown in principle and is usually statistically estimated based on its historical data (Shapiro et al., 2009). Nevertheless, the statistical inference of historical data to the underlying distribution is dubious or rather controversial (Shapiro & Nemirovski, 2005). Besides, medical history data is not always sufficient or complete (Priya et al., 2017). For example, the outbreak of COVID-19 has led to disruptive changes in surgical procedures and entrenched preoperative preparation and postoperative disinfection time for most patients. There are also some elective patients who are unable to be hospitalized in a timely manner and their condition deteriorates, making their operation time and LOS even more uncertain. In this case, the surgery duration and LOS can only be estimated by experts (e.g., surgeons) based on their experience and expertise, which may vary significantly from person to person, and this intensifies modeling difficulty.
Methodologically, Fuzzy theory is embraced to be an effective way to handle such variations in estimation (Bellman & Zadeh, 1970), and has gained a wide range of applications in job scheduling (Abdullah & Abdolrazzagh-Nezhad, 2014), production management (Han et al., 2020), among many others. Fuzzy estimation of uncertain durations does not require a large amount of historical data, hypothetical data distribution or complex probability calculations—only the inference of a panel consensus, which significantly reduces data bias and alleviates the complexity of the scheduling model (Gonzalez-Rodriguez et al., 2008). In practice, the fuzzy estimates of surgery duration and LOS made by panel consensus can truthfully reflect the heterogeneity of patients. Nevertheless, there has been scarce research investigating the impacts of uncertainty in surgical management by adopting Fuzzy theory. This study aims to bridge the gap in the extant literature.
In this paper, we study elective surgical schedulingFootnote 1 by considering downstream inpatient beds shortage under an uncertain environment. As mentioned before, although the overflow strategy has been proven to be effective in solving the issue of downstream bed shortage and simultaneously improving upstream resource utilization, it unavoidably comes with a cost, e.g., exacerbating the readmission rate and LOS. This study is dedicated to analyzing these tradeoffs pertaining to the overflow strategy and to finding the optimal policy for overflow bed allocation. Furthermore, given that both the uncertainty of surgery duration and LOS radically impact the effectiveness of surgery scheduling, this study investigates the feasibility of using a scheduling technique fueled with fuzzy data to mitigate the uncertainty. To the best of our knowledge, this is the first study to streamline upstream and downstream medical resources in a fuzzy environment.
Our study can be broken down into three efforts. First, we develop a multi-day scheduling model for elective surgery implementing the overflow strategy. Second, the multi-day scheduling model is then extended into a Fuzzy model by considering uncertainties of surgery durations. Third, the model is transformed and processed, whereby a hybrid GA algorithm (GA-P) is proposed based on Genetic Algorithm (GA) to solve the Fuzzy model.
A Monte Carlo simulation is performed to evaluate the model performance. The experimental results show that our developed algorithm GA-P delivers the best and the most reliable performance. Moreover, the overflow cost is shown to play a critical role in determining the effectiveness of the overflow strategy. For a large-scale problem, the overflow strategy is shown to be only effective when the unit overflow cost is not too high. However, for a small-scale problem, the overflow strategy is always effective, regardless of the overflow cost. Finally, the Fuzzy model, which requires only minimum scheduling uncertainty data, is shown to be the most reliable, without compromising the patient admission rate.
The remaining of this paper is organized as follows. Section 2 reviews the related literature and points out the contribution of our study, following which the deterministic scheduling model with the overflow strategy is devised in Sect. 3. The Fuzzy model is developed in Sect. 4, and its solutions of the Fuzzy model is addressed in Sect. 5. Computational experiments are performed in Sect. 6. Finally, Sect. 7 presents the conclusion and points out some future research directions. Additional materials are relegated in Appendix, including a brief review of Fuzzy theory, details of algorithm and some auxiliary numerical results.
2 Literature review
This study mainly focuses on the optimization of hospital service capacity considering two aspects: the shortage of downstream resources and the uncertainty of processing time in surgical scheduling. In what follows, we shall review the literature along each stream.
2.1 Shortage of downstream resources in surgical scheduling
Our model considers two homogeneous resources, inpatient wards and operating rooms (ORs). Surgical scheduling with limited beds has been studied extensively in the healthcare operations management literature. For example, Min and Yih (2010) focus on the shortage of downstream stroke intensive care unit (SICU) beds. By considering the number of SICU beds as a constraint, they develop a stochastic mixed-integer linear programming model to minimize the total of patient treatment costs and expected overtime costs. Recently, Zhang et al. (2019) propose a two-level optimization model considering the limited capacity of downstream SICU for elective surgery planning in a single department. Vancroonenburg et al. (2019) employ a chance constraint method to tackle the shortage of downstream inpatient beds in surgical admission scheduling. The important feature shared by these studies is the “exogenous” capacity constraint of the number of beds—once the bed capacity is fully used, patients can no longer be admitted. In contrast, our study considers bed utilization as an optimization objective, in lieu of a capacity constraint. There are some other studies, which also consider bed utilization as an objective function of optimization. For example, Schneider et al. (2020) study the impact of inpatient bed resources (such as the utilization of inpatient beds in both the wards and the ICU) on surgical planning, and propose a single-step scheduling model with the objectives of minimizing bed variations and maximizing OR utilization. Fügener et al. (2014) propose an MSS method, which aims to minimize downstream costs by balancing bed demand and reducing weekend bed demand. d’Obrenan et al. (2020) propose a model with the objective of variation minimization in the required bed capacity to solve the stochastic scheduling problem. Beliën and Demeulemeester (2007) assume that the bed shortage, such as expected shortage and the probability of shortage, is predictable if the daily bed demand can be stabilized, which further enables them to build a cyclical master surgery schedule with an estimate of bed utilization and shortages. All these aforementioned studies share the common goal of mitigating the impact of uncertain surgical scheduling on downstream resource management. Although their efforts to alleviate bed shortage is shown effective under some condition, we believe that further improvement can be achieved by consolidating the interaction of upstream and downstream management. Departing from the aforementioned studies, this paper considers the surgical process as a two-stage (upstream and downstream) system that also includes the management of multiple medical resources such as ORs and inpatient beds.
Some other studies propose innovative solutions to tackle the issue of bed shortages. For example, Augusto et al. (2010) recommend allowing patients to recover in the OR whenever a recovery bed is unavailable, but even though this approach might alleviate the strain on downstream resources, it incurs additional OR costs or other burden. In addition, Bekker et al.(2017) propose a shared ward strategy to allocate medical resources among different wards; this sharing strategy has been widely studied in patient admission scheduling problems (including both surgical and non-surgical patients). However, so far there has been only a few studies that have considered resource sharing in surgical scheduling. This study investigates the overflow strategy for sharing homogeneous beds to balance upstream and downstream demand and to increase patient admission rates as well as the number of surgeries.
2.2 Uncertainty of durations in surgery scheduling
The uncertainty in operation scheduling usually involves the surgery durations, LOS in the ICU, and LOS in the wards. Given that the upstream OR is one of the most expensive resources, most of the current research focuses on the OR scheduling and management. For example, Eun et al. (2019) define an OR planning problem in which uncertainty in surgery duration is incorporated. In addition, Denton et al. (2010), Min and Yih (2010), Freeman et al. (2016), Wang et al. (2022) and Atighehchian et al. (2020) also consider the uncertainty of surgery duration. Given the intertwined connection between the upstream and the downstream in a hospital system, some studies extend to consider the uncertainty from both stages. For instance, Saadouli et al. (2015) assume the durations of surgery and recovery to be stochastic, and develop a model to optimize surgery and recovery planning that avoids OR occupation by recovery patients. Considering the surgery and recovery uncertainties, Zhang et al. (2020) employ a stochastic programming model, and propose several heuristics to solve the elective surgery scheduling problems. Unlike the abovementioned studies, this paper integrates the surgery durations and LOS in the ICU as the overall surgery duration, and also takes LOS into consideration. In principle, risk and uncertainty is a primary consideration in the broad context of operations management; readers are referred to Shi and Zhao (2010), Shi Katehakis, and Melamed (2013), Shi, Katehakis, et al. (2014), Shi, Yue, et al. (2014), Shi et al. (2019)), Chang et al. (2019), Katehakis et al., (2015, 2016), Shi (2016), Xiao and Shi (2016), Qi, et al. (2015), Chang and Shi (2017), among many others.
