Abstract
Background
Precision medicine, targeting treatments to individual genetic and clinical profiles, faces challenges in data collection, costs, and privacy. Generative AI offers a promising solution by creating realistic, privacy-preserving patient data, potentially revolutionizing patient-centric healthcare.
Objective
This review examines the role of deep generative models (DGMs) in clinical informatics, medical imaging, bioinformatics, and early diagnostics, showcasing their impact on precision medicine.
Methods
Adhering to PRISMA guidelines, the review analyzes studies from databases such as Scopus and PubMed, focusing on AI's impact in precision medicine and DGMs' applications in synthetic data generation.
Results
DGMs, particularly Generative Adversarial Networks (GANs), have improved synthetic data generation, enhancing accuracy and privacy. However, limitations exist, especially in the accuracy of foundation models like Large Language Models (LLMs) in digital diagnostics.
Conclusion
Overcoming data scarcity and ensuring realistic, privacy-safe synthetic data generation are crucial for advancing personalized medicine. Further development of LLMs is essential for improving diagnostic precision. The application of generative AI in personalized medicine is emerging, highlighting the need for more interdisciplinary research to advance this field.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Precision medicine marks a departure from traditional clinical care, focusing on tailoring the most effective therapeutic interventions to the unique genetic and clinical profiles of individual patients. This approach involves personalizing clinical decisions based on each patient's specific medical history and current condition, integrating clinical parameters with genomic profiling to formulate innovative diagnostic and therapeutic strategies (Ali and Aittokallio 2019; Collins and Varmus 2015; Moon et al. 2023; Shin et al. 2017).
Generative AI, a specialized subset of artificial intelligence (AI), is dedicated to the creation of new content, data, or solutions encompassing diverse forms such as text, images, and synthetic data. This innovative field employs machine learning models, with a strong emphasis on deep learning techniques, to produce outputs that are both novel and realistic. These outputs are derived from discerning and replicating patterns and structures found in existing data. In precision medicine, generative AI, particularly through advanced deep generative models (DGMs) such as Generative Adversarial Networks (GANs) and Variational Autoencoders (VAEs), has become an indispensable technology. These sophisticated AI models adeptly tackle difficult challenges such as data scarcity, privacy issues, and the complexities of modeling complex human health data. By generating synthetic patient data that maintains realism and authenticity, these models significantly enhance data analysis and interpretation, thereby advancing precision medicine (Barbiero et al. 2021; Goodfellow et al. 2014; Kipf and Welling 2016; Openai 2016).
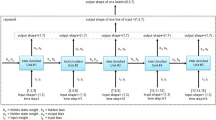
In Fig. 1 we illustrate a comprehensive overview of the versatile applications of generative AI in the context of precision medicine. It visually portrays the extensive utilization of generative AI techniques across a spectrum of critical healthcare domains. These include the analysis of Electronic Health Record (EHR) data, the interpretation and generation of medical imaging, the exploration of omics and biomarkers for drug discovery and response prediction, as well as the examination of physiological data and patient-reported information for digital diagnosis and decision-making support.
This review paper systematically examines the role of generative AI in precision medicine, focusing on its performance, limitations, and future directions. It highlights how generative AI, particularly DGMs, contributes to advancing personalized healthcare by generating high-quality synthetic data, enhancing diagnostic accuracy, and facilitating the development of personalized treatment plans. Unlike other reviews that broadly cover AI or machine learning in precision medicine, this paper specifically concentrates on the unique and up-to-date contributions and challenges of generative AI methodologies. It underscores the need for further interdisciplinary research to fully realize the potential of generative AI in transforming healthcare practices (Balla et al., n.d.; Bečulić et al., n.d.; Davri et al. 2022; Egger et al. 2022; Giannakopoulou et al. 2022; Kloczkowski et al. 2023; Rezayi et al. 2022; Sallam 2023; Song et al. 2023; Zerka et al. 2020).
To encapsulate the vast array of terminologies and core themes prevalent within the field of generative AI and its applications to precision medicine, we have synthesized a comprehensive word cloud from a curated collection of article titles, abstracts, and keywords. This visualization, represented in Fig. 1, serves not only as a testament to the rich vocabulary intrinsic to this interdisciplinary field but also as a beacon guiding us through the dense fog of complex concepts that characterize current research trends (Fig. 2).
Upon examination of the word cloud, several key findings emerge, painting a vivid picture of the current state of research in generative AI and precision medicine. Firstly, the prominence of terms related to "AI", "data", and "model" show a significant leaning towards computational methodologies and the harnessing of vast datasets to drive innovation in healthcare. This indicates not only the centrality of data-driven approaches but also the reliance on advanced modeling techniques to interpret complex biological information. Secondly, the visibility of specific methodologies, such as "deep learning" and "bioinformatics", hints at the cutting-edge tools being employed to navigate and decipher the multi-layered information intrinsic to personalized medicine. These tools represent the sharpened edge of the scalpel, meticulously carving out new pathways for treatment and diagnosis.
Moreover, the commonality of certain terms within the word cloud suggests a shared language that bridges diverse research efforts, pointing towards a consensus on the critical elements and challenges that define the field. This shared vocabulary facilitates a cohesive understanding and collaboration across disciplines, essential for tackling the multifaceted problems at the intersection of technology and healthcare. Finally, the word cloud subtly reveals potential research gaps and areas of saturation through the distribution of terms. While heavily represented terms suggest well-trodden paths, the less prominent ones may indicate niches or emerging areas ripe for exploration, guiding researchers towards uncharted territories that hold promise for groundbreaking discoveries.
In essence, this word cloud acts as both a mirror reflecting the current landscape of generative AI in precision medicine and a compass pointing towards future research directions. It encapsulates the collective knowledge and aspirations of the scientific community, offering insights into the dynamics of research trends and illuminating the way forward.
This review comprehensively analyzes literature from major databases such as Scopus (https://www.scopus.com/) and PubMed (https://pubmed.ncbi.nlm.nih.gov/), focusing specifically on the applications of generative AI in precision medicine. The paper is structured to provide an in-depth overview of generative AI technologies, their applications in various aspects of precision medicine, and the challenges they address, including data scarcity, privacy, and ethical considerations. The review then evaluates the performance of these AI models in enhancing patient care and discusses their current limitations. It concludes by exploring potential future advancements and advocating for more collaborative research efforts. The aim is to offer a detailed understanding of how generative AI is shaping the future of precision medicine, distinguishing this review from more general analyses of AI in healthcare and emphasizing the transformative potential of generative AI in this field.
2 Method
2.1 Overview
Adhering to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (Page et al. 2021) guidelines, the protocol for this paper was drafted and the review was conducted thoroughly. The approach involves a structured and transparent method for gathering, evaluating, and synthesizing the research study.
2.2 Information retrieval strategy
2.2.1 Search sources
A thorough search was conducted on the Scopus (https://www.scopus.com/) and PubMed (https://pubmed.ncbi.nlm.nih.gov/) databases to identify research papers specifically addressing the utilization of the generative AI models in personalized medicine on 06 December 2023. The initial search on these databases yielded 481 articles (129 from Scopus and 252 from PubMed).
2.2.2 Search terms
The search strategy for this systematic review was meticulously developed, drawing upon insights from existing literature. To capture the scope of our research accurately, we incorporated a range of terms specifically associated with generative AI, such as "generative adversarial networks" and "GANs," as well as terms relevant to precision medicine, including "personalized medicine" and "patient-centric medicine." Our searches in various bibliographic databases were conducted using carefully crafted combinations of these key terms, which are detailed in Appendix 1. We refined our search criteria further by focusing exclusively on English-language journal articles published within the last decade. This time frame was chosen to ensure that our review includes the most recent advancements and trends in the rapidly evolving fields of generative AI and precision medicine. The rationale behind these search parameters and the specific databases searched are outlined in the methodology section, ensuring transparency and reproducibility of our research process.
2.2.3 Study eligibility criteria
The inclusion criteria for this research article include original research papers that have been accepted and published, with a specific focus on exploring Generative AI and individualized medicine. The selected studies should fall within the 10-year timeframe spanning from 2013 to 2023. The chosen period coincides with the emergence of modern generative models (Bao et al. 2017; Naveed et al. 2023; Goodfellow et al. 2014). Any articles in the English language, without country restrictions, and those involving proposals and forecasting generative-aimed subject topics are eligible for inclusion. Conversely, the exclusion criteria involve studies that concentrate on generic AI in precision medicine. Publications in languages other than English, those not peer-reviewed, and those derived from unreliable or non-original sources (such as Wikipedia, posters, reviews and survey studies, editorials, commentaries, or duplications) are excluded from consideration. These criteria aim to ensure the selection of high-quality and relevant research for the comprehensive review. Some of the eligibility criteria are applied utilizing the database search filter, and the study eligibility criteria are summarized in Table 1.
