Abstract
The purpose of the study was to assess the effects of advanced HIV disease (AHD) on health-related quality of life (HRQoL) in PLHIV, the changes in HRQoL outcomes over the last 25 years, and the differences between countries according to level of economic development. We conducted a systematic review and meta-analysis. The search was conducted in PubMed and Web of Science using the terms: “health-related quality of life”, “HQRoL”, “HIV”, “AIDS”, “advanced HIV disease” and “low CD4 cells”. Studies inclusion criteria were: adult population; initiated after 1996 and published before July 2021; clinical trials, cross-sectional, cohort, and case–control studies; studies analyzing the relationship between AHD and HRQoL; English or Spanish language. Standardized mean differences (d+) were calculated to estimate the effect size for the meta-analyses. Summary statistics were calculated using a random-effects model, and analyses of effect moderators, using mixed-effects models. The meta-analysis included 38 studies. The results indicated that HRQoL is worse in patients with AHD compared to those without. The main HRQoL domains affected were overall health perception and concern and physical and functional health and symptoms. We found a moderate impact for age and gender on some HRQoL domains. There were no differences in relation to socioeconomic inequities, country of residence, or time period analyzed. In conclusion, advanced HIV disease has a negative impact on health and well-being in PLHIV. Our results show that despite all the advances in antiretroviral treatments over the last 25 years, AHD persists as a source of extreme vulnerability, regardless of where PLHIV live.
Resumen
El objetivo del estudio fue evaluar los efectos de la enfermedad avanzada de sida (EAS) en la calidad de vida relacionada con la salud (CVRS) en personas que viven con el VIH (PVVIH), los cambios experimentados en la CVRS en los últimos 25 años y las diferencias entre países. Realizamos una revisión sistemática y metaanálisis. La búsqueda se llevó a cabo en PubMed y Web of Science utilizando los términos: “calidad de vida relacionada con la salud”, “CVRS”, “VIH”, “SIDA”, “enfermedad avanzada por VIH” y “células CD4 bajas”. Los criterios de inclusión de los estudios fueron: población adulta; iniciado después de 1996 y publicado antes de julio de 2021; ensayos clínicos, estudios transversales, de cohorte y de casos y controles; estudios que analizan la relación entre EAS y CVRS; idioma inglés o español. Se calcularon diferencias de medias estandarizadas (d+) para estimar el tamaño del efecto para los metaanálisis. Los efectos promedios se calcularon utilizando un modelo de efectos aleatorios, y el análisis de moderadores utilizando modelos de efectos mixtos. El metaanálisis incluyó 38 estudios. Los resultados indicaron que la CVRS es peor en pacientes con EAS en comparación con aquellos sin EAS. Los principales dominios de CVRS afectados son la percepción de salud general y su preocupación, y la función física y de salud y los síntomas asociados. Encontramos un impacto moderado por edad y género en algunos dominios de CVRS. No encontramos diferencias en cuanto a las desigualdades socioeconómicas, país de residencia o período de tiempo analizado. En conclusión, la enfermedad avanzada por VIH tiene un impacto negativo en la salud y el bienestar en las personas con VIH. Nuestros resultados muestran que, a pesar de todos los avances en los tratamientos antirretrovirales en los últimos 25 años, el EAS persiste como una fuente de extrema vulnerabilidad, independientemente de dónde vivan las personas con VIH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1996, the advent of combined active antiretroviral therapy (cART) dramatically transformed the face of AIDS [1]. Since then, the efficacy and safety of antiretroviral therapies have improved, while AIDS-related morbidity and mortality have continued to decrease [1]. In 2014, the World Health Organization (WHO) approved a new goal on HIV and AIDS: “to end the AIDS epidemic as a public health threat by 2030”. To achieve this objective, UNAIDS established the “90-90-90” targets, defined as: “90% of people living with HIV (PLHIV) will know their HIV status, 90% of people who know their HIV-positive status will be accessing treatment, and 90% of PLHIV on treatment will have suppressed viral loads.” This strategy promoted healthy lives and well-being for all PLHIV, regardless of their age or country of residence [2, 3]. Recently, these objectives have become more ambitious, raising the bar to ensure that 95% of PLHIV achieve these targets [4]. Since then, different authors have highlighted the importance of health-related quality of life (HRQoL) for attaining long-term health and well-being in PLHIV, calling on health systems, international societies, and others providing AIDS services to include it as an essential goal of HIV care [5, 6].