There are a variety of methods developed for tackling the uncertainties in surgical scheduling, among which the most common two are stochastic optimization and robust optimization. For the former, Kumar et al. (2018) develop a stochastic mixed-integer programming model to balance the patient flow under downstream capacity constraints. Stochastic optimization considers hospitalization time to be stochastic, but assumes that the distribution is known, which might not be true in practice. Denton et al. (2010) compare a stochastic optimization model with a robust optimization model for the uncertainty of the surgery durations. The results show that robust optimization is suitable for those in which parameter distributions are unknown. Neyshabouri and Berg (2017) propose a two-stage robust optimization model that considers both the uncertain surgery durations and the LOS to develop robust schedules for a surgical ICU. Moosavi and Ebrahimnejad (2020) assume the surgery time, LOS, and emergency to be stochastic, and develop a robust multi-objective programming model for elective and emergency surgery scheduling. In contrast to the assumption of known distribution for stochastic optimization, robust optimization is more resilient when the information about data distribution is scarce (Rachuba & Werners, 2017; Wang et al., 2022). Given limited information, robust optimization needs to estimate the range of uncertain parameters from panel consensus, while epistemic uncertainties among panel experts are usually ignored. To accommodate such epistemic uncertainties and minimize model errors, we employ fuzzy sets for surgery scheduling. There have been several studies adopting fuzzy sets to tackle surgical uncertainties (e.g., De & Sana, 2015; Singh & Yadav, 2018; Tavana et al., 2013). Moreno and Blanco (2018) propose a fuzzy planning method for multi-objective patient appointment scheduling in large hospitals. Lee and Yih (2014), Behmanesh and Zandieh (2019), and Wang, Li, et al. (2020) , Wang, Qin, et al. (2020) ) describe the uncertain duration of operations with fuzzy numbers to build fuzzy models, which proves to be effective in the application of surgical scheduling. In contrast, previous studies focused on the daily scheduling of surgery with limited ORs and ICU beds (i.e., the upstream), whereas we study multi-day scheduling for a planned time horizon with a focus on the limited recovery beds (i.e., the downstream). In this paper, we leverage triangular fuzzy numbers (TFN) to model the surgery duration and LOS. TFN and trapezoidal fuzzy numbers are two representative and commonly-used types of fuzzy numbers (Seyfi-Shishavan et al., 2021; Sun et al., 2019; S. Wang et al., 2013). Actually, TFN can be treated as a special case of trapezoidal fuzzy number, which is characterized by its simple expression for a complex model. For our problem under this study, a surgical scheduling problem is usually complicated for its joint considerations of various resources (including upstream and downstream) and uncertainties of surgery and hospitalization durations. In this sense, TFN is selected for our proposed surgical scheduling problem to reduce its computational complexity.
3 Deterministic scheduling model with the overflow strategy
In this study, we consider a medical specialty as a focal scheduling unit where each specialty has its own inpatient wards. Considering the constraints of fixed ORs and inpatient beds, the surgical planner needs to select some patients from the patient pool to schedule first and postpone the rest until the next scheduling cycle. Once admitted, a patient’s surgery must be performed by the scheduled due date; if the designated ward is full, the patient must be re-assigned to another ward. To minimize non-designated assignments, hospital management needs to allocate resources efficiently and strategically.
This study attempts to address the following questions in sequence: (1) What kind of patients deserve admission with priority? (2) Which OR to be assigned to the admitted patients? (3) When to schedule the surgery? (4) What type of inpatient bed to be allocated to the patient? The objectives of the model are to minimize the total cost, consisting of the overtime costs of ORs and the overflow costs of inpatient beds, while maximizing the profit of ORs. The rationale behind the objectives is to reflect the close interplay between a hospital’s upstream and downstream medical resources. The two-side impacts of the patient admission decision are straightforward: over-admission of patients will lead to an overtime utilization in ORs and overflow of inpatient beds; under-admission of patients will dwindle OR profit.
It is worth noting that surgery costs can be classified into two parts: general costs and additional costs. The former covers the open costs of ORs and inpatient beds, and the latter refers to the overtime costs of ORs and the overflow costs of inpatient beds. Given that the numbers of ORs and inpatient beds are fixed in the short term, we only consider the additional costs in our model.
The assumptions of our model are described as follows:
-
(1)
Since the pre-operative cleaning and post-operative anesthesia recovery are performed in an OR and require OR resources, surgery duration is considered to include these processes.
-
(2)
Planning and scheduling of emergency patients is not considered in our model, because hospitals usually reserve dedicated ORs and wards for emergencies (Freeman et al., 2016).
The main symbols and notations used in this paper are summarized as follows.
The objective of the deterministic surgery scheduling model is to maximize the surgery’s total profit. There are two factors affecting surgery profit: (1) regular surgery profit, which increases linearly in surgery duration; (2) additional surgery costs, which is the sum of overtime costs of ORs and overflow costs of inpatient beds. Accordingly, we have the following mixed-integer programming (MIP).
The model is subject to the following constraints:
Constraints (2, 3) below require that the non-negative variable of the total overtime of the OR \(j\) on day \(d\) must be larger than or equal to the excess usage of the operating room over the regular opening duration.
Constraint (4) requires that the expected discharge day for each patient must be equal to the surgery time plus the total length of stay (LOS).
Constraint (5) confines that a patient must stay in only one bed from the time of being admitted to the time of being discharged.
Constraint (6) is a bed capacity constraint, which states that the total number of inpatients must be less than the total number of beds.
Equation (7) is a constraint of bed occupancy for each patient and each type of bed.
Constraint (8) is a capacity constraint for each type of inpatient bed.
Constraint (9) is a capacity constraint of surgery teams, which requires that the total number of surgeries performed each day must be less than the total number of available surgery teams.
Constraint (10) is a constraint on the daily maximum surgery hours for each surgery team.
Constraint (11) is a constraint on the maximum overtime for each OR.
Elective surgery, although is less time-sensitive compared to emergency surgery, it still requires judicious attention. Surgical patients are usually assigned a surgery due date, by which time the surgery must be performed in an OR. Constraints (12)-(13) require that each patient must be assigned by the due date and must only be assigned once.
Constraint (14) states that each patient must be discharged and be discharged only once.
4 Scheduling model based on fuzzy duration
The surgical department and the inpatient department are inherently connected, and both are faced with great uncertainties in scheduling. In the above MIP model for a deterministic environment, we assume that surgery durations and LOS are known. Nevertheless, they are uncertain in practice. Surgery durations and LOS are usually estimated from historical data, but heterogeneity in patients makes the estimations biased and modeling accuracy compromised. In addition, dynamical changes in the medical environment will further decrease predicting efficacy of historical data. For example, the outbreak of COVID-19 has caused disruptive changes in surgical procedures, and increased preoperative preparation and postoperative disinfection time for most patients. There are also some elective patients who are unable to be admitted to the hospital in time, and their condition deteriorates, making their operation time and LOS even more uncertain. In this study, we thus employ possibility theory to treat expert estimations as a set of fuzzy numbers so as to improve modeling accuracy and efficiency.
Following prior literature on medical scheduling (Behmanesh & Zandieh, 2019; Lee & Yih, 2014), this study employs triangular fuzzy numbers (TFN) to model the expert’s estimates of surgery duration for each patient as \(\tilde{\xi }_{i} = (\xi_{i}^{l} ,\xi_{i}^{m} ,\xi_{i}^{r} )\), where \(\xi_{i}^{m}\) represents the most plausible value of the surgery duration of the patient \(i\), and \(\xi_{i}^{l}\), \(\xi_{i}^{r}\) denote the optimistic (the best case) and pessimistic (the worst case) values, respectively. In a similar vein, the LOS can be expressed as \(\tilde{L}_{i} = (L_{i}^{l} ,L_{i}^{m} ,L_{i}^{r} )\). A detailed definition of TFN (Laarhoven & Pedrycz, 1983) is provided in Appendix A.1.
The rules of fuzzy operations in our model involve the arithmetic of TFNs, the ranking method of fuzzy numbers, and the operation of expectation value of fuzzy numbers (Chen & Hwang, 1992; Jiménez et al., 2007; Lei, 2010). The details of fuzzy operations are provided in Appendix A.2, A.3 and A.4.
In the Fuzzy model, the objective function is expressed as Eq. (15).
Constraints (2)-(3) in the MIP formulated in Sect. 3 are reformulated as,
However, when the operation duration parameter \(\xi_{i}\) is replaced with a fuzzy number \(\tilde{\xi }_{i}\), we need to devise a new expression incorporating the fuzzy sets. Therefore, the OR overtime constraint with fuzzy surgery duration is revamped as Eq. (16):
where \(P\left[ \cdot \right]\) represents the rule that generates the positive part of the triangular fuzzy number. In the following Sect. 5.1, we will elaborate on the rule \(P\left[ \cdot \right]\) in more detail. The constraint of the patient discharge day with a fuzzy length of stay is expressed as Eq. (17).
The above Eq. (17) states that, given the fuzzy numbers of surgery duration and LOS, the patient discharge time becomes a fuzzy number. This can be further expressed as a function that converts the fuzzy discharge day into the most possible day, as formulated in Eq. (18) below. In fuzzy related research, many different conversion functions have been proposed and studied. The transfer functions used in this paper will be elaborated further in Sect. 5.1.
where,\(f\left( \cdot \right)\) represents the conversion function. Constraint (19) is the capacity constraint of working hours with a fuzzy surgery duration.
where, \(\tilde{W}_{sd}\) is the maximum fuzzy working time of the surgery team \(s\) on day \(d\). Constraint (20) refers to the capacity constraint of OR overtime in terms of a fuzzy surgery duration.
where \(\tilde{M}_{dj}^{o}\) is the maximal fuzzy overtime of the OR \(j\) on day \(d\).