2.2.4 Study selection and screening process
Our study selection and screening process was meticulously designed and executed in three distinct phases to ensure only the most relevant and high-quality studies were considered. Initially, targeted searches were conducted to compile an initial set of studies. This was followed by a rigorous process to remove any duplicates. Subsequently, titles and abstracts were reviewed to filter out studies that did not align with our research objectives, allowing for a more focused evaluation of potentially relevant studies. The final phase involved a detailed full-text review against a set of predefined criteria, ensuring the inclusion of studies that truly contributed to our understanding of the field. Throughout this process, any discrepancies between reviewers were resolved through discussion to maintain the integrity of the research selection process. By adhering to these stringent criteria, we aimed to construct a solid foundation for our analysis, free from the influence of less relevant or lower-quality studies.
2.2.5 Data items and data extraction process
A granular exploration of the included papers was achieved through a meticulous and systematic analysis. This task was undertaken by a designated author for each study, ensuring a focused and comprehensive analysis. The extraction covered bibliographic details (including the first author, publication year, and title), specifics of the generative model employed (detailing any modifications and the intended purpose of the model), key findings, datasets and their characteristics, evaluation metrics used, and the reported accuracy, strengths, and limitations of each study. This comprehensive examination ensured a deep understanding of each study's unique contribution to the field, revealing the intricacies and unique contributions of the research, thereby laying the groundwork for a substantive synthesis of the gathered insights.
2.2.6 Data synthesis
Upon analyzing the extracted data, we identified four clearly defined themes present in the selected papers. These themes encompassed various aspects of the application of generative AI in the medical field. The first theme highlighted the use of bioinformatics in the context of personalized medicine, showcasing how AI can be leveraged to tailor treatments to individual patients. The second theme focused on clinical informatics, illustrating how AI technologies can be utilized to streamline healthcare processes and improve patient care. The third theme delved into medical imaging informatics, demonstrating the role of AI in enhancing diagnostic capabilities through advanced imaging analysis. Lastly, the fourth theme highlighted the emergence of Large Language Models (LLMs) as a pivotal force in transforming personalized medical research and practice, indicating a significant shift in the field towards more sophisticated AI-driven approaches. These themes highlight the breadth and depth of current research endeavors and underscore the diverse applications of generative AI in the realm of personalized medicine.
2.2.7 Quality assessment
The assessment of each study incorporated into the review involved a careful examination of various factors to ensure a thorough and unbiased evaluation of the prevailing state of generative AI applications in the context of personalized medicine. This involved exploring several key factors, including the clarity and transparency of results presentation, ensuring that findings were easily understandable. Another critical criterion was the relevance of each study to the overarching theme of the review, highlighting the necessity of aligning the research with the specific focus on generative AI applications in precision medicine. This multifaceted approach to quality assessment was instrumental in curating a selection of studies that not only meet high academic standards but also directly contribute to the discourse on generative AI's applications in precision medicine.
2.2.8 Review of literature
The use of generative AI in patient-centric medicine is still in its early stages, but it has the potential to revolutionize the way we diagnose and treat diseases. The utilization of generative AI models within the domain of precision medicine has experienced exponential growth in the past decade. This significant interest can be attributed to the unique capabilities of these models in generating diverse data types, encompassing fundamental microscopic details, intricate imaging, varied modalities, and multidimensional representations. Figure 3 presents a quantitative analysis of publications dedicated to generative AI in personalized medicine, employing search data retrieved from the Scopus database for the period 2014–2023.
The graph shows that the number of publications on generative AI in personalized medicine has increased dramatically in recent years. In 2014, there were only 2 publications on the topic. However, by 2023, there were 52 publications. This represents a more than 25-fold increase in the number of publications in just 9 years. This suggests that generative AI is having a major impact on the field of individualized medicine.
3 Results
3.1 Search and selection
From the initial search of the two electronic databases retrieved 481 citations, we meticulously applied our selection criteria. Following the removal of duplication and title/abstract screening, 407 were excluded. A close examination of the 50 remaining full-text articles resulted in the further exclusion of 21, as depicted in Fig. 4. Ultimately, only 29 studies aligned with our stringent criteria and were included in this systematic review.
3.2 Characteristics of the included studies
As shown in Table 2 it is evident from the increasing number of publications in this domain, rising from one article in 2019 to 17 articles in 2023. The global nature of this research is highlighted by the diverse geographic distribution of publications, with leading contributions from the United States and China. All the included publications that have been reviewed are journal articles with the majority focusing on applications in clinical informatics (31%), medical imaging (28%), and bioinformatics (31%).
3.3 Findings of the included studies
DGMs are advancing in clinical informatics, including the generation of synthetic patient data, data anonymization, and the development of predictive models, contributing significantly to realizing the potential of precision medicine while addressing privacy and ethical concerns. These models also contribute significantly to advancing our understanding of complex biological processes, enabling personalized medicine, and improving treatment outcomes across various diseases. Meanwhile, in imaging informatics, the convergence of generative AI and medical imaging is propelling advancements in disease prognosis and diagnosis, exemplified by studies in pulmonary imaging, neuroimaging, retinal imaging, and cancer molecular imaging. These breakthroughs underscore the transformative potential of informatics and advanced technologies in shaping the future of healthcare and personalized medicine. In bioinformatics, the spotlight is mainly on VAEs and GANs, but also foundation models like LLMs for gene prioritization and knowledge-driven candidate selection, highlighting the capability of these models in uncovering hidden patterns within multi-omics data. Furthermore, the integration of LLMs in precision oncology and radiation oncology presents a paradigm shift in decision support for healthcare practitioners, offering complementary insights and potentially enhancing individualized clinical decision-making.
Table 3 provides an overview of the 29 included papers, presenting the applied DGMs or LLMs along with their modified or underlying generative model and the focus of their application.
3.3.1 Clinical informatics
Synthetic patient data generation
A recent study (El Emam 2023) developed a method using conditional generative adversarial networks (cGANs) to generate synthetic patient data from real-world clinical and genomic sources for myeloid malignancies such as myelodysplastic syndromes and acute myeloid leukemia. The model was trained on over 7,000 patients’ data and evaluated the synthetic data using a synthetic validation framework, finding high fidelity for clinical, demographic, genomic, and outcome features as well as strong privacy preservability. They showed the synthetic data could effectively recapitulate disease subgroups, prognostic scoring systems, and results from clinical trials. Additionally, generating augmented synthetic cohorts allowed for predicting later insights years ahead of real studies. A web portal (https://sdg-webserver-cloudrun-xkb3corsxq-ew.a.run.app/) was created for clinicians to generate synthetic cohorts, demonstrating the potential of this approach to enhance data use and accelerate personalized medicine in hematology.
By injecting realistic EHR data, clinical informatics can overcome data scarcity and unlock new possibilities for analysis and prediction. The paper (Bernardini et al. 2023) addresses this by proposing a data imputation technique called ccGAN (clinical conditional Generative Adversarial Network) to handle missing values in EHR datasets. The ccGAN approach conditions the generation of missing values for partially observed features (called yellow predictors) on both the available values of fully observed features (called green predictors) as well as other clinical information that is usually available in EHR datasets like age, weight, etc. This allows the ccGAN to capture nonlinear relationships between features when imputing missing values. The ccGAN approach is evaluated on a real multi-center diabetic dataset and is shown to outperform other state-of-the-art imputation techniques in terms of both imputation accuracy and predictive performance for diabetic retinopathy detection tasks. An additional experiment on a benchmark MIMIC-III dataset (Purushotham et al. 2018) further demonstrates the robustness of ccGAN across different missingness rates. Furthermore, the paper (Li et al. 2023) proposes a novel approach to improving prediction models trained on EHRs when labeled data is limited. The researchers introduce a network-based generative adversarial semi-supervised method that leverages graph representations of EHR datasets and a GAN to generate synthetic data. This synthetic data aims to bridge the density gap between classes in the real sample data. The approach also incorporates a modified discriminator loss to enhance semi-supervised learning performance while generating privacy-preserving data. The paper presents experimental results on four datasets, demonstrating that their approach outperforms other semi-supervised methods and achieves performance comparable to supervised learning using only 10% of the labels. Similarly, the research paper (Ahuja et al. 2022) explores an automatic phenotyping method called MixEHR-Guided (MixEHR-G), which is a multimodal hierarchical Bayesian topic model that can efficiently model large-scale EHR data to identify latent phenotype structure and simultaneously predict up to 1515 well-defined phenotypes. MixEHR-G uses priori information from clinical coding systems like PheCodes (Wei et al. 2017) to guide the posterior topic inference, allowing it to learn interpretable and clinically meaningful phenotypes in an unsupervised manner. It was applied to the Population Health Record (PopHR) dataset (Yuan et al. 2017) containing administrative claims data for 1.3 million patients in Quebec, and the MIMIC-III dataset (Purushotham et al. 2018) containing intensive care unit observations.