As a multidimensional concept, HRQoL can be influenced by many factors in PLHIV, including ageing, gender, HIV-related symptoms, comorbidities, substance abuse, depression, socioeconomic inequalities, employment, and financial stress, among others [7,8,9,10,11,12,13]. Understanding all the factors associated with HRQoL is critical for designing interventions to support PLHIV, especially vulnerable populations such as those accessing services with advanced HIV disease (AHD).
The WHO defines AHD as a CD4 + count under 200 cells/µL or a WHO clinical stage 3 or 4 [14]. Despite all the efforts to rapidly diagnose HIV infection, diagnosis of AHD is still frequent in PLHIV even in middle- and high-income countries. In 2021, of the 692,000 people in the European Union/European Economic Area who received a new HIV diagnosis, 35% were diagnosed with AHD [15]. Worldwide, about 650,000 people died from AIDS-related diseases in 2021, over half in the WHO Africa and Asia–Pacific regions [16].
AHD has a negative impact on the health of PLHIV [17, 18], increasing the likelihood of developing AIDS- and non-AIDS-defining disease, especially cancer, cardiovascular disease, kidney disease, and neurocognitive impairment. All these risks predispose those with AHD to experience more physical symptoms, including chronic pain, extreme fatigue, and a significant decline in their ability to carry out activities of daily living [19,20,21,22]. Moreover, AHD is associated with an increased risk of mortality and reduced life expectancy [21, 23]. Managing multiple medical conditions simultaneously can be overwhelming, negatively impacting emotional and psychological quality of life [24]. Furthermore, the stigma and discrimination associated with HIV may intensify in this advanced stage, leading to social isolation, self-esteem issues, and difficulties in accessing adequate medical care [25]. All these aspects suggest that AHD has a devastating impact on the physical, mental, and emotional health in PLHIV, ultimately affecting HRQoL.
However, there is no consensus on the impact of AHD on HRQoL. Different authors have reported that PLHIV with low CD4 counts or an AIDS-defining event have worse HRQoL than those with higher CD4 counts or without AIDS. However, there is no agreement about which HRQoL domains are most affected in people with AHD. Regarding CD4 + cells counts, studies by Fuster-RuizdeApodaca et al., Venturi et al., and Aden et al. have reported that lower CD4 counts correspond to lower overall HRQoL scores [26,27,28]. Emuren et al. and Liu et al. described that CD4 + cell counts below 200/µL were associated with worse physical but not mental health [29, 30]. On the other hand, Fumaz et al., focusing on gender differences, observed that poorer mental health in women was associated with lower CD4 cell counts but not with physical health [31]. Préau et al. reported similar results in a mixed-gender sample, finding a negative association between low CD4 cells and mental HRQoL [32]. Regarding AIDS, Emuren et al. and Préau et al. found that an AIDS diagnosis was linked with worse physical health but not with the mental health dimension [29, 33], while Fumaz et al. reported that AIDS-defining events were similarly associated with unacceptably low physical and mental HRQoL, regardless of gender [31]. Nevertheless, Badia et al. did not find any relationship between low CD4 counts or AIDS with lower HRQoL scoring [34]. The variability of these findings in different settings supports the need to synthesize all available evidence to clarify how AHD affects HRQoL.