It should be noted that in practice, if a decision maker does not provide the fuzzy number \(\tilde{W}_{sd}\) or \(\tilde{M}_{dj}^{o}\), but instead providing the exact number \(W_{sd}\) and \(M_{dj}^{o}\), then according to the definition of fuzzy number, the latter can still be transformed into triangular fuzzy numbers. For example, the exact number 5 can be transformed into a triangular fuzzy number (5, 5, 5). All other constraints, ceteris paribus, remain the same as those formulated in the deterministic scheduling model in Sect. 3.
5 Solution approach for the fuzzy model
Fuzzy models involve complex computation, and its solution typically requires a two-stage treatment. First, the Fuzzy models must be transformed. Second, considering that OR scheduling is an NP-hard problem and that the decision variables of the Fuzzy model involve multiple dimensions (e.g., a selection of surgical patients, OR and bed assignments, etc.), the solution approach of a traditional algorithm may not be efficient or satisfactory. To address the challenge, we propose a hybrid GA (GA-P) algorithm which extends the Genetic Algorithm (GA) algorithm framework by integrating heuristic rules (Lu et al., 2020).
5.1 Transformation of the fuzzy model
The objective function in this study is composed of three terms, namely, surgery profit, overtime costs of OR, and bed overflow costs, wherein the first two are associated with TFNs, and the last is a crisp number. As depicted in Fig. 2, in light of the fuzzy arithmetic rules, the objective function can be expressed as a TFN, \(\tilde{G} = (g_{l} ,g_{m} ,g_{r} )\), where \(g_{l}\), \(g_{m}\), and \(g_{r}\) represent the optimistic, the most plausible, and the pessimistic estimates, respectively.
In Fig. 2, \(LA\) (the left area) denotes the triangular area under the curve from \(g_{l}\) to \(g_{m}\), and \(RA\) (the right area) refers to the triangular area from \(g_{m}\) to \(g_{r}\). According to Buckley and Feuring (2000), our objective function of the maximization of \(\tilde{G}\) in Eq. (15) can then be transformed into three sub-optimization functions: (1) \({\text{Max}}(g_{m} )\), (2) \({\text{Max}}(RA)\), and (3) \({\text{Min}}(LA)\).
Triangular fuzzy numbers (TFNs) are bounded; we use \(\overline{L}\) to denote the upper bound of LA and \(LA^{\prime} = \overline{L} - LA\). In this case, the objective function of \({\text{Min}}(LA)\) can then be transformed into \({\text{Max}}(LA^{\prime})\), and the overall objective of the model can now be expressed to maximize \(g_{m} , \, RA,\) and \(LA^{\prime}\). To transform a multi-objective problem into a single-objective problem, the most straightforward and efficient way is to assign different weight \(\psi_{i} \ge 0\) to each sub-objective function. In this fashion, the objective function is then formulated as follows:
where the constant \(\psi_{i} \ge 0\) denotes the weight associated with each sub-objective function \(i = 1,2,3\) such that \(\sum\nolimits_{i} \psi_{i} = 1\) Given that both \(RA\) and \(LA^{\prime}\) are TFN, increasing linearly in the length g, the objective function can be consequently rewritten as (Chang et al., 2021):
In our numerical experiments, we implement the method of TFN via taking the weights as \(\psi_{1} { = }\psi_{3} { = }0.25\) and \(\psi_{2} { = }0.5\). Here, the selection of \(\psi_{i}\)’s values is determined mainly based on the decision maker's risk tolerance and attitude toward OR overtime and bed overflow. In particular, a larger \(\psi_{2}\) reflects that the manager tends to rely more on the plausible estimates of surgery duration and LOS. In contrast, larger values of \(\psi_{1}\) and \(\psi_{3}\) mean that the manager inclines to conservatively control OR overtime and bed overflow by relying more on pessimistic estimates.
The OR overtime Constraint (16) is rather difficult to tackle since it only considers the positive part of the difference between the total usage and the availability of ORs. In this case, the negative fuzzy numbers must be discarded, and it can then be summarized into five scenarios, as illustrated in Fig. 3. After removing the negative parts, the shaded area represents the solution for each scenario, wherein Case 1 and Case 3 are no longer TFNs. Next, an approximate conversion, \(P\left[ \cdot \right]\), is performed in order to initiate the fuzzy operations. In particular, for Cases 1–2, we convert them into \((0,g_{m} ,g_{r} )\) and \((g_{m} ,g_{m} ,g_{r} )\), respectively; Case 3 can be transformed into \((0,0,g_{r} )\); Case 4 remains unchanged; Case 5 is transformed into \((0,0,0)\). Approximations for each scenario are presented in red lines in Fig. 3.
In the Fuzzy model, the number of discharged patients per day cannot be calculated only based on the fuzzy discharge date of each patient. Therefore, for operational simplicity, the fuzzy discharge day in Eq. (18) are converted to the following, based on Eq. (23) by referring to the rules of fuzzy expectation (cf. Appendix "Ranking of TFNs"), wherein the estimated discharge day is de-fuzzified into a specific day.
where \(\left\lceil {EV\left( {\tilde{N}_{{_{i} }}^{{}} } \right)} \right\rceil\) is the ceiling number of \(EV\left( {\tilde{N}_{{_{i} }}^{{}} } \right)\).
Next, considering the computational complexity of Constraints (19) and (20), they are respectively converted to:
5.2 Hybrid GA algorithm
In this section, we propose a hybrid GA algorithm (GA-P) to solve the model. The framework is delineated in Fig. 4. The motivation for developing the GA-P algorithm is based on the following two reasons: (1) surgical scheduling is an NP-hard problem with high dimensionality, which intensifies the difficulty level, and (2) there have not been any methodology in the extant literature to solve the fuzzy problems directly.
5.2.1 Algorithm steps
-
(1)
Encoding:
Terminologically, a chromosome (a.k.a. genotype) in a Genetic Algorithm (GA) is a set of parameters which define a proposed solution to the problem that the GA aims to solve. In our setting, each chromosome \(I\) is a three-string vector, composed of OR assignment, scheduled surgery day, and bed assignment, denoted by \(\beta\), \(\eta\), and \(\gamma\) respectively, as shown in the following Eq. (27).
$$ I = \left( \begin{gathered} \beta \hfill \\ \eta \hfill \\ \gamma \hfill \\ \end{gathered} \right) = \left( \begin{gathered} o_{1} ,...,o_{N} \hfill \\ d_{1} ,...,d_{N} ,d_{{N{ + 1}}} \hfill \\ v_{1} ,...,v_{N} \hfill \\ \end{gathered} \right). $$(27)
In particular, the vector (\(o_{1}\),\(d_{1}\),\(v_{1}\)) encapsulates the variables of the assigned operation room, scheduled surgery day and allocated bed type for patient number one. It is worth noting that a dummy variable \(d_{{N{ + 1}}}\) is added to accommodate unselected patients (due to limited medical resources) who will be postponed to the next surgery cycles. One example is illustrated in Fig. 5.
-
(2)
Population initialization:
The diversity in patient population may pose a problem in the process of population initialization, which can directly affect the results of a global search. Therefore, we should generate as many feasible solutions as possible to avoid any bias in population initialization. Moreover, some constraints in surgery scheduling must be taken into consideration, such as the due dates of patient surgeries and the availability of surgery teams. In this case, the substrings \(\eta\) can only be randomly generated within a limited time range. For example, if the due day of a surgery is day 4, the admission schedule for the patient must be randomly generated within the first four days. Besides, this study also considers outpatients, who do not require hospitalization and can be discharged on the same day of their surgery. The model assigns those outpatients to the dummy day \(d_{{N{ + 1}}}\). The other two assignments, operation room (\(\beta\)) and impatient bed (\(\gamma\)), are generated by the heuristic rules.
-
(3)
Fitness function
$$ g(I) = \left\{ \begin{gathered} U(I){\text{, if }}I{\text{ is feasible}} \hfill \\ S^{T} ,{\text{ otherwise}} \hfill \\ \end{gathered} \right., $$(28)
where \(U(I)\) is the objective function, and constant \(S^{T} \ge 0\) denotes a constraint penalty.
-
(4)
Crossing
The algorithm employs a two-point crossover method. However, when the number of patients is growing, the length of chromosomes may increase dramatically, and the computation efficiency may thus be devastated. In this case, we treat different parts of the chromosome separately: \(\gamma\) and \(\beta\) leverage the heuristic rules, while \(\eta\) follows a standard crossover method.
In order to preserve the diversity of patient population, duplications are removed and replaced with new patients. The algorithm in this study adopts tournament selection. Readers are referred to Bonabeau et al. (1999) for more details.