Data anonymization and privacy preservation
While EHRs offer exciting possibilities in the digital healthcare arena, data privacy and ethical issues remain a prominent concern. To address this, research papers (Yoon et al. 2020) and (Piacentino et al. 2021) propose utilizing GANs in the synthetize of data in the health sector focusing on anonymizing users' information. The paper (Yoon et al. 2020) discusses a novel framework called ADS-GAN (Anonymization through Data Synthesis using Generative Adversarial Networks) to generate synthetic EHR data that closely approximates the joint distributions of variables in the original dataset while satisfying a quantifiable definition of identifiability. ADS-GAN modifies the conditional GAN framework by optimizing the conditioning variables for each patient to improve data quality while ensuring no combination of features can readily identify a patient. It also uses WGAN-GP to improve training stability. Experiments on four real-world healthcare datasets demonstrate that ADS-GAN outperforms other methods in preserving data characteristics and distributions across different identifiability levels. Predictive models trained on ADS-GAN synthetic data perform similarly to those trained on real data, showing it can generate useful realistic synthetic datasets that address both data distribution and privacy constraints to enable wider sharing of healthcare data for AI research and development while protecting patient confidentiality. Following the same line of thought, the paper (Piacentino et al. 2021) proposes a procedure for GAN-based anonymization of general health data, including both images and raw static data in the context of electrocardiograms (ECGs).
Prediction models and prognosis
DGMs have also proven instrumental in enhancing the understanding and prediction of prognosis and diagnosis from patients' data. In the study (Hsu and Lin 2023), a new machine learning framework called SCAN (Semi-supervised Cancer prognosis classifier with Bayesian variational auto-encoder) was proposed for predicting cancer patient prognosis using small medical datasets. SCAN utilizes both labeled and unlabeled patient data in a semi-supervised manner to train deep-learning models. It is tested on breast cancer and non-small cell lung cancer (NSCLC) datasets for predicting 5-year survival rates. The performance gains observed stemmed from SCAN's ability to fully leverage all available patient information. Another innovative framework is demonstrated in the paper (Zhu et al. 2023) in which a deep learning model GluGAN is designed for generating personalized glucose time series data using GANs that provide additional support for decision-making in the management of Type 1 Diabetes (T1D). GluGAN incorporates recurrent neural network modules and a combination of unsupervised and supervised training to learn the temporal dynamics in latent spaces. It is evaluated on three clinical datasets containing data from 47 T1D patients, and it outperforms four baseline GAN models according to various quantitative metrics assessing the quality of synthetic data. The paper also discusses an application of GluGAN, where it is used to augment training data for glucose prediction algorithms, leading to a significant reduction in prediction errors.
Counterfactual explanations and causal inference
A recent scholarly article (Pearl 2018) contended that counterfactual explanations have the potential to offer the utmost interpretability in machine learning models and can serve as a foundation for generating causal inferences. Taking this into account the paper (Zhou et al. 2023) proposes a new method called Sparse CounteRGAN (SCGAN) to generate counterfactual explanations for predicting the response of breast cancer patients to neoadjuvant systemic therapy (NST). SCGAN aims to overcome the limitations of existing counterfactual generation methods by producing counterfactuals that are sparse, diverse, and plausible while maintaining proximity to the original instances. It utilizes a generative adversarial network with dropout training of the discriminator to encourage sparsity and introduces a diversity term to maximize the distance between counterfactuals. A masking approach is also used to handle immutable features and ensure plausibility. Evaluation of benchmark datasets and a breast cancer MRI dataset shows SCGAN can generate counterfactuals with higher prediction probabilities and sparser feature changes compared to baseline methods, helping to identify causal relationships between imaging phenotypes, clinical information, molecular features, and pathologic response to NST to guide more informed treatment decisions.
3.3.2 Medical imaging informatics
Pulmonary imaging
Generative models have seen a lot of use recently in a variety of medical imaging domains, such as label-to-image, mask-to-image, and medical cross-modality translation (Ben-Cohen et al. 2017; Goodfellow et al. 2014; Nie et al. 2017; Schlegl et al. 2017; Uzunova et al. 2020; Wang et al. 2021; Yao et al. 2021). Consequently, the paper (Sui et al. 2021) proposes a deep learning-based radio genomics framework to analyze the correlation between lung cancer imaging and gene expression data. It first segments tumor regions from CT images using U-Net. Then it uses a conditional auto-encoder to extract multi-level image features under gene expression conditions. Various analyses like survival prediction and genomic data using gene-set enrichment analysis (GSEA) are performed to validate the correlation between image features, genes, and prognosis data. Finally, a modified CVAE-GAN (Bao et al. 2017) is used to generate tumor images from gene expression data for visualization purposes. This allows for the exploration of the relationship between gene expression and tumor imaging, providing a means to visually represent the correlation between the two data modalities. Similar to this theme, a recent study (Rafael-Palou et al. 2022) also discusses U-HPNet, a deep-learning model created to forecast the advancement of lung nodules identified in CT scans. U-HPNet is specifically designed to tackle the uncertainty linked to image noise in medical images and discrepancies in annotations made by different radiologists. It takes an initial CT image of a nodule as its input and generates predictions regarding its growth, estimates the anticipated size of future growth, and provides a probabilistic segmentation outlining the expected appearance of the nodule at a later time point. The paper's primary emphasis is on the creation and assessment of U-HPNet as a tool for foreseeing lung nodule progression, with potential implications for influencing clinical decision-making and enhancing patient care.
Longitudinal neuroimaging analysis of glioblastoma patients
(Elazab et al. 2020) introduced GP-GAN, a novel brain tumor growth prediction method using 3D generative adversarial networks. By analyzing longitudinal MRI scans, GP-GAN predicts future tumor boundaries more accurately, utilizing a modified 3D U-Net generator and a new L1 and Dice loss-based objective function. Tested on 18 subjects, it surpassed traditional and deep learning models in several metrics, showcasing GANs' effectiveness in data-driven tumor prediction. Further advancements are seen in (Strack et al. 2023), where personalized neural networks, trained on individual patient MRI data, utilized Wasserstein GANs for tumor change detection, proving effective in glioblastoma tumor monitoring and modified RANO classification.
Alzheimer’s’ disease and mild cognitive impairment detection
The importance of individualized brain atrophy in Alzheimer's disease (AD) and mild cognitive impairment (MCI) lies in its ability to underscore the variability in disease progression and treatment responses among patients. This understanding could lead to more personalized treatments, enhancing their effectiveness. The paper by (Gao et al. 2023) introduces a novel method, BrainStatTrans-GAN, to decode individual brain atrophy in AD and MCI. This approach uses a generative adversarial network to transform diseased brain images into healthy ones, with a residual-based multi-level fusion network (RMFN) aiding in disease classification and diagnosis. Tested on 1,739 subjects across three datasets, this method outperforms others in the classification and interpretation of individual changes, underlining its significance in tackling the complexity of neurodegenerative diseases.
Furthermore, (R. Shi et al. 2023a, b) present another framework, GANCMLAE (Generative Adversarial Network Constrained Multiple Loss Autoencoder), focusing on detecting individual atrophy patterns in AD and MCI. Leveraging structural MRI data from the ADNI cohort, this model demonstrates robust image reconstruction and the potential to identify varying MCI atrophy patterns, surpassing traditional group-level methods. Its ability to predict MCI conversion to dementia highlights GANCMLAE's promise for precise, individualized diagnosis and monitoring of AD and MCI.