Another aspect that warrants investigation is the effect of improvements in antiretroviral therapy (ART) over time. Since its introduction in 1996, ART has made gradual but steady advances in terms of better efficacy, decreased toxicity, and improved convenience. In the first years of the AIDS pandemic, surviving to an AHD diagnosis was the main challenge for PLHIV. In the mid-1990s, treatments with protease inhibitors (PIs) like ritonavir and indinavir plus zidovudine represented a significant breakthrough in the approach to HIV/AIDS, providing an opportunity for people with AHD [1]. The first PIs were associated with diarrhea, metabolic side effects, and lipodystrophy [35], while zidovudine, the first nucleoside retrotranscriptase inhibitor (NRTI), was associated with anemia and bone marrow suppression [36]. Other first-generation NRTIs, such as stavudine, zalcitabine, and didanosine were associated with severe mitochondrial toxicity, peripheral neuropathy, and pancreatitis [37]. Efavirenz, the first non-nucleoside retrotranscriptase inhibitor (NNRTI), and nevirapine were approved at the end of the twentieth century. Efavirenz was associated with neurotoxicity, while nevirapine could cause severe hypersensitivity hepatitis [38, 39]. In the 2000s, the second-generation therapies appeared, including boosted PI, new NRTI, and NNRTI, with greater efficacy, tolerance, and convenience [40]. These new drugs permitted the development of fixed-dose combination (FDC) therapies, reducing the pill burden and improving adherence. In this second period, PIs were associated with a higher cardiovascular risk [41], and tenofovir disoproxil-fumarate (TDF) with renal and bone toxicity [42]. In the mid-2000s, raltegravir, the first integrase strand-transfer inhibitor (INSTI), was approved for the treatment of HIV infection, followed by elvitegravir, dolutegravir, and bictegravir. The introduction of the latest INSTIs marked the third period of antiretroviral treatment, characterized by the widespread use of FDC in therapies and the development of potent bi-therapies for treating HIV infection. INSTIs have been associated with neuropsychiatric toxicity and obesity, but these adverse effects rarely lead to treatment discontinuation [43]. The success of ART has permitted the establishment of new strategies, such as early treatment of HIV infection regardless of the number of CD4 cells and the “test and treat” strategy, initiating ART as soon as possible [44, 45]. These extraordinary changes could support the hypothesis that improvements in ART from 1996 to 2021 have impacted HRQoL even in PLHIV with AHD. Unfortunately, access to remarkable advances in antiretroviral therapy has been disparate worldwide due to the varying availability and allocation of economic resources in different countries [46].
The aims of this meta-analysis are: (1) to assess the effects of AHD on HRQoL in PLHIV; (2) to analyze the probable changes on HRQoL outcomes over the last 25 years, from the advent of the first cART with PI to the new regimens of ART that use INSTI; (3) to assess heterogeneity among studies; and (4) to evaluate potential differences in HRQoL in PLHIV with AHD from different countries and levels of economic development: low-, middle- and high- income countries, and to examine the influence of other potential variables.
Methods
Protocol and Registration
The study protocol was pre-registered on the Open Science Framework (see https://doi.org/10.17605/OSF.IO/RZAMQ for further information). Furthermore, this systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA guidelines) [47]. Supplementary Table 1 presents the PRISMA checklist.
Study Eligibility Criteria
Our study population was adults with AHD defined as AIDS (CDC clinical stage C3 or WHO HIV-clinical stage 3/4), or CD4 < 200 cells/µL [14]. Inclusion criteria were: (1) studies in PLHIV aged 18 years or older; (2) studies initiated after the advent of cART in 1996, and published before 31 July 2021; (3) clinical trials, cross-sectional, cohort, and case–control studies that included any HRQoL intervention; (4) studies analyzing the relationship between AHD and HRQoL, comparing HRQoL with versus without AIDS and/or with CD4 + lymphocytes ≥ 200 cells/μL versus < 200 cells/μL; (5) studies written in English or Spanish. Exclusion criteria were: (1) meta-analyses, systematic reviews, and secondary studies; (2) abstracts, conference proceedings, books or book chapters, unpublished material, and reports that were not peer-reviewed; (3) articles reporting HRQoL measures obtained using a non-validated questionnaire; (4) articles reporting lymphocyte CD4 + counts, not categorized as having more or less than 200 CD4 + cells/μL.
Search Strategy
The search was conducted from 1 to 31 July 2021 in PubMed and the Web of Science. The following keywords were used: “health-related quality of life” [OR] “HQRoL” AND “HIV” [OR] “AIDS” [OR] “advanced HIV disease” [OR] “low CD4 cells”. Supplementary Table 2 presents the full search strategy. Additionally, the reference lists of the selected articles were handsearched to identify other potentially eligible studies.