5.2.2 Heuristic algorithm
-
(1)
OR assignment
The OR assignment can be formulated as a daily OR assignment problem, which shares a similarity with the number partition problem. For such setting, the process can be divided into two stages: initial assignment stage and readjustment stage. The first stage employs a greedy algorithm for an initial assignment, and then the swap rule is used for readjustment. An example is illustrated in Fig. 6, in which there are 9 patients scheduled for surgeries within a 2-day time frame, and there are two available ORs with a capacity of 45 time units per room per day. The first stage is performed through Steps 1–2, and the second stage is executed by Steps 3–6.
Initial assignment stage (Steps 1-2): Patients are first grouped according to admission day, and then each group is sorted by the length of surgery duration in a descending order. The first patient in the first group is assigned to the freest OR and then removed from the assignment waiting list. The process is repeated and performed with the second group until all patients are properly assigned.
Readjustment stage (Steps 3-6): First, ORs are ranked by the total duration of surgery in a descending order. In the illustrative example in Figure 6, OR1 is suffering from overtime, while OR2 has idling capacity. Next step is to calculate the overtime of the first-ranked OR (OR_1) and the idling time of last-ranked OR (OR_J). The overtime of OR_1 in the example is 1 (i.e., 46-45=1), and the idling time of OR_J is 6 (i.e., 45-39=6). Third, the patient with shortest surgery duration in bottom-ranked OR are selected (patient 1, p1, with 19 time units of surgery in the illustrative example) to calculate the threshold duration L, which is the sum of overtime of OR_1, idling time of OR_J, and the shortest surgery duration in OR_J. In the example, L=1+6+19=26. Fourth, if the patient’s surgery duration is less than L, then we swap the patient with the longest surgery duration in OR_1 (patient 9, p9, with 21 time units in the example) with the selected patient with shortest surgery in OR_J. In the example, since the surgery duration of patient 9 (p9) is less than the threshold duration of L=26, he/she is selected to be swapped with patient 1 (p1). Lastly, the process is repeated until all the overtimes of the OR are resolved.
The algorithm process of OR assignment is curated in Algorithm 1 in Appendix "Heuristic of OR assignment and bed assignment".
-
(2)
Ward assignment
Each specialty has a set of designated wards (beds) as its primary wards, and its patients can also be routed to nonprimary wards (beds) when it goes out of capacity. Ward assignment can be divided into two stages: initial allocation stage and readjustment stage. The first stage is to maximize occupation of the primary beds. In the second stage, beds released from discharged patients can be redistributed. An illustrative example is described in in Fig. 7, which also delineates the basics of the bed assignment algorithm.
In the aforementioned example, there are 9 patients and two types of beds (a primary bed is denoted as type 1 and a non-primary bed denoted as type 2) over a 5-day planning horizon. It is worth noting that bed type 0 and day 6 are dummy variables to accommodate patients who cannot be assigned in the current planning horizon. The number of the primary beds (type 1) is two, and the other (type 2) is seven. The bed assignment process can be described as follows:
-
Step 1: Estimate the expected time of discharge for each patient based on his/her admission day and LOS, and then sort by days in an ascending order. The sorting rule is to sort first by discharge day, and then by admission day.
-
Step 2: Allocate primary beds to the first two patients.
-
Step 3: If the inpatient bed capacity, B, has not been reached, then continue bed assignment with the next patients until either all beds are allocated, or all patients are assigned (whichever comes first).
-
Step 4: Step 3 has only achieved utilization maximization of the primary beds without considering patient discharge. Once a patient is discharged, a bed is released and becomes available for the next patient. It is worth noting that the bed can only be reassigned to a patient who is admitted later than the day on which the bed is released. The next patient candidate for receiving the bed can be selected by sorting the dummy pool of patients that was not assigned a bed at the initial assignment stage, the sorting rule remaining the same as in Step 1. Once the candidate patient is added, it is necessary to determine whether the daily bed occupancy within the planning horizon is less than two (i.e., the number of primary beds). If it is less than two, then the current candidate patient is added successfully; otherwise, the current candidate patient cannot be added.
-
Step 5: Update with the next released bed, and repeat Step 4 until either all the patients with bed assignments are discharged or no candidate patient qualifies for a primary bed.
-
Step 6: Assign non-primary beds to the remaining patients. For clarity, note that the allocation of a dummy bed and dummy day does not affect bed scheduling.
To implement those steps, we develop a bed assignment algorithm which is reported as Algorithm 2 in Appendix "Heuristic of OR assignment and bed assignment".
6 Computational experimentations
In this section, we implement our developed solution approaches in numerical experiments.
6.1 Experimental design
The experimental data is classified as follows: (1) By level of surgery: The level of surgery is classified as basic, moderate, normal, difficult, and superior based on the surgery duration and its estimated length of stay (cf. Table 8 in Appendix C). (2) By hospital size: The size of hospital is classified as extra small, small, medium, and large size based on the number of surgeries, operating rooms, surgeons, types of surgery and LOS (cf. Tables 9 and 10 in Appendix C).
Based on the data classification scheme, a set of experimental data is randomly generated, so-called the waiting list. Next, the waiting list is randomly put into several sets and divided into two groups, the mimic group and the simulation group. In particular, the former imitates real patients with similar medical conditions; the latter simulates possible actualizations of patients. In compliance with the practice of experts estimating surgery durations and LOS from historical data, fuzzy data is generated accordingly from the mimic group’s data. The experiment process is demonstrated in Fig. 8. It is worth noting that although a lack of expert’s rationale in estimations might diminish the performance of the model, the robustness of the model is not affected.
In the sequel, we shall consider random durations of the form m + v, where v is a random number with an interval of 1% to 30% of the average duration, m. This setting has been adopted in other works in the medical scheduling literature, e.g., Behmanesh and Zandieh (2019).
Fuzzy models are considered as a-priori solutions (Gonzalez-Rodriguez et al., 2008). To evaluate an a-priori solution, we can find a series of U crisp problems caused by fuzzy estimation, and then define them as realizations of fuzzy problems. To simulate such possible realizations, we randomly generate fuzzy estimations following a selected probability distribution.
Finally, we apply the data, including a training dataset and a testing dataset controlled by the levels of surgery and hospital sizes, through the following models: 1) GA-P method; 2) Overflow model; 3) Fuzzy model. The algorithm in this study is programmed with Python, and it is performed on a 3.7 GHz Intel Core i7 CPU computer with 16 GB memory, and employs the solver CPLEX in the Python docplex package to solve the MIP model.
The parameters and their values in the model are summarized in Table 1. There are two types of planning horizons considered, 5 days and 10 days. There are two types of beds (primary and non-primary), which could be easily extended to a more generic setting with multiple types. The ratio of available primary beds and nonprimary beds is 1:2. In particular, there are 30 available inpatient beds in the hospital, including 10 primary beds and 20 nonprimary beds. The normal operating hours for an OR is 8 h per day, with maximum overtime of 3 h per day, and the surgery team follows the OR schedule scheme. To ensure practicality of our model, several hospitals are interviewed, which reveals that the unit cost of OR overtime and unit revenue of OR do likely vary hospital by hospital. However, several hospitals have a similar cost and revenue structure, as examined by the study of Gerchak et al. (1996). Following their work, we set the unit cost of OR overtime to $15/min (\(p^{o} = \$ 15\)), and the unit revenue of an OR to $10/min (\(r = \$ 10\)). The per-bed costs at the stages of intensive care, step-down, acute care, and post-acute care, are set as $4000/day, $2500/day, $1000/day, and $600/day, respectively, by following the estimations in Bretthauer et al. (2011). The unit overflow cost is set as $500 (\(p_{1}^{h} = \$ 500\)) by referring to Dai and Shi (2019). The overflow cost is equal to the cost difference between a primary bed and an overflow bed, and various levels of unit overflow cost will be examined later in Sect. 6.2.
In addition to the parameters previously discussed, we need to further define the following parameters in the algorithm: population size (pop), crossover rate (cp), mutation rate (mp) and number of iterations (iter). Given that the meta-heuristic algorithm is sensitive to the change of parameters, we instead employ the Taguchi Method (Mountgomery, 2005; Taguchi, 1987) to configure these parameters to minimize the number of experiments. In this case, we fix cp = 1, mp = 0.1 and vary the values of population size and the number of iterations by the scale of problem.
6.2 Model performance and evaluation
6.2.1 GA-P versus other algorithms
Several algorithms are selected and performed as benchmarks in comparison with our GA-P. Specifically, the Genetic Algorithm (GA) is a classic algorithm, which is widely applied in combinatorial optimization in various fields. GA has also been introduced to operations management. For example, hybrid particle swarm PSO-GA is proposed by Niu et al. (2008) to solve the problem of job shop scheduling, which shares similarities with surgery scheduling.
Several measurements are employed to evaluate the performance of the algorithms. The relative percentage deviation (RPD), as a common performance indicator, is applied to evaluate the optimization effect of algorithms. Since each algorithm needs to run M times, we further use ARPD (AR), BRPD (BR), and WRPD (WR) as performance evaluation indicators by following the work of Wang et al. (2017). More details of each performance measurement can be found in Appendix D.