Retinal imaging
Age-related macular degeneration (AMD) with new blood vessel growth can cause severe vision loss (Bressler et al. 1982). For neovascular AMD, blocking anti-vascular endothelial growth factor (VEGF) remains the primary line of defense (Solomon et al. 2019). The paper (Moon et al. 2023) proposes an AI model using attentionGAN to predict short-term anatomical treatment outcomes for different anti-VEGF agents in patients with neovascular age-related macular degeneration (AMD). The researchers aim to leverage the capabilities of attentionGAN, a type of generative adversarial network (GAN), to make predictions related to treatment outcomes for this specific medical condition. Notably, the AI model outperforms human examiners in predicting the presence of residual fluid after treatment, but its performance is comparable to or slightly lower than examiners in predicting the complete resolution of retinal fluid. The study emphasizes the importance of predicting fluid status for selecting appropriate anti-VEGF agents in personalized medicine.
Synthesis and biodistribution of QDs for breast cancer imaging
The frontiers of cancer molecular imaging are being pushed by nanotechnology, with quantum dots (QDs) emerging as a groundbreaking probe due to their exceptional optical and electronic properties, unlocking possibilities for both in vitro and in vivo applications (Fang et al. 2012). The study (Tang et al. 2021) introduces a sophisticated generative model named Generative Adversarial Network for Distribution Analysis (GANDA) to characterize and conditionally produce the intertumoral distribution of QDs in breast cancer tissues following intravenous administration. GANDA undergoes training by breaking down entire-slide images of tumor sections into patches that carry information about tumor vessels, cell nuclei, and QDs. It is capable of accurately generating the QD distribution based on the provided vessels and nuclei channels. The generated QD distribution allows for quantitative analysis, enabling calculations such as extravasation distance and subarea distribution. GANDA showcases the potential for predictive modeling of nanoparticle distribution, offering insights into customizing the design of nanomedicine for individual cases.
3.3.3 Bioinformatics: integrated omics and biomarkers profiling
Drug response prediction
Predicting prognostic outcomes is crucial for advancing personalized medicine in cancer treatment, as emphasized by (M. Shi et al. 2023a, b). (Wang et al. 2023) contributed significantly to this field by developing the Multi-Omics Integrated Collective Variational Autoencoders (MOICVAE), a novel deep-learning framework for predicting cancer drug sensitivity. This model utilizes integrated genomic and transcriptomic data, employing a multimodal deep autoencoder and a collective variational autoencoder for enhanced prediction accuracy. MOICVAE's effectiveness was demonstrated through high AUCs on major datasets such as the Genomics of Cancer Drug Sensitivity (GDSC) and the Cancer Cell Line Encyclopedia (CCLE), highlighting its potential in personalizing cancer treatment. Additionally, (Rampášek et al. 2019) introduced Dr.VAE, a semi-supervised variational autoencoder designed for generative modeling of post-treatment gene expression. Dr.VAE's capability was evaluated through the expected root mean square error (RMSE) in both latent and gene spaces, offering valuable insights into its predictive accuracy compared to baseline models.
Further advancements in the field were made by (Xue et al. 2020), who utilized two different deep generative models (DGMs)—the variational autoencoder (VAE) and the supervised vector-quantized variational autoencoder (S-VQ-VAE). These models were trained on datasets containing gene expression profiles influenced by small molecules or gene knockdowns. Their study revealed that VAE could effectively regenerate gene expression data and encapsulate relationships between perturbagens. Additionally, S-VQ-VAE’s embedding space sheds light on the shared mechanisms of action among perturbagen classes. In a separate study, (M. Shi et al. 2023a, b) developed CSAM-GAN, a generative adversarial network enhanced with sequential channel-spatial attention modules. This method, applied to datasets like lower-grade glioma (LGG) and kidney renal clear cell carcinoma (KIRC) from TCGA, showcased the potential of integrating multimodal data (miRNA expression, mRNA expression, histopathological images) for accurate cancer prognostic outcome prediction.
Drug candidate generation
The paper (Yamanaka et al. 2023) introduces a novel computational method named DRAGONET, designed for the generation of potential drug candidates tailored to specific diseases using patient gene expression profiles and deep learning. DRAGONET leverages gene expression data from patients afflicted with a particular disease to discern patterns associated with that ailment. Subsequently, it employs a generative neural network, specifically a variational autoencoder, to create novel molecular structures that are computationally predicted to target disease-associated genes and biological processes. The underlying transformer-based variational autoencoder (TransVAE) maps these molecules to latent space coordinates, facilitating the production of new molecules through the exploration of this space. The methodology was applied to generate drug molecules targeting gastric cancer, atopic dermatitis, and Alzheimer's disease. The newly generated drugs exhibited a closer structural resemblance to registered drugs for each disease when compared to the initial disease reversal molecules.
Individualized treatment effects
The publication (Ge et al. 2020) examines the development of precision oncology through the use of a counterfactual generator in the imputation block, which is a non-linear function of various vectors and random variables. The proposed MGANITE was applied to 256 newly diagnosed acute myeloid leukemia (AML) patients, and the data were obtained from the M. D. Anderson Cancer Center database. MGANITE is a modified GAN that refers to “Modified Generative Adversarial Network for Individualized Treatment Effects”. MGANITE is designed to improve the accuracy of estimating individualized treatment effects and to identify optimal treatment strategies for patients, particularly in the context of precision oncology. It extends the capabilities of previous conditional generative adversarial network (CGAN)-based causal inference methods by enabling the estimation of individualized effects of continuous treatments, developing new network structures for the generator and discriminator in the CGANs, and combining sparse techniques for the selection of biomarkers to predict the best treatment for each patient.
Clinical endpoints simulation
The study (Barbiero et al. 2021) demonstrates the development of a digital patient model using a Generative Adversarial Network (GAN) and a Graph Neural Network (GNN) to simulate and forecast clinical endpoints. The model is trained and validated using a learning process lasting 50 epochs with a learning rate of 0.01. The results include a 95% confidence interval estimation of the evolution of each variable over time, and the assessment of prediction uncertainty using dropout techniques. In the study, the GAN model is extended to address the specific problem related to gene expression samples by simultaneously accounting for multiple tissue types from the same donor. The extension involves building on a Wasserstein GAN with gradient penalty (WGAN-GP) to estimate a generative model via an adversarial process. The generator in the GAN model aims at producing samples from the conditional P (X|M, R, Q), where X represents gene expression values, and M, R, and Q are different parameters. The model is designed to generate gene expression data for synthetic patients in every modeled tissue type without any missing values, facilitating the cross-tissue analysis of gene expression. This extension allows for a more comprehensive and integrated analysis of gene expression samples across multiple tissue types, addressing the specific problem of joint observations for different tissue types in real datasets. In addition, the generative model is used for transcriptomics data to simulate the effects of SARS-CoV-2 (i.e., the virus responsible for the COVID-19 pandemic) infection on gene expression in various tissues.
Gene expression augmentation
It is prominent to develop frameworks for generating and evaluating diverse and reliable genetic data, which can ultimately lead to more personalized and effective medical interventions for individual patients. The paper (Jahanyar et al. 2023) proposes a modified auxiliary classifier generative adversarial network (MS-ACGAN) for augmenting schizophrenia gene expression microarray data. It downloaded two schizophrenia microarray datasets from NCBI and performed feature selection to select the most relevant genes. It then implemented four types of GANs (basic GAN, modified GAN, ACGAN, MS-ACGAN proposed in the paper) and five classifiers (LR, KNN, SVM, MLP, 1DCNN) for generating and evaluating samples. Five different volumes of data were generated based on stopping criteria. The proposed MS-ACGAN feeds the generator with restricted Gaussian noise based on mean and standard deviation of training data. The model was evaluated using quantitative measures like GAN-test, and GAN-train that measure the quality and diversity of generated data respectively. Statistical techniques like calibration plots, Brier score, and confidence intervals were used to characterize model reliability. The experimental results showed that MS-ACGAN outperformed other models in terms of data quality, diversity, and reliability.
Multi-omics data integration
The study (Ahmed et al. 2022) shows a framework known as omicsGAN, designed to revolutionize the integration of multi-omics data and their biological interaction networks. The process began by inputting mRNA and miRNA expression data, along with their interaction network, into the omicsGAN model. Through an intricate series of iterations, an adversarial game unfolded between a generator and a critic, resulting in the updating of the input omics data. In study refers to a component of the omicsGAN model, which is a generative adversarial network (GAN) tailored for multi-omics data integration. The generator meticulously synthesized new mRNA and miRNA datasets, harnessing information from the input omics and their interaction network, while the critic diligently discerned between real and synthetic data. Upon completion of the training, the model produced final synthetic mRNA and miRNA datasets, enriched with comprehensive information from the input omics and their interactions. These synthetic datasets were then leveraged for disease phenotype prediction tasks, such as cancer classification and survival analysis. The study's experiments on real cancer datasets showcased the remarkable predictive performance of the synthetic omics data generated by omicsGAN, underscoring its success in seamlessly integrating multi-omics and network information to enhance disease outcome predictions.