Search results were de-duplicated, and four review authors (IPT, JP, JPT and SR) independently screened the titles and abstracts against the selection criteria. All relevant or potentially relevant records were retrieved for full-text review, which was also performed independently by IPT, JP, JPT, and SR. Disagreements were resolved by discussion between the four review authors. Doubts about HRQoL measures or meta-analysis methods were resolved by MJF, an expert in HRQoL, and MRA, an expert in meta-analyses.
Data Extraction and Quality Assessment
Study characteristics were independently extracted by the researchers according to a predefined protocol and categorized based on article characteristics. The following information was collected: first author, publication year, country, World Bank income level, sample size, demographic characteristics of the sample (i.e., age, sex), study design, HRQoL questionnaires, recruitment period, percentage of patients on ART, percentage of patients with undetectable viral load, percentage of patients with CD4 + < 200 cells/µL, and percentage of patients with AIDS (Table 1). Information was also extracted on study funding, authors’ conflict of interests, and other ethical issues.
The methodological quality of the studies included in the meta-analysis was assessed with the Newcastle–Ottawa Scale (NOS), adapted for cross-sectional studies [48]. This scale uses a “star system” to judge quality based on three dimensions: selection, comparability, and outcome. The NOS consists of 7 items, and the total maximum quality score is 9 stars. Study quality was classified as “high” (8–9 stars), “moderate” (5–7 stars) or “low” (≤ 4 stars).
To assess the reliability of the data extraction, all studies were independently coded by two review authors (IPT and MRA), and inconsistencies were resolved by consensus. For categorical variables, kappa coefficients ranged between 0.810 and 1.0 (M = 0.931), and for continuous variables all intra-class correlations were equal to 1.0.
Computation of Effect Sizes
Usually, HRQoL is measured as a multidimensional concept; however, not all HRQoL questionnaires have exactly the same dimensions. To make the comparison feasible, we categorized the most frequent dimensions according to similarity or by grouping the instruments containing summary indexes. Three review authors (PI, RM, MJF) carried out this process, discussing and resolving discrepancies by consensus. Six outcome domains were finally created: overall health perception and concern, physical and functional health and symptoms, psychological health, social relationships, mental health summary, and physical health summary. Supplementary Table 3 provides details on how dimensions of the questionnaires were categorized.
Once the six HQRoL domains were summarized, the effect sizes for each individual study were calculated. The effect size index was the standardized mean difference (d), defined as the difference between the mean of the treatment group and the mean of the control group, divided by a pooled standard deviation (SD): d = c(m)(yT − yC)∕SD, with c(m) representing a correction factor for small sample sizes [49]. In this meta-analysis, the d index was calculated to compare the differences in HRQoL between people diagnosed versus not diagnosed with AIDS, and between those with CD4 counts under versus over 200 cells/µL. In any case, the mean values of the non-AIDS and the CD4 ≥ 200 cells/µL groups (control groups) were subtracted from the means for the AIDS and the CD4 < 200 cells/µL groups (treatment groups), respectively, such that negative d values indicate poorer HRQoL in the AIDS and CD4 < 200 cells/µL groups. For standardization, absolute values of d of around 0.2, 0.5, and 0.8 were interpreted as small, moderate, and large magnitudes of effect, respectively [50]. For effect size calculations, the available means and SDs for each group were used. When this information was not reported, the corresponding authors were contacted to request the required values. In some studies, when the results were reported by means of odds ratio or correlation, conversion formulas were applied to obtain the corresponding d value [51, 52].
Statistical Analysis
Separate meta-analyses were carried out with the effect sizes for each of the six outcome measures, for the comparison of the AIDS versus non-AIDS groups, and for the comparison of the CD4 < 200 cells/µL versus CD4 ≥ 200 cells/µL groups.