The results of each algorithm are summarized in Table 2. As the scale of setting increases, the algorithm of CPLEX cannot find the optimal solution, and the algorithm of PSO-GA and GA cannot even (in 10 h) find a feasible solution. Importantly, the errors of GA-P do not increase significantly with setting scale. Therefore, we conclude that GA-P delivers the most plausible and reliable performance regardless of the growing scale of problem.
In order to further gauge the performance of the heuristic rules in the GA-P algorithm, we compare GA-P with GA-OR and GA-BED, where GA-OR refers to the traditional GA algorithm combined with the OR assignment heuristic algorithm, and GA-BED refers to the GA algorithm combined with bed assignment heuristic algorithm. As exhibited in Table 3, it is shown that the OR assignment algorithm and the bed assignment algorithm can enhance the algorithm's solving capability. Importantly, if both the heuristic algorithms are incorporated into the GA algorithm simultaneously, the solution efficiency can be greatly improved.
6.2.2 Overflow policy versus Naive policy
In order to examine the effectiveness of the overflow strategy, we conduct an experimental analysis and compare it with the benchmark strategy, which only admits and schedules patients on primary beds. It is worth noting that the experiments are conducted without any consideration of uncertain surgery duration and LOS in order to mitigate the interference of uncertainty.
The experiment results are summarized in Table 4. OR-O represents the overtime of the OR; OR-U denotes the idle time of the OR; and B represents the number of overflow beds. In this numerical comparison, we examine the idle rate of ORs (R1), the relative reduction of idle rate with an implementation of the overflow strategy (R2), and the relative reduction of idle rate per overflow bed (R3). The idle rate of ORs is successfully reduced by 7.2−29.6% with an implementation of the overflow policy. Furthermore, an overflow bed can reduce the idle rate of the OR by 0.87%-2.99% on average.
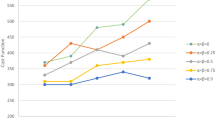
In practice, the unit cost of the ORs is steady and could be easily assessed, but the unit overflow cost may vary significantly from one hospital to another. Therefore, it is our intension to conduct a sensitivity analysis in terms of the unit overflow cost to examine the robustness of the model. The results are summarized in Table 5 and Fig. 9. There are several interesting findings in the sensitivity analysis. To begin with, it is shown that the higher the unit overflow cost, the fewer the surgeries can be scheduled, and the surgery revenue decreases accordingly. Moreover, when the unit overflow cost reaches around $3000, the advantage of the overflow strategy is almost fully compromised. The advantage of the overflow strategy tends to be salient when the scale of problem is small. Even when the unit overflow cost skyrockets to extremely high as $999,999 or beyond, the overflow strategy still successfully assigns an overflow bed in Problem 1. This would happen when a hospital has exhausted the bed capacity, but some patients remain and must undergo surgeries within the planning horizon. In summary, the overflow strategy will benefit for either a small hospital or one with lower overflow costs, and the overflow strategy empowers a hospital with more flexibility to handle excessive demand.
6.3 Fuzzy model
For performance evaluation, several models are selected and run as benchmarks in conjunction with the Fuzzy model; cf. Lee and Yih (2014). As depicted in Fig. 10, we select the following key statistical measurements to model the crisp time decision: mode (MODE), median of the interval (MED), maximum (MAX) and minimum (MIN) of the support of the triangular fuzzy number (TFN). Given that the uncertainties considered in this model are from the surgery duration and LOS, minimum estimates lead to the most optimistic and aggressive scheduling plan, and maximum estimates yield the most pessimistic and conservative scheduling plan, while the plans of median and mode likely stay in between.
Fuzzy theory is leveraged in this study for its advantage in analyzing data with limited size and uncertain probability distribution. It can model multiple probability distributions, while simulation experiments can only be performed with a single predetermined probability distribution. The probability distributions considered in this study for the surgery duration are assumed to be normal, lognormal, and uniform distribution (Lee & Yih, 2014; Marcon & Dexter, 2006; Min & Yih, 2010); those for the length of stay (LOS) are normal, exponential, and uniform distribution (Augusto et al., 2010; Best et al., 2015; Moosavi & Ebrahimnejad, 2020). Fuzzy theory allows us to consider various combinations of distributions for surgery duration and LOS, e.g., lognormal distribution for surgery duration and exponential distribution for LOS. In other words, Fuzzy theory enables scenario analysis of probability distribution for the combined uncertainty of surgery duration and LOS to make a thorough examination of the model robustness.
To deliver the best performance in Sect. 6.2.1, we employ our GA-P for all the Fuzzy models in this section. In practice, surgical and bed scheduling must be done prior to the realizations of uncertain surgery duration and LOS, which intensifies the difficulty in performance evaluation of scheduling models. In this case, Monte Carlo simulation with U (U = 100) repetitions is adopted for simulating the realizations of uncertainties.
To evaluate the performance of the selected models via the simulation experiments, we consider the following evaluation measures:
where \(n\) indicates the nth problem setting generated in the simulation experiment, \(ob_{n}\) and \(consnum_{n}\) denote the objective value and the number of constraint violations of solutions, respectively. In particular,\(cons\) implies the adaptability of the solution scheme in an uncertain environment, which can also be considered as a measurement of model robustness.
We have conducted extensive numerical studies. The complete result of scenario analysis for various combinations of various probability distributions can be retrieved in the Appendix C (Table 11, 12 and 13). The scenario of normal distribution for both surgery duration and LOS is reported in Table 6 for elaboration purposes. The number assigned to the problem is chosen to reflect the problem scale in an ascending order; RP is the average ratio of surgery duration to total OR capacity; ob and cons denote the objective value in the simulation experiment and adaptability of the solution scheme respectively, as defined in Eqs. (29) and (30). Results of minimum (MIN), maximum (MAX), median of the interval (MED), mode (MODE) in crisp time decision accompanied with the Fuzzy model are listed in Table 6. For ease of exposition, we use “Normal distribution + Normal distribution’’ to represent the combination of both normal distributions of the surgery duration and LOS.
In viewing the results of Problems 1 and 2, it is shown that the difference among various schemes is trivial, and a similar pattern can be observed in the other scenarios (cf. Table Table 11, 12 and 13 in Appendix C). The difference among various schemes is shown to be significant only comparing Problems 3 to 10. This can be intuitively explained by that when the problem scale is small, OR utilization is relatively slack, and surgery demand is relatively modest, the number of admitted patients is not sensitive to the estimation models. In other words, the effect of various models becomes significant only when the resource shortage emerges in play.
The maximum estimation scheme, MAX, provides a conservative estimate of surgery duration and LOS, and thus admits and schedules the least number of patients, while enjoying a minimum of constraint violations. On the contrary, the minimum estimation scheme, MIN, yields an aggressive estimate of surgery duration and LOS, and thus admits and schedules the most patients, while suffering from more constraint violations. The MAX scheme is obviously not favorable to hospital management for its poor profitability, but the patient satisfaction level may be compromised by the MIN scheme. In addition, the MAX scheme is more susceptible to extreme values, so hospitals are generally not able to adopt it. Surprisingly, the MIN scheme does not always bring the highest income for each problem setting, from Problems 1 to 10, compared to the MED, MODE, and FUZZY. For example, for Problem NO.10, the ob of MIN is not the largest in all distributions. The reason for this is that the MIN scheme admits too many patients and exhausts the bed capacity, which erodes the profitability by additional OR overtime costs.
An analysis of variance and the least significant difference (LSD) test are further performed to rigorously examine the model performance (specifically, MED, MODE, and FUZZY). The results show that there is no significant difference in the number of admitted patients among the various schemes and models, while the number of constraint violations does vary significantly by schemes/models as shown in Table 7. This implies that the Fuzzy model and the crisp models (MODE and MED) have similar effects on the value of the objective function, while departing in their robustness. Among the schemes/models of FUZZY, MODE, and MED, the fuzzy model (FUZZY) consistently gives the best reliability performanceFootnote 2 across the various combinations of probability distributions (Figs. 11, 12, 13 and 14). For ease of exposition, we use ‘’Normal distribution + Normal distribution’’ to denote the combination of the normal distributions of both the surgery duration and LOS. The same abbreviation applies for other combinations. We can thus draw the conclusion that the fuzzy model outperforms the others and delivers the most desirable result, in terms of both performance and reliability. More importantly, the fuzzy data generated in the experiments is to simulate the estimations made by inexperienced physicians (without any self-corrections); in practice, however, physicians repeatedly refine their estimations based on their accumulation of experience, which can be leveraged gradually to improve the performance of the Fuzzy model.