3.3.4 Utilization of foundation models (FMs)
Precision oncology with LLMs support
Healthcare practitioners can utilize the support of LLMs like ChatGPT to enhance clinical decision-making, despite their current limitations compared to human experts. (Benary et al. 2023) demonstrated this in precision oncology, comparing treatment recommendations from human experts and LLMs for ten fictional cancer cases. The LLMs evaluated were BioMed LM (MosaicML; Stanford University), Perplexity.ai (University of California, Berkeley), ChatGPT (OpenAI), and Galactica (Meta), using metrics like precision and recall. A molecular tumor board (MTB) then assessed the usefulness of these recommendations. Although LLMs didn't reach the expertise level of humans, they offered valuable complementary insights. Another study by (Huang et al. 2023)benchmarked ChatGPT-4 in radiation oncology. Using the radiation oncology in-training exam (TXIT) with 300 questions and 15 clinical cases, ChatGPT-4's performance in providing accurate treatment recommendations was evaluated, underscoring its potential in complex medical fields.
LLMs in multi-omics data exploration
As microscopes of information, LLMs are also peering into the depths of multi-omics data, revealing hidden patterns and connections that hold the key to unleashing the next level of personalized medicine. The paper (Toufiq et al. 2023) explored the potential for using LLMs to assist with knowledge-driven candidate gene prioritization and selection. The researchers first evaluated four LLMs (GPT-3.5, GPT-4, Gemini, and Claude) on tasks like identifying functional relationships between genes and scoring genes against relevance criteria. This identified GPT-4 and Claude as the best-performing models. They then established a multi-step workflow for these LLMs, which involved: selecting candidate gene modules, identifying functional themes among genes, scoring genes, summarizing justifications, validating justifications with references, and selecting top candidates. This workflow was applied to prioritize 11 modules associated with an erythroid signature. The LLMs incorporated profiling data to finalize top gene picks, and their selections were generally agreed upon. The results demonstrated LLMs can enhance gene prioritization efficiency with minimal human input, though limitations like the potential for incorrect information still need addressing for broader adoption as research tools. As the field of artificial intelligence continues to evolve, studies like these contribute significantly to our understanding of the capabilities and limitations of LLMs, paving the way for their responsible integration into various personalized medical disciplines.
Table 4 compiles the results from the examination of 29 papers focused on generative artificial intelligence in precision medicine. It provides comprehensive analysis aims to understand how these methods operate in diverse datasets, assesses the metrics used to quantify their performance, and quantifies the accuracy levels attained in the context of personalized medicine applications. The papers under consideration make use of diverse datasets for their studies in generative artificial intelligence across various domains. These datasets encompass a wide range of medical and biological contexts. Notable datasets include: The Cancer Genome Atlas (TCGA), Medical Information Mart for Intensive Care (MIMIC-III), the Gene-expression omnibus (GEO) archive, Library of Integrated Network-based Cellular Signatures (LINCS), and Alzheimer's Disease Neuroimaging Initiative (ADNI). In the publications, various performance metrics were reported based on the nature of the tasks and the type of data being analyzed. The selection of metrics is contingent upon the particular tasks and goals of each study. These metrics encompass standard machine learning measures such as accuracy (AUC), precision, F1 score, recall, and extend to task-specific metrics like imputation accuracy, clinical and genomic syntenic fidelity, Jaccard index, Dice coefficient, among others. The collective outcomes of these studies highlight a varied and encouraging scenario for the utilization of generative methods across diverse domains, especially within the realm of personalized medicine.
In Table 5 we present a concise summary of various research studies focusing on the development and application of generative models in the field of precision medicine. Each row of the table highlights a specific study, citing its strengths and limitations. The strengths column emphasizes the key advancements and achievements of each model, such as improved performance, ability to handle complex data, or generation of synthetic data for rare clinical scenarios. The limitations column, on the other hand, outlines the challenges or potential areas of improvement for each model, including issues like data limitations, training instability, generalizability concerns, and interpretability challenges. This comparative analysis provides a highlight of the current state of research in generative models within precision medicine, offering insights into both their potential and the hurdles that need to be addressed.
4 Discussion
This paper lightens the developments of the cutting-edge applications of generative AI methods in precision medicine, to improve treatment outcomes and patient care through personalized and data-driven approaches. The methodology employed in the review involved a systematic and rigorous approach to identify, select, and evaluate relevant literature on the applications of generative models in personalized medicine, ensuring a comprehensive and unbiased review of the current state of the field. The review of the 29 final refined papers focuses on exploring the potential of DGMs and LLMs in various aspects that constitute individualized medicine including clinical informatics, bioinformatics, and medical imaging.
4.1 Interpretation of results
A diverse array of generative models is tailored for personalized clinical, biomedical, and healthcare applications. These models, such as omicsGAN, GANITE, Dr.VAE, and innovativeGAN architectures with attention mechanisms, demonstrate their efficacy in tasks ranging from cancer phenotype classification to drug response prediction. Notably, they contribute to data generation, simulation of rare scenarios, and personalized experiments in a virtual environment. These models exhibit capabilities in handling high-dimensional biological data, predicting disease progression, and generating synthetic healthcare data for translational research. In imaging informatics generative models such as are U-HPNet model are designed to forecast lung nodule progression, addressing uncertainties in medical images and radiologist annotations. For brain tumor growth prediction, the Growth Prediction (GP-GAN) method utilizes stacked 3D generative adversarial networks, outperforming existing models in accuracy. In Alzheimer's and mild cognitive impairment, BrainStatTrans-GAN and GANCMLAE are proposed to decode individualized brain atrophy patterns, providing a crucial approach for more personalized interventions. Other studies introduced ccGAN for imputing missing values in EHR datasets, excelling in diabetic retinopathy detection, while others proposed a semi-supervised method using graph representations and GANs to generate synthetic data, outperforming alternative approaches with minimal labels. Generative models, such as MixEHR-G, a Bayesian topic model facilitating automatic phenotyping in large-scale EHR data, have proven effective in predicting phenotypes and revealing associations between diseases. In data anonymization, ADS-GAN and extended GAN-based approaches demonstrated anonymization for general health data, ensuring patient confidentiality and preserving data characteristics while maintaining identifiability. For prognosis, modified generative models such as SCAN, a semi-supervised cancer prognosis classifier, and GluGAN, for personalized glucose time series data generation, have shown promise in enhancing glucose prediction algorithms, respectively. In counterfactual explanations, SCGAN has provided sparse, diverse, and plausible explanations for breast cancer patient response predictions, contributing to interpretability and causal understanding. These advancements collectively drive progress in precision medicine, clinical research, and healthcare data privacy. Additionally, the review highlights the potential of advanced foundation models like ChatGPT-4 in medical decision-making and proposes standardized workflows for integrating large language models into candidate gene prioritization processes. The collective impact of these models spans diverse aspects of healthcare, from molecular interactions to patient prognosis and diagnostic support.
4.2 Challenges and limitations of deep generative models in precision medicine
Challenges in the present ecosystem of deep generative modes and foundation models like LLMs in precision medicine are underscored by their limitations. These challenges involve issues such as restricted generalizability, insufficient model evaluation metrics, and a lack of external validation across diverse datasets. The intricacies of model architectures and their dependencies on data quality and representativeness present additional hurdles, along with concerns about training process stability, potential biases stemming from unobserved confounders, and the risk of overfitting. Additionally, the limited consideration of multi-omics complexities, sparse modeling techniques, and a focus on specific diseases emphasize the necessity for improvements in the applicability, interpretability, and clinical validation of these models for practical use in healthcare settings. These limitations of the studies highlight the complexities and challenges faced in various scientific and medical research endeavors, emphasizing the ongoing need for careful consideration, validation, and improvement in generative methodologies. Table 5 provides a comprehensive overview of the strengths and limitations of each generative model. Addressing these limitations and implementing the suggested recommendations will contribute to the advancement of generative AI and its potential for personalized clinical applications is provided in Table 6.