Random-effects models were used to account for the expected variability in effect sizes. This model involves weighting each effect size by its inverse variance, defined as the sum of the within-study variance and between-study variance, the latter being estimated by restricted maximum likelihood [53]. For each meta-analysis, the method proposed by Hartung was applied to compute the mean effect size along with its 95% confidence intervals (CI) [54]. To check the variability in effect sizes, Cochran’s heterogeneity Q statistic and the I2 index (values of 0%, 25%, 50% and 75% representing no, low, moderate, and high heterogeneity, respectively) were calculated [55]. For each meta-analysis, a forest plot was also constructed.
Publication bias was assessed by constructing funnel plots using the trim-and-fill method, which consists of imputing missing effect sizes to achieve symmetry [56]. Furthermore, Egger’s regression test was also applied [57]. Evidence of publication bias was defined as a statistically significant result for Egger’s test, defined at p < 0.10 instead of the usual p < 0.05 because of the lower statistical power with a small number of studies [58].
Finally, in the presence of heterogeneity and at least 10 studies for the outcome, analyses of potential effect moderators were performed [59]. Meta-regressions and weighted ANOVAs for continuous and categorical moderators, respectively, were applied by assuming mixed-effect models by means of the F statistic, described by Knapp-Hartung [60, 61]. The QE statistic was calculated to assess the model misspecification of the moderator analyses, together with an estimate of the percentage of variance accounted for by the moderator, R2 [62].
All statistical analyses were carried out with the metafor package in R version 3.2.3 [63].
Results
Characteristics of Included Studies
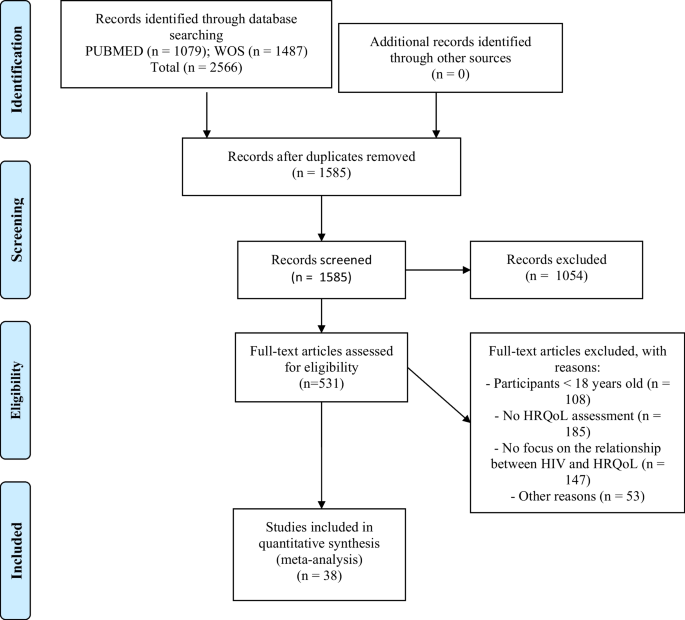
The search yielded 1079 results in PubMed and 1487 in the Web of Science. After removing duplicates, a total of 1585 unique records were screened, and 531 full-text articles were assessed for eligibility (see Fig. 1). After exclusion criteria were applied, 38 studies were included in the meta-analysis. One of these used two samples, so a total of 39 samples were analyzed (Table 1; Fig. 1) [24, 27,28,29, 33, 64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81, 81,82,83,84,85,86,87,88,89,90,91,92,93,94,95]. According to the assessment with the NOS scale, 18 studies (46.2%) were deemed high quality and 21 (53.8%) moderate quality (Supplementary Table 4). All studies met criteria for the ascertainment of the exposure and the assessment of the outcome, as these were inclusion criteria for the study.