7 Concluding remarks
Operating rooms, as one type of the most expensive medical resources, easily draw research attention, and the scheduling and management of the OR has been comprehensively studied. However, the strong nexus between the upstream ORs and the downstream inpatient beds is often overlooked. Operationally, a surgery cannot be performed if a downstream inpatient bed is not available. Some studies have revealed that the overflow strategy, albeit an effective solution for a resource balancing between the upstream and downstream, leads to an increase in both readmission rate and LOS. Additionally, surgery duration and LOS are both critical data needed for medical resource scheduling and allocation, but they are simply assumed to be deterministic or stochastic with pre-determined distributions. In practice, surgery duration and LOS are uncertain and estimated by doctors, and the estimation distributions are also usually unknown. Fuzzy theory is well known for its advantages in analyzing data with limited size and with uncertain probability distributions. This study thus leverages the fuzzy theory, and at the same time incorporates an additional cost of the overflow strategy to consider the strong upstream and downstream nexus. As a solution approach, a hybrid heuristic method (GA-P) is proposed to solve the NP-hard resource scheduling and allocation problem.
Computational experiments are carefully designed to test the proposed GA-P heuristic model with the overflow strategy in a fuzzy environment. Several interesting observations are made from the experiments. First, the algorithm of GA-P delivers the best and most reliable model compared to the benchmark algorithms of CPLE, PSO-GA and GA, especially when the scale of problem becomes large. Further, the overflow strategy is shown to be effective in terms of improving utilization of operating room, but its advantages are compromised as the unit overflow cost increases. However, the overflow strategy is still beneficial for small hospitals even when the overflow cost is abnormally high. Finally, although the number of admitted patients is indifferent to estimation models, our Fuzzy model outperforms the crisp models in terms of reliability, having less constraint violations. More importantly, the fuzzy scheduling can be constantly improved with the continuously accumulated and refined estimations of physicians.
Several managerial insights can be gleaned from the study: (1) Our GA-P is an effective and reliable algorithm for upstream and downstream scheduling of medical resources, especially for large hospitals. (2) The overflow cost (including the intangible cost of increasing both re-admission rate and LOS) plays a critical role in the effectiveness of the overflow strategy. For large hospitals, the overflow strategy is effective in solving the imbalance between the upstream and downstream resources only if the unit overflow cost is not too high. Therefore, it is recommended that large hospitals must carefully examine their overflow costs prior to implementing the overflow strategy. However, the overflow strategy is always effective for small hospitals, regardless of the unit overflow cost. (3) The Fuzzy model requires merely minimum knowledge of the estimations for surgery durations and patient LOS, yet yields a viable and reliable scheduling solution, while maintaining a satisfactory level of patient admission rate.
The hospital management system is full of uncertainties, and both surgery duration and patient LOS are considered in this study. Future research may empirically study these time durations, as well as the inherent overflow costs, so as to further mitigate the uncertainties involved in medical resource management.
Notes
Elective surgery is defined as a surgery that can be scheduled in advance. It usually refers to a surgery for improving life quality (e.g., cosmetic procedures), but sometimes can refer to surgery for a serious condition (e.g., cancer treatment).
It has been discussed that the effect of schemes/models only becomes significant with an increase in the scale of the problem (from Problems 3 to 10). Therefore, the conclusion on reliability performance is made based only on the results from Problem 3 to Problem 10.
Abbreviations
- \(N\) :
-
Total number of patients seeking healthcare (patient is indexed as \(i\)\(= 1,2,...,N\)).
- \(S\) :
-
The total number of surgery teams available (team is indexed as \(s\)\(= 1,...,S\)).
- \(J\) :
-
The total number of available ORs at the hospital (OR is indexed as \(j\)\(= 1,...,J\)).
- \(D\) :
-
Planning horizon (indexed with \(d\)).
- \(D^{\prime}\) :
-
A dummy day after planning horizon to accommodate excessive demand.
- \(E\) :
-
Longest length of stay (LOS) among all patients (indexed with \(e\)).
- \(V\) :
-
Types of inpatient beds (indexed with \(v\))
- \(B_{v}\) :
-
The number of beds of type \(v\).
- \(M_{dj}^{o}\) :
-
Maximal overtime of OR \(j\) on the day \(d\).
- \(p^{o}\) :
-
Unit overtime cost per operating room (OR).
- \(r\) :
-
Unit revenue per surgery.
- \(p_{v}^{h}\) :
-
Unit overflow cost per bed of type \(v\).
- \(\rm{\{ }Y_{is} \rm{\} }_{N \times S}\) :
-
Assignment matrix, where \(Y_{is}^{{}}\) = 1 if the surgery team \(s\) is assigned to treat patient \(i\); otherwise \(Y_{is}^{{}}\) = 0.
- \(Z_{i}^{{}}\) :
-
Type of patient; \(Z_{i}^{{}}\) = 1 if a patient is an inpatient; otherwise \(Z_{i}^{{}}\) = 0.
- \(\xi_{i}\) :
-
Surgery duration of patient \(i\).
- \(\beta_{sd}^{{}}\) :
-
Availability of surgical team; \(\beta_{sd}^{{}}\) = 1 if surgery team \(s\) is available on day \(d\); otherwise \(\beta_{sd}^{{}}\) = 0.
- \(T_{dj}\) :
-
The open duration of OR \(j\) on day \(d\).
- \(L_{i}\) :
-
The length of stay (LOS) of patient \(i\).
- \(W_{sd}\) :
-
The maximum working time of surgery team \(s\) on day \(d\).
- \(Due_{i}\) :
-
The assigned due date for patient \(i\)
- \(X_{idjv}^{{}}\) :
-
Binary variable; \(X_{idjv}^{{}}\) = 1, if the patient \(i\) is assigned to the OR \(j\), of bed type \(v\), on day d; otherwise \(X_{idjv}^{{}}\) = 0.
- \(N_{iev}^{{}}\) :
-
Binary variable; \(N_{iev}^{{}}\) = 1, if patient \(i\) of inpatient bed type \(v\) is discharged on day \(e\); otherwise \(N_{iev}^{{}}\) = 0.
- \(O_{dj}\) :
-
The total overtime of OR \(j\) on day \(d\).
- \(H_{iv}\) :
-
Binary variable; \(H_{iv}\) = 1 if the bed assigned to patient \(i\) is of the type \(v\); otherwise \(H_{iv}\) = 0
References
Abdullah, S., & Abdolrazzagh-Nezhad, M. (2014). Fuzzy job-shop scheduling problems: A review. Information Sciences, 278, 380–407.
Atighehchian, A., Sepehri, M. M., Shadpour, P., & Kianfar, K. (2020). A two-step stochastic approach for operating rooms scheduling in multi-resource environment. Annals of Operations Research, 292(1), 191–214.
Augusto, V., Xie, X., & Perdomo, V. (2010). Operating theatre scheduling with patient recovery in both operating rooms and recovery beds. Computers & Industrial Engineering, 58(2), 231–238.
Bastos, L. S. L., Marchesi, J. F., Hamacher, S., & Fleck, J. L. (2019). A mixed integer programming approach to the patient admission scheduling problem. European Journal of Operational Research, 273(3), 831–840.
Bazzoli, G., Brewster, L., Liu, G., & Kuo, S. (2003). Does U.S hospital capacity need to be expanded? Health Affairs, 22, 40–54.
Behmanesh, R. & Zandieh, M. (2019). Surgical case scheduling problem with fuzzy surgery time : An advanced bi-objective ant system approach. Knowledge-Based Systems, 186, 104913.
Bekker, R., Koole, G., & Roubos, D. (2017). Flexible bed allocations for hospital wards. Health Care Management Science, 20(4), 453–466.
Beliën, J., & Demeulemeester, E. (2007). Building cyclic master surgery schedules with leveled resulting bed occupancy. European Journal of Operational Research, 176, 1185–1204.
Bellman, R., & Zadeh, L. A. (1970). Decision-making in fuzzy environment. Management Science, 17(4), B141–B164.
Best, T. J., Sand, B., Eisenstein, D. D., & Meltzer, D. O. (2015). Managing hospital inpatient bed capacity through partitioning care into focused wings. Manufacturing & Service Operations Management, 17(2), 157–176.
Bonabeau, E., Dorigo, M., & Theraulaz, G. (1999). Swarm intelligence: From natural to artificial systems. Oxford University Press.
Bretthauer, K. M., Heese, H. S., Pun, H., & Coe, E. (2011). Blocking in healthcare operations: A new heuristic and an application. Production and Operations Management, 20(3), 375–391.
Buckley, J. J., & Feuring, T. (2000). Evolutionary algorithm solution to fuzzy problems: Fuzzy linear programming. Fuzzy Sets and Systems, 109(1), 35–53.
Chang, A., Katehakis, M.N., Shi, J. & Yan, Z. (2021). Blockchain-empowered Newsvendor optimization, International Journal of Production Economics, 238, 108144.
Chang, A., Lu, H., & Shi, J. (2019). Stock-out risk control of the production/inventory system with Compound Poisson demands”. Omega, the International Journal of Management Science, 83, 181–198.
Chang, A. & Shi, J. (2017). Pricing American option via the Transform-Expand-Sample forecasting methods, Journal of Business and Economics, Paper ID: JBE20160824–2, http://www.academicstar.us/issueshow.asp?daid=1942
Chen, S. J. & Hwang, C. L. (1992). Fuzzy multiple attribute decision making methods. Lecture Notes in Economics and Mathematical Systems, 375.
d’Obrenan, A., Ridder, A., Roubos, D., & Stougie, L. (2020). Minimizing bed occupancy variance by scheduling patients under uncertainty. European Journal of Operational Research, 286(1), 336–349.
