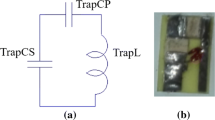
Abstract
This paper examines the design and implementation of a 21 MHz CMOS differential or so-called dual-path receiver (DPR) dedicated to nuclear magnetic resonance (NMR) spectrometer. This receiver features a CMOS chip incorporated with two mini-Coils and other circuitries. Herein we discuss the design and implementation of the DPR chip as the core part of this new NMR system. The DPR consists of two differential low-noise amplifiers, voltage buffers, phase shifters and variable gain amplifiers in order to accurately cancel the effect of the background NMR signal. We put forward the design and analysis of the DPR chip and thereafter demonstrate and discuss the simulation and experimental results. Based on these results, the front-end receiver achieves a voltage gain of 80 dB at a low input referred noise of 2.7 nV/√Hz. The chip is designed in a 0.13-µm CMOS technology and occupies an area of 1 mm × 2 mm. As per experimental results, this device can be used n the future low-cost NMR technologies.


















Similar content being viewed by others
References
Rosenstein, J. K., et al. (2012). Integrated nanopore sensing platform with sub-microsecond temporal resolution detection. Nature Methods, 9, 487–492.
Chen, Y., et al. (2012). CMOS high density electrical impedance biosensor array for tumor cell detection. Sensors and Actuators B: Chemical, 173, 903–907.
Ahmadi, M. M., et al. (2009). A wireless-implantable microsystem for continuous blood glucose monitoring. IEEE Transactions on Biomedical Circuits and Systems, 3, 169–180.
Scott, A., et al. (2013). A microfluidic microelectrode array for simultaneous electrophysiology, chemical stimulation, and imaging of brain slices. Lab on a Chip, 13, 527–535.
Ghafar-Zadeh, E., et al. (2010). Bacteria growth monitoring through a differential CMOS capacitive sensor. IEEE Transactions on Biomedical Circuits and Systems, 4(4), 232–238.
Eltoukhy, H., et al. (2006). A 0.18-µm CMOS bioluminescence detection lab-on-chip. IEEE Journal of Solid-State Circuits, 41, 651–662.
Hall, D. A., et al. (2013). A 256 pixel magnetoresistive biosensor microarray in 0.18 µm CMOS. IEEE Journal of Solid-State Circuits, 48, 1290–1301.
Stangi, C., et al. (2006). CMOS DNA sensor array with integrated A/D conversion based on label-free capacitance measurement. IEEE Journal of Solid-State Circuits, 41, 2956–2964.
Ghafar-Zadeh, E., et al. (2007). A hybrid microfluidic/CMOS capacitive sensor dedicated to lab-on-chip applications. IEEE Transactions on Biomedical Circuits and Systems, 1(4), 270–277.
Huang, Y., et al. (2011). Lab-on-CMOS integration of m. JM Rothberg et al. “An integrated semiconductor device enabling non-optical genome sequencing”. Nature, 475, 345–372.
Sun, N. (2011). Palm NMR and 1-Chip NMR. IEEE Journal of Solide State Circuits, 46, 1.
Pellecchia, M., Sem, D. S., & Wüthrich, K. (2002). NMR in drug discovery. Nature Reviews Drug Discovery, 1, 211–219.
Cabrita, L. D., Hsu, S.-T. D., Launay, H., Dobson, C. M., & Christodoulou, J. (2009). Probing ribosome–nascent chain complexes produced in vivo by NMR spectroscopy. Proceedings of the National Academy of Sciences of the United States of America, 106, 22239–22244.
Rutkowska, A., Beerbaum, M., Rajagopalan, N., Fiaux, J., Schmieder, P., Kramer, G., et al. (2009). Large-scale purification of ribosome–nascent chain complexes for biochemical and structural studies. FEBS Letters, 583, 2407–2413.
Maguire, Y., et al. (2007). Ultra-small-sample molecular structure detection using microslot waveguide nuclear spin resonance. PNAS, 104, 9198–9203.
Lee, H., Sun, E., Ham, D., & Weissleder, R. (2008). Chip-NMR biosensor for detection and molecular analysis of cells. Nature Medicine, 14, 869–874.
Hoult, D. I., & Richards, R. E. (1969). The signal-to-noise ratio of the nuclear magnetic resonance experiment. Journal of Magnetic Resonance, 24(1), 71–85.
Haken, R., et al. (2000). Anisotropy in tendon investigation in vivo by a portable NMR scanner, the NMR-MOUSE. Journal Magnetic Resonance, 144, 195–199.
Coil-on-a-chip, Broker Inc. https://www.bruker.com/products/mr/nm/probes/probes/coil-on-a-chip/overview.html.
Pourmodheji H., et al. (2015). Dual-path NMR receiver using double transceiver microcoils. IEEE EMBC.
Pourmodheji H., et al. (2015) Active nuclear magnetic resonance probe: A new multidiciplinary approach toward highly sensitive biomolecoular spectroscopy. IEEE Conference, ISCAS.
Anders, J., Chiaramonte, G., SanGiorgio, P., & Boero, G. (2009). A single-chip array of NMR receivers. Journal of Magnetic Resonance, 201, 239–249.
Cherifi, T., Abouchi, N., Lu, G. N., Bouchet-Fakri, L., Quiquerez, L., Sorli, B., et al. (2005). A CMOS microcoil-associated preamplifier for NMR spectroscopy. IEEE Transaction on Circuits and Systems, 52(12), 2576–2583.
Anders, J., Handwerker, J., Ortmanns, M., & Boero, G. (2013). A fully-integrated detector for NMR microscopy in 0.13 μm CMOS. In Solid-State Circuits Conference (A-SSCC), 2013 IEEE Asian (pp. 437–440).
Eichmann, C., Preissler, S., Riek, R., & Deuerling, E. (2010). Cotranslational structure acquisition of nascent polypeptides monitored by NMR spectroscopy. Proceedings of the National Academy of Sciences of the United States of America, 107, 9111–9116.
Benesi, A. J. (2015). A primer of NMR theory with calculation in mathematica Google Books.
Krojanski, H. G., Lambert, J. R., Gerikalan, Y., Suter, D., & Hergenröder, R. (2008). Microslot NMR probe for metabolomics studies. Analytical Chemistry, 80, 8668–8672.
Wensink, H., Benito-Lopez, F., Hermes, D. C., Verboom, W., Gardeniers, H. J., Reinhoudt, D. N., et al. (2005). Measuring reaction kinetics in a lab-on-a-chip by microcoil NMR. Lab on a Chip, 5, 280–284.
Kong, T. F., Peng, W. K., Luong, T. D., Nguyen, N.-T., & Han, J. (2012). Adhesive-based liquid metal radio-frequency microcoil for magnetic resonance relaxometry measurement. Lab on a Chip, 12, 287–294.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourmodheji, H., Ghafar-Zadeh, E. & Magierowski, S. A CMOS differential receiver dedicated to nuclear magnetic resonance applications. Analog Integr Circ Sig Process 91, 97–109 (2017). https://doi.org/10.1007/s10470-016-0901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10470-016-0901-3