Mean Effect Sizes and Heterogeneity
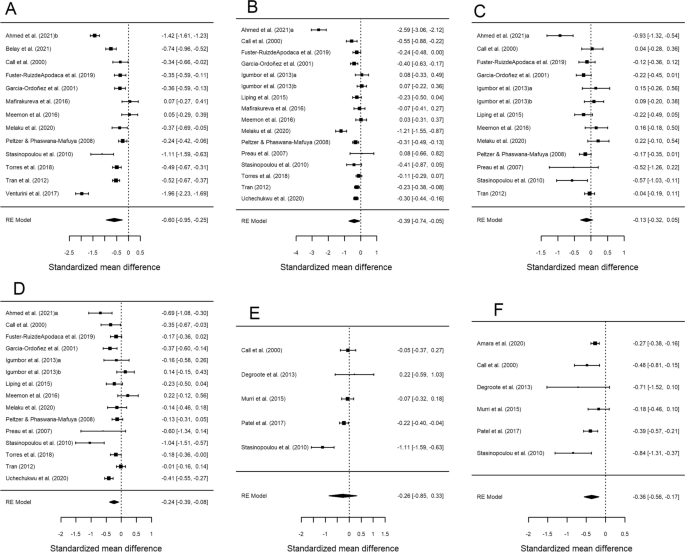
Table 2 presents the results of the meta-analysis for the comparison of the groups with CD4 < 200 cells/µL versus CD4 ≥ 200 cells/µL, for each of the six HRQoL outcome domains. Forest plots are displayed in Fig. 2. The mean effect sizes for all six outcomes were negative, indicating worse HRQoL in the group with lower CD4 counts. All results were statistically significant except for psychological health (d+ − 0.133, 95% CI − 0.319, 0.054) and mental health summary (d+ − 0.260, 95% CI − 0.851, 0.332). The highest mean effect size was found for overall health perception and concern (d+ − 0.598, 95% CI − 0.949, − 0.248), followed by physical and functional health and symptoms (d+ − 0.395, 95% CI − 0.740, − 0.049) and physical health summary (d+ − 0.362, 95% CI − 0.557, − 0.167), with similar magnitudes. The meta-analysis showed heterogeneity (I2 > 70% and statistically significant Q), except for physical health summary, where the I2 value was 36.7% and Cochran’s Q was not significant (Table 2). This large variability is also reflected in the forest plots (Fig. 2).
Forest plots displaying the standardized mean differences with 95% confidence intervals for the comparison of the CD4 < 200 cells/μL and CD4 > 200 cells/μL groups for each of the six outcome measures: overall health perception and concern (A), physical and functional health and symptoms (B), psychological health (C), social relationship (D), mental health summary (E) and physical health summary (F). RE (random-effects) model refers to the statistical model used in the calculations
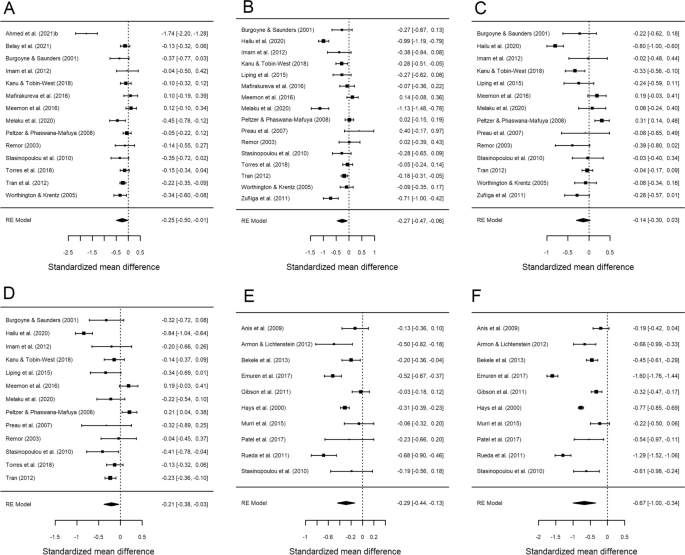
Table 3 presents the results of the meta-analysis comparing AIDS versus non-AIDS groups for each of the six HRQoL outcome measures. Forest plots are displayed in Fig. 3. As previously, the mean effect sizes were negative for all six outcomes, indicating poor HRQoL in the AIDS group. All analyses were statistically significant with the exception of psychological health (d+ − 0.136; 95% CI − 0.305, 0.032). In this case, the highest mean effect size was found for physical health summary (d+ − 0.670; 95% CI − 1.002, − 0.338), while the rest of the mean effect sizes showed a low to moderate magnitude. Regarding the variability among effect sizes, very high heterogeneity was found for all outcomes (I2 > 80% and statistically significant Q) (Table 3; Fig. 3).