Dai, J. G., & Shi, P. (2019). Inpatient overflow: An approximate dynamic programming approach. Manufacturing & Service Operations Management, 21(4), 894–911.
De, S. K., & Sana, S. S. (2015). Backlogging EOQ model for promotional effort and selling price sensitive demand-an intuitionistic fuzzy approach. Annals of Operations Research, 233, 57–76.
Denton, B. T., Miller, A. J., Balasubramanian, H. J., Huschka, T. R., Denton, B. T., & Miller, A. J. (2010). Optimal allocation of surgery blocks to operating rooms under uncertainty. Operations Research, 58(4), 802–816.
Durán, G., Rey, P. A., & Wolff, P. (2017). Solving the operating room scheduling problem with prioritized lists of patients. Annals of Operations Research, 258(2), 395–414.
Eun, J., Kim, S. P., Yih, Y., & Tiwari, V. (2019). Scheduling elective surgery patients considering time-dependent health urgency: Modeling and solution approaches. Omega, 86, 137–153.
Freeman, N. K., Melouk, S. H., & Mittenthal, J. (2016). A scenario-based approach for operating theater scheduling under uncertainty. Manufacturing and Service Operations Management, 18(2), 245–261.
Fügener, A., Hans, E. W., Kolisch, R., Kortbeek, N., & Vanberkel, P. T. (2014). Master surgery scheduling with consideration of multiple downstream units. European Journal of Operational Research, 239(1), 227–236.
Gao, L., Shi, J., Gorman, M., & Luo, T. (2020). Business analytics for intermodal capacity management. Manufacturing & Service Operations Management, 22(2), 310–329.
Gerchak, Y., Gupta, D., & Henig, M. (1996). Reservation planning for elective surgery under uncertain demand for emergency surgery. Management Science, 42(3), 321–334.
Gonzalez-Rodriguez, I., Puente, J., Vela, C. R., & Varela, R. (2008). Semantics of schedules for the fuzzy job-shop problem. IEEE Transactions on Systems, Man, and Cybernetics, 38(3), 655–666.
Green, L. (2012). The vital role of operations analysis in improving healthcare delivery. Manufacturing & Service Operations Management, 14(4), 488–494.
Gupta, D. (2007). Surgical suites’ operations management. Production & Operations Management, 16(6), 689–700.
Han, J., Liu, Y., Luo, L. & Mao, M. (2020). Integrated production planning and scheduling under uncertainty: A fuzzy bi-level decision-making approach. Knowledge-Based Systems, 201, 106056.
Izady, N., & Israa, M. (2021). A clustered overflow configuration of inpatient beds in hospitals. Manufacturing & Service Operations Management, 23(1), 139–154.
Jiménez, M., Arenas, M., & A. (2007). Linear programming with fuzzy parameters: An interactive method resolution. European Journal of Operational Research, 177(3), 1599–1609.
Katehakis, M. N., Melamed, B., & Shi, J. (2015). Optimal replenishment rate for inventory systems with compound Poisson demands and lost sales: A direct treatment of time-average cost. Annals of Operations Research. https://doi.org/10.1007/s10479-015-1998-y
Katehakis, M. N., Melamed, B., & Shi, J. (2016). Cash-flow based dynamic inventory management. Production and Operations Management, 25(9), 1558–1575.
Kumar, A., Costa, A. M., Fackrell, M., & Taylor, P. G. (2018). A sequential stochastic mixed integer programming model for tactical master surgery scheduling. European Journal of Operational Research, 270(2), 734–746.
Laarhoven, P. M. J., & Pedrycz, W. (1983). A fuzzy extension of Saaty’s priority theory. Fuzzy Sets and Systems, 134, 365–385.
Lee, S., & Yih, Y. (2014). Reducing patient-flow delays in surgical suites through determining start-times of surgical cases. European Journal of Operational Research, 238(2), 620–629.
Lei, D. (2010). A genetic algorithm for flexible job shop scheduling with fuzzy processing time. International Journal of Production Research, 48(10), 2995–3013.
Liu, N., Truong, V. A., Wang, X., & Anderson, B. R. (2019). Integrated scheduling and capacity planning with considerations for patients’ length of stays. Production and Operations Management, 28(7), 1735–1756.
Lu, H., Chen, X., Shi, J., Vaidya, J., Atluri, V., Hong, Y., & Huang, W. (2020). Algorithms and applications to weighted rank-one binary matrix factorization. ACM Transactions on Management Information Systems (TMIS), 11(2), 1–33.
Marcon, E., & Dexter, F. (2006). Impact of surgical sequencing on post anesthesia care unit staffing. Health Care Management Science, 9(1), 87–98.
Min, D., & Yih, Y. (2010). Scheduling elective surgery under uncertainty and downstream capacity constraints. European Journal of Operational Research, 206(3), 642–652.
Moosavi, A. & Ebrahimnejad, S. (2020). Robust operating room planning considering upstream and downstream units : A new two-stage heuristic algorithm. Computers & Industrial Engineering, 143, 106387.
Moreno, M. S., & Blanco, A. M. (2018). A fuzzy programming approach for the multi-objective patient appointment scheduling problem under uncertainty in a large hospital. Computers and Industrial Engineering, 123, 33–41.
Mountgomery, D. (2005). Design an analysis of experiments (6th ed.). John Wiley & Sons.
Neyshabouri, S., & Berg, B. P. (2017). Two-stage robust optimization approach to elective surgery and downstream capacity planning. European Journal of Operational Research, 260(1), 21–40.
Niu, Q., Jiao, B., & Gu, X. (2008). Particle swarm optimization combined with genetic operators for job shop scheduling problem with fuzzy processing time. Applied Mathematics and Computation, 205(1), 148–158.
Priya, R. D., Sivaraj, R., & Priyaa, N. S. (2017). Heuristically repopulated bayesian ant colony optimization for treating missing values in large databases. Knowledge-Based Systems, 133(1), 107–121.
Qi, L., Shi, J., & Xu, X. (2015). Supplier competition and its impact on firm’s sourcing strategy. Omega, the International Journal of Management Science, 55, 91–110.
Rachuba, S., & Werners, B. (2017). A fuzzy multi-criteria approach for robust operating room schedules. Annals of Operations Research, 251(1), 325–350.
Roshanaei, V., Luong, C., Aleman, D. M., & Urbach, D. R. (2017). Collaborative operating room planning and scheduling. INFORMS Journal on Computing, 29(3), 558–580.
Saadouli, H., Jerbi, B., Dammak, A., Masmoudi, L., & Bouaziz, A. (2015). A stochastic optimization and simulation approach for scheduling operating rooms and recovery beds in an orthopedic surgery department. Computers & Industrial Engineering, 80, 72–79.
Schneider, A. J., Theresia van Essen, J., Carlier, M., & Hans, E. W. (2020). Scheduling surgery groups considering multiple downstream resources. European Journal of Operational Research, 282(2), 741–752.
Seyfi-Shishavan, S. A., Donyatalab, Y., Farrokhizadeh, E. & Satoglu, S. I. (2021). A fuzzy optimization model for designing an efficient blood supply chain network under uncertainty and disruption. Annals of Operations Research. Springer US.
Shapiro, A., Dentcheva, D., & Ruszczyński, A. (2009). Lectures on stochastic programming. Modeling and theory. SIAM.
Shapiro, A. & Nemirovski, A. (2005). On complexity of stochastic programming problems. In Continuous Optimization (pp. 111–146). Springer.
Shi, J. (2016). Optimal production/inventory system subject to stock-out risk. Annals of Operations Research. https://doi.org/10.1007/s10479-016-2339-5
Shi, J., Katehakis, M. N., & Melamed, B. (2013). Martingale methods for pricing inventory penalties under continuous replenishment and compound renewal demands. Annals of Operations Research, 208(1), 593–612.
Shi, J., Katehakis, M. N., Melamed, B., & Xia, Y. (2014a). Optimal continuous replenishment for a production-inventory system with Compound Poisson demands and lost-sales. Operations Research, 6(5), 1048–1063.
Shi, J., Yue, X., & Zhao, Y. (2014b). Operations sequencing under yield loss. Naval Research Logistics, 61(2), 144–154.
Shi, J., & Zhao, Y. (2010). Some structural results on acyclic supply chains. Naval Research Logistics, 57(6), 605–613.
Shi, J., & Zhao, Y. (2014). The value of component commonality under Non-Holdback Allocation rules”. Operations Research Letters, 42(6–7), 409–413.
Shi, J., Zhao, Y., Kiwanuka, K., & Chang, A. (2019). Optimal selling policies for farmer cooperatives. Production and Operations Management, 28(12), 3060–3080.
Shi, P., Chou, M. C., Dai, J. G., Ding, D., & Sim, J. (2016). Models and insights for hospital inpatient operations: Time-dependent ED boarding time. Management Science, 62(1), 1–28.