4.3 Limitations of the review process
This systematic review is subject to several limitations that warrant acknowledgment. Firstly, there is a possibility that the review might have overlooked certain publications that could contribute valuable insights and data pertinent to the scope of the study. The vast and continually evolving nature of medical literature poses a challenge, and despite comprehensive search strategies, some relevant studies may have been inadvertently omitted. Recognizing these limitations is crucial for a subtle interpretation of the review's findings and underscores the importance of future updates or supplementary reviews conducted with the input of medical professionals to enhance the comprehensiveness and accuracy of the analysis.
4.4 Future research and directions
In navigating the future trajectory of research, numerous pathways emerge with the potential to transcend current boundaries and rectify existing limitations. These avenues necessitate a collaborative effort to confront prevailing constraints, bolster model robustness, enhance generalizability, and advocate for the conscientious and effective utilization of DGMs and FMs in precision medicine. One key direction involves cultivating enhanced generalizability through the development and training of models on diverse datasets encompassing a spectrum of cancer types and diseases. Moreover, adopting quantitative metrics beyond accuracy scores and emphasizing external validation on independent datasets becomes imperative for rigorous evaluation. Simultaneously, prioritizing model interpretability, investing in research to elucidate generative model decision-making processes, and fostering transparency cater to the needs of healthcare practitioners. This involves simplifying model architectures, implementing standardized validation procedures, and addressing challenges related to data quality to contribute to the reliability and accuracy of models, facilitating their adoption in clinical settings. The integration of multi-omics data and an understanding of system biology expands the depth of disease comprehension, ensuring clinical applicability through validation and focusing on ethical considerations, patient privacy, and continuous evaluation underscore the commitment to responsible model deployment. Strategies such as heterogeneous data utilization, improved model robustness, and interdisciplinary collaboration further fortify the models' efficacy. Furthermore, the exploration of advanced architectures, refined validation criteria, disease-specific tailoring, and real-world implementation considerations contributes to the evolution of generative models. Continuous learning from new data, advancements in foundation models for medical imaging, exploration of new model paradigms, and human-in-the-loop approaches propel the field forward, anticipating and addressing challenges for a comprehensive and impactful advancement in healthcare technology.
5 Conclusion
In conclusion, despite certain limitations such as restricted generalizability, insufficient model evaluation metrics, and a lack of external validation across diverse datasets, the integration of generative artificial intelligence (AI) models into personalized healthcare holds immense potential to revolutionize disease diagnosis and treatment. The continuous advancement and sophistication of generative AI models are expected to fuel further growth in precision medicine. Various future paths and recommendations outlined in this review offer opportunities to expand research boundaries and overcome existing limitations. A collective effort is required to confront current constraints, enhance model robustness, improve generalizability, and promote the responsible and effective application of deep generative models and foundation models in this field. Overcoming challenges such as data scarcity and ensuring the generation of realistic, privacy-preserving synthetic patient data are crucial steps toward surpassing limitations in real patient data collection. Additionally, the ongoing development and refinement of LLMs-based diagnostics are essential for improving diagnostic accuracy, precision, and medical recommendation capabilities across various medical conditions. The application of generative AI methods to personalized medicine is still in its early stages, highlighting the need for more interdisciplinary and collaborative research efforts to advance this field collectively.
Abbreviations
- 3D:
-
Three-Dimensional
- ACC:
-
Accuracy
- ADS-GAN:
-
Anonymization through Data Synthesis using Generative Adversarial Networks
- AD:
-
Alzheimer's Disease
- AI:
-
Artificial Intelligence
- AMD:
-
Age-Related Macular Degeneration
- AUC:
-
Area Under the Curve
- AUC_RANO_criteria:
-
Area Under the Curve for RANO Criteria
- AUC_Wasserstein-GAN:
-
Area Under the Curve for Wasserstein-GAN
- AIBL:
-
Australian Imaging, Biomarkers & Lifestyle Flagship Study of Aging
- BN:
-
Bayesian Network
- BRATS:
-
Brain Tumor Segmentation
- CCGAN:
-
Clinical Conditional Generative Adversarial Network
- CNN:
-
Convolutional Neural Network
- CSE:
-
Clinical Synthetic Fidelity
- CT:
-
Computed Tomography
- CVAE-GAN:
-
Conditional Variational Autoencoder-Generative Adversarial Network
- DGMs:
-
Deep Generative Models
- Dr.VAE:
-
Doctor Variational Autoencoder
- EHR:
-
Electronic Health Record
- FCN:
-
Fully Connected Network
- FM:
-
Foundation Model
- GDSC:
-
Genomics of Cancer Drug Sensitivity
- GEO:
-
Gene Expression Omnibus
- GFN:
-
Genomic Synthetic Fidelity
- GNN:
-
Graph Neural Network
- GP:
-
Gene Expression Profiles
- GANDA:
-
Generative Adversarial Network for Distribution Analysis
- GAN:
-
Generative Adversarial Network
- GANCMLAE:
-
Generative Adversarial Network Constrained Multiple Loss Autoencoder
- GP-GAN:
-
Growth Prediction Generative Adversarial Network
- GSEA:
-
Gene-Set Enrichment Analysis
- GTEx:
-
Genotype-Tissue Expression
- ICC:
-
Intraclass Correlation Coefficient
- KIRC:
-
Kidney Renal Clear Cell Carcinoma
- KNN:
-
K-Nearest Neighbors
- LGG:
-
Lower-Grade Glioma
- LLMs:
-
Large Language Models
- LINCS:
-
Library integrated Network-based Cellular Signatures
- LR:
-
Logistic Regression
- MAE:
-
Mean Absolute Error
- MAGGIC:
-
Meta-Analysis Global Group In Chronic Heart Failure
- MCI:
-
Mild Cognitive Impairment
- MIMIC-III:
-
Medical Information Mart for Intensive Care III
- MLP:
-
Multi-Layer Perceptron
- MOICVAE:
-
Multi-Omics Integrated Collective Variational Autoencoders
- MRI:
-
Magnetic Resonance Imaging
- MSE:
-
Mean Squared Error
- NST:
-
Neoadjuvant Systemic Therapy
- NIH:
-
National Institutes of Health
- NS-VQ-VAE:
-
Supervised Vector-Quantized Variational Autoencoder
- NSCLC:
-
Non-Small Cell Lung Cancer
- OCT:
-
Optical Coherence Tomography
- OASI:
-
Open Access Series of Imaging Studies
- PATE-GAN:
-
Private Aggregation of Teacher Ensembles—Generative Adversarial Network
- PopHR:
-
Population Health Record
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- PSNR:
-
Peak Signal-to-Noise Ratio
- QDs:
-
Quantum Dots
- RForest:
-
Random Forest
- RMFN:
-
Residual-Based Multi-Level Fusion Network
- RNA-seq:
-
Ribonucleic Acid Sequencing
- S-VQ-VAE:
-
Supervised Vector-Quantized Variational Autoencoder
- SDI:
-
Standard Deviation
- SMP:
-
Small Molecule Perturbation
- SVM:
-
Support Vector Machine
- TXIT:
-
Radiation Oncology In-Training Exam
- U-HPNet:
-
Uncertainty Handling Prediction Network
- UNOS:
-
United Network for Organ Sharing
- VEGF:
-
Vascular Endothelial Growth Factor
- VAE:
-
Variational Autoencoder
- ZINC:
-
Zinc Database
References
Ahmed KT, Sun J, Cheng S, Yong J, Zhang W (2022) Multi-omics data integration by generative adversarial network. Bioinformatics 38(1):179–186. https://doi.org/10.1093/bioinformatics/btab608
Ahuja Y, Zou Y, Verma A, Buckeridge D, Li Y (2022) MixEHR-Guided: A guided multi-modal topic modeling approach for large-scale automatic phenotyping using the electronic health record. J Biomed Inform, 134. https://doi.org/10.1016/j.jbi.2022.104190
Ali M, Aittokallio T (2019) Machine learning and feature selection for drug response prediction in precision oncology applications. In Biophys Rev (Vol. 11, Issue 1). https://doi.org/10.1007/s12551-018-0446-z
Balla, Y., Tirunagari, S., & Windridge, D. (n.d.). Pediatrics in Artificial Intelligence Era: A Systematic Review on Challenges, Opportunities, and Explainability. https://github.com/
Bao J, Chen D, Wen F, Li H, Hua G (2017) CVAE-GAN: Fine-Grained Image Generation through Asymmetric Training. Proceedings of the IEEE International Conference on Computer Vision, 2017-October. https://doi.org/10.1109/ICCV.2017.299
Barbiero P, Viñas Torné R, Lió P (2021) Graph Representation Forecasting of Patient’s Medical Conditions: Toward a Digital Twin. Front Gen, 12. https://doi.org/10.3389/fgene.2021.652907
Bečulić H, Begagić E, Skomorac R, Mašović A, Selimović E, Pojskić M (n.d.). ChatGPT’s contributions to the evolution of neurosurgical practice and education: a systematic review of benefits, concerns and limitations. https://doi.org/10.17392/1661-23
Benary M, Wang XD, Schmidt M, Soll D, Hilfenhaus G, Nassir M, Sigler C, Knödler M, Keller U, Beule D, Keilholz U, Leser U, Rieke DT (2023) Leveraging Large Language Models for Decision Support in Personalized Oncology. JAMA Netw Open 6(11):e2343689. https://doi.org/10.1001/jamanetworkopen.2023.43689
Ben-Cohen A, Klang E, Raskin SP, Amitai MM, Greenspan H (2017) Virtual PET images from CT data using deep convolutional networks: Initial results. Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 10557 LNCS. https://doi.org/10.1007/978-3-319-68127-6_6
Bernardini M, Doinychko A, Romeo L, Frontoni E, Amini MR (2023) A novel missing data imputation approach based on clinical conditional Generative Adversarial Networks applied to EHR datasets. Comput Biol Med, 163. https://doi.org/10.1016/j.compbiomed.2023.107188
Bressler SB, Bressler NM, Fine SL, Hillis A, Murphy RP, Olk RJ, Patz A (1982) Natural course of choroidal neovascular membranes within the foveal avascular zone in senile macular degeneration. Am J Ophthalmol, 93(2). https://doi.org/10.1016/0002-9394(82)90410-X
Collins FS, Varmus H (2015) A New Initiative on Precision Medicine. New England J Med, 372(9). https://doi.org/10.1056/nejmp1500523
Davri A, Birbas E, Kanavos T, Ntritsos G, Giannakeas N, Tzallas AT, Batistatou A (2022) Deep Learning on Histopathological Images for Colorectal Cancer Diagnosis: A Systematic Review. In Diagnostics (Vol. 12, Issue 4). MDPI. https://doi.org/10.3390/diagnostics12040837
Egger J, Gsaxner C, Pepe A, Pomykala KL, Jonske F, Kurz M, Li J, Kleesiek J (2022) Medical deep learning—A systematic meta-review. In Comput Meth Programs Biomed (Vol. 221). Elsevier Ireland Ltd. https://doi.org/10.1016/j.cmpb.2022.106874
El Emam K (2023) Status of Synthetic Data Generation for Structured Health Data. JCO Clinical Cancer Informatics, 7. https://doi.org/10.1200/cci.23.00071
Elazab A, Wang C, Gardezi SJS, Bai H, Hu Q, Wang T, Chang C, Lei B (2020) GP-GAN: Brain tumor growth prediction using stacked 3D generative adversarial networks from longitudinal MR Images. Neural Netw 132:321–332. https://doi.org/10.1016/j.neunet.2020.09.004
Fang M, Peng CW, Pang DW, Li Y (2012) Quantum dots for cancer research: current status, remaining issues, and future perspectives. Cancer Biol Med, 9(3). https://doi.org/10.7497/j.issn.2095-3941.2012.03.001
Gao X, Liu H, Shi F, Shen D, Liu M (2023) Brain Status Transferring Generative Adversarial Network for Decoding Individualized Atrophy in Alzheimer’s Disease. IEEE J Biomed Health Inform 27(10):4961–4970. https://doi.org/10.1109/JBHI.2023.3304388
Ge Q, Huang X, Fang S, Guo S, Liu Y, Lin W, Xiong M (2020) Conditional Generative Adversarial Networks for Individualized Treatment Effect Estimation and Treatment Selection. Front Gen, 11. https://doi.org/10.3389/fgene.2020.585804
Giannakopoulou KM, Roussaki I, Demestichas K (2022) Internet of Things Technologies and Machine Learning Methods for Parkinson’s Disease Diagnosis, Monitoring and Management: A Systematic Review. In Sensors (Vol. 22, Issue 5). MDPI. https://doi.org/10.3390/s22051799
Goodfellow IJ, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y (2014) Generative adversarial nets. Adv Neural Inform Process Syst, 3(January). https://doi.org/10.1007/978-3-658-40442-0_9
Hsu TC, Lin C (2023) Learning from small medical data - Robust semi-supervised cancer prognosis classifier with Bayesian variational autoencoder. Bioinform Adv, 3(1). https://doi.org/10.1093/bioadv/vbac100
Huang Y, Gomaa A, Semrau S, Haderlein M, Lettmaier S, Weissmann T, Grigo J, Ben TH, Frey B, Gaipl U, Distel L, Maier A, Fietkau R, Bert C, Putz F (2023) Benchmarking ChatGPT-4 on a radiation oncology in-training exam and Red Journal Gray Zone cases: potentials and challenges for ai-assisted medical education and decision making in radiation oncology. Front Oncol, 13. https://doi.org/10.3389/fonc.2023.1265024
Jahanyar B, Tabatabaee H, Rowhanimanesh A (2023) MS-ACGAN: A modified auxiliary classifier generative adversarial network for schizophrenia’s samples augmentation based on microarray gene expression data. Comput Biol Med, 162. https://doi.org/10.1016/j.compbiomed.2023.107024
Kipf TN, Welling M (2016) Variational Graph Auto-Encoders. https://arxiv.org/abs/1611.07308v1
Kloczkowski A, Peña C, Al-Tashi Q, Saad MB, Muneer A, Qureshi R, Mirjalili S, Sheshadri A, Le X, Vokes NI, Zhang J, Wu J (2023) Machine Learning Models for the Identification of Prognostic and Predictive Cancer Biomarkers: A Systematic Review. Int J Mol Sci 2023:7781. https://doi.org/10.3390/ijms
Li R, Tian Y, Shen Z, Li J, Li J, Ding K, Li J (2023) Improving an Electronic Health Record–Based Clinical Prediction Model Under Label Deficiency: Network-Based Generative Adversarial Semisupervised Approach. JMIR Med Inform, 11. https://doi.org/10.2196/47862
Moon S, Lee Y, Hwang J, Kim CG, Kim JW, Yoon WT, Kim JH (2023) Prediction of anti-vascular endothelial growth factor agent-specific treatment outcomes in neovascular age-related macular degeneration using a generative adversarial network. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-32398-7
Naveed H, Khan AU, Qiu S, Saqib M, Anwar S, Usman M, Akhtar N, Barnes N, Mian A (2023) A Comprehensive Overview of Large Language Models. https://arxiv.org/abs/2307.06435v6
Nie D, Trullo R, Lian J, Petitjean C, Ruan S, Wang Q, Shen D (2017) Medical image synthesis with context-aware generative adversarial networks. Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 10435 LNCS. https://doi.org/10.1007/978-3-319-66179-7_48
Openai IG (2016) NIPS 2016 Tutorial: Generative Adversarial Networks. https://arxiv.org/abs/1701.00160v4
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S … Moher D (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. In PLoS Medicine (Vol. 18, Issue 3). https://doi.org/10.1371/JOURNAL.PMED.1003583
Pearl J (2018) Theoretical Impediments to Machine Learning With Seven Sparks from the Causal Revolution.https://doi.org/10.1145/3159652.3176182
Piacentino E, Guarner A, Angulo C (2021) Generating synthetic ecgs using gans for anonymizing healthcare data. Electronics (switzerland) 10(4):1–21. https://doi.org/10.3390/electronics10040389
Purushotham S, Meng C, Che Z, Liu Y (2018) Benchmarking deep learning models on large healthcare datasets. J Biomed Inform, 83. https://doi.org/10.1016/j.jbi.2018.04.007
Rafael-Palou X, Aubanell A, Ceresa M, Ribas V, Piella G, Ballester MAG (2022) Prediction of Lung Nodule Progression with an Uncertainty-Aware Hierarchical Probabilistic Network. Diagnostics, 12(11). https://doi.org/10.3390/diagnostics12112639