Shylo, O. V., Prokopyev, O. A., & Schaefer, A. J. (2013). Stochastic operating room scheduling for high-volume specialties under block booking. INFORMS Journal on Computing, 25(4), 682–692.
Singh, S. K., & Yadav, S. P. (2018). Intuitionistic fuzzy multi-objective linear programming problem with various membership functions. Annals of Operations Research, 269(1), 693–707.
Song, H., Tucker, A., Graue, R., Moravick, S., & Yang, J. (2020). Capacity pooling in hospitals: The hidden consequences of off-service placement. Management Science, 66(9), 3799–4358.
Sun, L., Lin, L., Gen, M., & Li, H. (2019). A hybrid cooperative coevolution algorithm for fuzzy flexible job shop scheduling. IEEE Transactions on Fuzzy Systems, 27(5), 1008–1022.
Taguchi, G. (1987). The system of experimental design engineering methods to optimize quality and minimize cost. White Plains, N. Y.: UNIPUB.
Tavana, M., Zandi, F., & Katehakis, M. N. (2013). A hybrid fuzzy group ANP- TOPSIS framework for assessment of e-government readiness from a CiRM perspective. Information & Management, 50(7), 383–397.
Vancroonenburg, W., Causmaecker, P. De, & Berghe, G. Vanden. (2019). Chance-constrained admission scheduling of elective surgical patients in a dynamic, uncertain setting. Operations Research for Health Care, 22, 100196.
Wang, J., Chang, A., Li. Z., Shi, J. & Liu, H. (2022). Robust Surgical Scheduling for Non-Operating Room Anesthesia (NORA) under Surgical Duration Uncertainty, working paper, New Jersey Insitute of Technology.
Wang, J., Li, Z., Chang, A., & Shi, J. (2021). Capacity reallocation via sinking high-quality resource in a hierarchical healthcare system. Annals of Operations Research, 300(1), 97–135.
Wang, J., Li, Z., Shi, J. & Chang, A. (2020a). Hospital referral and capacity strategies in two-tier healthcare systems”, Omega, The International Journal of Management Science. 100, 102229.
Wang, K., Qin, H., Huang, Y., Luo, M. W. & Zhou, L. (2020b). Surgery scheduling in outpatient procedure centre with re-entrant patient flow and fuzzy service times. Omega, 102350.
Wang, K., Luo, H., Liu, F., & Yue, X. (2018). Permutation Flow Shop Scheduling With Batch Delivery to Multiple Customers in Supply Chains. IEEE Transactions on Systems, Man, and Cybernetics: Systems, 48(10), 1826–1837.
Wang, S., Wang, L., & Xu, Y. (2013). An estimation of distribution algorithm for solving hybrid flow-shop scheduling problem with stochastic processing time. International Journal of Production Research, 51(12), 2456–2461.
Xiao, T., & Shi, J. (2016). Pricing and supply priority in a dual-channel supply chain. European Journal of Operational Research, 254(3), 813–823.
Zhang, J., Dridi, M., & El Moudni, A. (2019). A two-level optimization model for elective surgery scheduling with downstream capacity constraints. European Journal of Operational Research, 276(2), 602–613.
Zhang, J., Dridi, M. & Moudni, A. El. (2020). Column-generation-based heuristic approaches to stochastic surgery scheduling with downstream capacity constraints. International Journal of Production Economics, 229(201604490106), 107764.
Acknowledgements
The authors are indebted to Dr. Endre Boros, Dr. Michael N. Katehakis, the Editor and the anonymous reviewer for their constructive comments and helpful suggestions that helped improve this paper considerably. This research is supported by the National Natural Science Foundation of China (71672019, 71903020), the Fundamental Research Funds for the Central Universities (DUT21RW406), the United States Department of Agriculture (USDA Award No. AM21TMATRD00C003), the Leir Research Institute and the Hurlburt Chair Endowment at NJIT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Appendix A: review of fuzzy theory
2.1 A.1 Definition of triangle fuzzy number
Denote \(T = \, \left( {a^{L} ,a^{M} ,a^{R} } \right)\), where \(0{ < }a^{L} \le a^{M} \le a^{R}\). T is called a triangular fuzzy number, and its membership function can be expressed as:
\( \mu _{A} (x) = \left\{ {\begin{array}{*{20}l} {\frac{{x - a^{L} }}{{a^{M} - a^{L} }},} & {{\text{if }}a^{L} {\text{ < }}x{\text{ < }}a^{M} } \\ {\frac{{x - a^{R} }}{{a^{M} - a^{R} }},} & {{\text{if }}a^{M} {\text{ < }}x{\text{ < }}a^{R} } \\ {0,} & {{\text{Otherwise}}} \\ \end{array} } \right.{\text{ }} \).
2.2 A.2 Fuzzy number operation rules
For any two TFNs, \(\tilde{M} = (m_{1} ,m_{2} ,m_{3} )\) and \(\tilde{N} = (n_{1} ,n_{2} ,n_{3} )\). Addition: \(\tilde{M} + \tilde{N} = (m_{1} + n_{1} ,m_{2} + n_{2} ,m_{3} + n_{3} )\). Subtraction: \(\tilde{M} - \tilde{N} = (m_{1} - n_{3} ,m_{2} - n_{2} ,m_{3} - n_{1} )\). Scalar multiplication:
\(\forall \lambda > 0,\lambda \in R,\lambda \tilde{M} = (\lambda m_{1} ,\lambda m_{2} ,\lambda m_{3} )\);
\(\forall \lambda < 0,\lambda \in R,\lambda \tilde{M} = (\lambda m_{3} ,\lambda m_{2} ,\lambda m_{1} )\).
2.3 A.3 Ranking of TFNs
For any two TFNs, \(\tilde{M} = (m_{1} ,m_{2} ,m_{3} )\) and \(\tilde{N} = (n_{1} ,n_{2} ,n_{3} )\). Fuzzy ranking can be expressed as:
Criterion 1: If \(C_{1} (\tilde{M}) = \frac{{m_{1} { + 2}m_{2} { + }m_{3} }}{4} > ( < )C_{1} (\tilde{N}) = \frac{{n_{1} { + 2}n_{2} { + }n_{3} }}{4},\) then \(\tilde{M} > ( < )\tilde{N}\).
Criterion 2: If \(C_{1} (\tilde{M}) = C_{1} (\tilde{N})\), then compare \(C_{2} (\tilde{M}) = m_{2}\) with \(C_{2} (\tilde{N}) = n_{2}\) to rank them.
Criterion 3: If \(C_{1} (\tilde{M}) = C_{1} (\tilde{N})\) and \(C_{2} (\tilde{M}) = C_{2} (\tilde{N})\), then \(C_{3} (\tilde{M}) = m_{3} - m{}_{1}\) is compared with \(C_{3} (\tilde{N}) = n_{3} - n{}_{1}\) as the last criterion.
2.4 A.4 Fuzzy expectation range and expectation interval
For any TFN, \(\tilde{M} = (m_{1} ,m_{2} ,m_{3} )\), expectation interval \(EI[\tilde{M}]\) and expectation value \(EV[\tilde{M}]\) can be expressed as: \(EI[\tilde{M}] = \left[ {E_{1}^{M} ,E_{2}^{M} } \right] = \left[ {\frac{{m_{1} + m_{2} }}{2},\frac{{m_{2} + m_{3} }}{2}} \right]\), \(EV[\tilde{M}] = \left[ {\frac{{E_{1}^{M} + E_{2}^{M} }}{2}} \right] = \left[ {\frac{{m_{1} + 2m_{2} { + }m_{3} }}{4}} \right]\).
Appendix B: heuristic of OR assignment and bed assignment



3.1 Appendix C
See Tables 8, 9, 10, 11, 12 and 13.
Appendix D: dexa evaluation criteria of the algorithm
The relative percentage deviation (RPD), as a common performance indicator, is used to evaluate the optimization efficiency of the algorithm. It is formulated as follows:
where \(R_{k}\) represents the target value of algorithm \(k\), and \(R_{best}\) denotes the target value of the optimal solution.
Due to the inherent randomness of meta-heuristics, each iteration of the experiment may produce different solutions; hence RPD cannot be directly applied to algorithm evaluation. Instead, we average out the above evaluation formulas to obtain the average relative percentage deviation (ARPD). Its formula is given as follows:
where n represents the number of iterations. Likewise, the best value for each run is the best relative percentage deviation (BRPD), with the formula given as follows:
where \(BR_{k}\) indicates the best target value among \(n\) runs.
Similarly, the worst value for each run is the worst relative percentage deviation (WRPD), the formula being defined as follows:
where \(WR_{k}\) represents the worst target value among \(n\) runs.
Rights and permissions
About this article
Cite this article
Wang, JJ., Dai, Z., Chang, AC. et al. Surgical scheduling by Fuzzy model considering inpatient beds shortage under uncertain surgery durations. Ann Oper Res 315, 463–505 (2022). https://doi.org/10.1007/s10479-022-04645-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10479-022-04645-z