Rampášek L, Hidru D, Smirnov P, Haibe-Kains B, Goldenberg A (2019) Dr.VAE: Improving drug response prediction via modeling of drug perturbation effects. Bioinformatics, 35(19), 3743–3751. https://doi.org/10.1093/bioinformatics/btz158
Rezayi S, R Niakan Kalhori S, Saeedi S (2022) Effectiveness of Artificial Intelligence for Personalized Medicine in Neoplasms: A Systematic Review. In BioMed Research International (Vol. 2022). Hindawi Limited. https://doi.org/10.1155/2022/7842566
Sallam M (2023) ChatGPT Utility in Healthcare Education, Research, and Practice: Systematic Review on the Promising Perspectives and Valid Concerns. In Healthcare (Switzerland) (Vol. 11, Issue 6). MDPI. https://doi.org/10.3390/healthcare11060887
Schlegl T, Seeböck P, Waldstein SM, Schmidt-Erfurth U, Langs G (2017) Unsupervised anomaly detection with generative adversarial networks to guide marker discovery. Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 10265 LNCS. https://doi.org/10.1007/978-3-319-59050-9_12
Shi M, Li X, Li M, Si Y (2023) Attention-based generative adversarial networks improve prognostic outcome prediction of cancer from multimodal data. Briefings in Bioinformatics, 24(6). https://doi.org/10.1093/bib/bbad329
Shi R, Sheng C, Jin S, Zhang Q, Zhang S, Zhang L, Ding C, Wang L, Wang L, Han Y, Jiang J (2023b) Generative adversarial network constrained multiple loss autoencoder: A deep learning-based individual atrophy detection for Alzheimer’s disease and mild cognitive impairment. Hum Brain Mapp 44(3):1129–1146. https://doi.org/10.1002/hbm.26146
Shin SH, Bode AM, Dong Z (2017) Addressing the challenges of applying precision oncology. Npj Precision Oncology, 1(1). https://doi.org/10.1038/s41698-017-0032-z
Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS (2019) Anti-vascular endothelial growth factor for neovascular agerelated macular degeneration. In Cochrane Database of Systematic Reviews (Vol. 2019, Issue 3). https://doi.org/10.1002/14651858.CD005139.pub4
Song C, Chen X, Tang C, Xue P, Jiang Y, Qiao Y (2023) Artificial intelligence for HPV status prediction based on disease-specific images in head and neck cancer: A systematic review and meta-analysis. J Med Virol, 95(9). https://doi.org/10.1002/jmv.29080
Strack C, Pomykala KL, Schlemmer HP, Egger J, Kleesiek J (2023) A net for everyone: fully personalized and unsupervised neural networks trained with longitudinal data from a single patient. BMC Medical Imaging, 23(1). https://doi.org/10.1186/s12880-023-01128-w
Sui D, Guo M, Ma X, Baptiste J, Zhang L (2021) Imaging Biomarkers and Gene Expression Data Correlation Framework for Lung Cancer Radiogenomics Analysis Based on Deep Learning. IEEE Access 9:125247–125257. https://doi.org/10.1109/ACCESS.2021.3071466
Tang Y, Zhang J, He D, Miao W, Liu W, Li Y, Lu G, Wu F, Wang S (2021) GANDA: A deep generative adversarial network conditionally generates intratumoral nanoparticles distribution pixels-to-pixels. J Control Release 336:336–343. https://doi.org/10.1016/j.jconrel.2021.06.039
Toufiq M, Rinchai D, Bettacchioli E, Kabeer BSA, Khan T, Subba B, White O, Yurieva M, George J, Jourde-Chiche N, Chiche L, Palucka K, Chaussabel D (2023) Harnessing large language models (LLMs) for candidate gene prioritization and selection. J Translation Med, 21(1). https://doi.org/10.1186/s12967-023-04576-8
Uzunova H, Ehrhardt J, Handels H (2020) Memory-efficient GAN-based domain translation of high resolution 3D medical images. Computerized Medical Imaging and Graphics, 86. https://doi.org/10.1016/j.compmedimag.2020.101801
Wang C, Yang G, Papanastasiou G, Tsaftaris SA, Newby DE, Gray C, Macnaught G, MacGillivray TJ (2021) DiCyc: GAN-based deformation invariant cross-domain information fusion for medical image synthesis. Information Fusion, 67. https://doi.org/10.1016/j.inffus.2020.10.015
Wang C, Zhang M, Zhao J, Li B, Xiao X, Zhang Y (2023) The prediction of drug sensitivity by multi-omics fusion reveals the heterogeneity of drug response in pan-cancer. Comput Biol Med, 163. https://doi.org/10.1016/j.compbiomed.2023.107220
Wei WQ, Bastarache LA, Carroll RJ, Marlo JE, Osterman TJ, Gamazon ER, Cox NJ, Roden DM, Denny JC (2017) Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS ONE, 12(7). https://doi.org/10.1371/journal.pone.0175508
Xue Y, Ding MQ, Lu X (2020) Learning to encode cellular responses to systematic perturbations with deep generative models. NPJ Syst Biol Appl 6(1):35. https://doi.org/10.1038/s41540-020-00158-2
Yamanaka C, Uki S, Kaitoh K, Iwata M, Yamanishi Y (2023) De novo drug design based on patient gene expression profiles via deep learning. Mol Inform, 42(8–9). https://doi.org/10.1002/minf.202300064
Yao S, Tan J, Chen Y, Gu Y (2021) A weighted feature transfer gan for medical image synthesis. Mach Vision Appl, 32(1). https://doi.org/10.1007/s00138-020-01152-8
Yoon J, Drumright LN, Van Der Schaar M (2020) Anonymization through data synthesis using generative adversarial networks (ADS-GAN). IEEE J Biomed Health Inform 24(8):2378–2388. https://doi.org/10.1109/JBHI.2020.2980262
Yuan M, Powell G, Lavigne M, Okhmatovskaia A, Buckeridge D L (2017) Initial Usability Evaluation of a Knowledge-Based Population Health Information System: The Population Health Record (PopHR). AMIA ... Annual Symposium Proceedings. AMIA Symposium, 2017
Zerka F, Barakat S, Walsh S, Bogowicz M, Ralph, Leijenaar TH, Jochems A, Miraglio B, David, Lambin P (2020) Systematic Review of Privacy-Preserving Distributed Machine Learning From Federated Databases in Health Care. In JCO Clin Cancer Inform (Vol. 4). 10.
Zhou S, Islam UJ, Pfeiffer N, Banerjee I, Patel BK, Iquebal AS (2023) SCGAN: Sparse CounterGAN for Counterfactual Explanations in Breast Cancer Prediction. IEEE Trans Autom Sci Eng, 1–12. https://doi.org/10.1109/TASE.2023.3333788
Zhu T, Li K, Herrero P, Georgiou P (2023) GluGAN: Generating Personalized Glucose Time Series Using Generative Adversarial Networks. IEEE J Biomed Health Inform 27(10):5122–5133. https://doi.org/10.1109/JBHI.2023.3271615
Acknowledgements
This research is partially supported by ASPIRE, the technology program management pillar of Abu Dhabi’s Advanced Technology Research Council (ATRC), via the ASPIRE Precision Medicine Research Institute Abu Dhabi (ASPIREPMRIAD) award grant number VRI-20-10.
Funding
This research is partially supported by ASPIRE. Award grant number VRI-20–10. In addition, this research was also partially funded by the United Arab Emirates University (UAEU), grant number G00003558.
Author information
Authors and Affiliations
Contributions
IG, NZ, and RD collaboratively contributed to the conceptualization, methodology, and the writing of the original draft of the paper, with NZ taking a leading role in formulating the research question, defining the scope of the literature review, and overseeing the project's supervision. All authors were actively involved in data curation, writing, review, and editing of the manuscript, with IG playing a pivotal role in gathering and curating relevant literature to ensure thorough coverage of the topic. Furthermore, each author equally contributed to the investigation and analysis aspects of the research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no potential conflicts or competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghebrehiwet, I., Zaki, N., Damseh, R. et al. Revolutionizing personalized medicine with generative AI: a systematic review. Artif Intell Rev 57, 128 (2024). https://doi.org/10.1007/s10462-024-10768-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s10462-024-10768-5
Keywords
- Generative AI
- Artificial Intelligence
- Deep Generative Models (DGMs)
- Foundation Models (FM)
- Precision Medicine
- Personalized Medicine
- Personalized treatment
- Generative Adversarial Networks (GANs)
- Chat Generative Pre-Trained Transformer (ChatGPT)
- Individualized Treatment Effect (ITE)
- Large Language Models (LLMs)