Forest plots displaying the standardized mean differences with 95% confidence intervals for the comparison of the AIDS with non-AIDS groups for each of the six outcome measures: overall health perception and concern (A), physical and functional health and symptoms (B), psychological health (C), social relationship (D), mental health summary (E) and physical health summary (F). RE (random-effects) model refers to the statistical model used in the calculations
Publication Bias
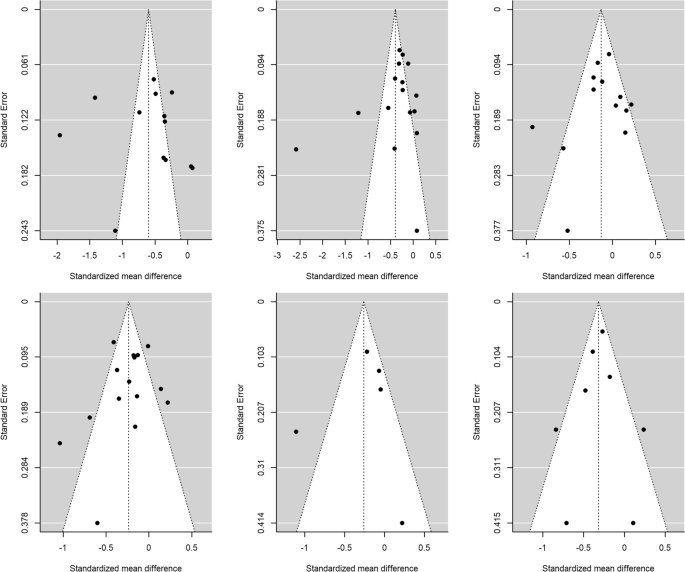
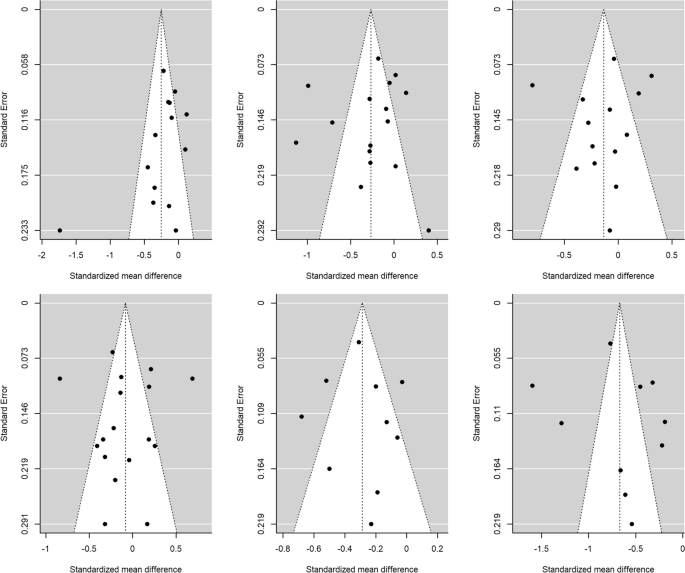
Publication bias was assessed through Egger’s tests and funnel plots, using the trim-and-fill method. Figure 4 presents the funnel plots for the comparison according to CD4 counts (< 200 cells/µL versus ≥ 200 cells/µL) for the HRQoL measures. For physical health summary, two additional effect sizes were imputed to the original set of estimates to achieve symmetry in the funnel plot (Fig. 4F). When a mean effect (and its 95% CI) was calculated using the six effect sizes plus the two imputed values, the mean effect was d+ − 0.317 (95% CI − 0.490, − 0.144). However, Egger’s test did not reach statistical significance (p = 0.117).
Funnel plots for the comparison of the CD4 < 200 cells/μL and CD4 > 200 cells/μL groups for overall health perception and concern (A), physical and functional health and symptoms (B), psychological health (C), social relationship (D), mental health summary (E) and physical health summary (F) measures. The white circles are the imputed standardized mean changes by means of the Duval and Tweedie's trim and-fill method
Figure 5 presents the funnel plots for the comparison of the AIDS versus non-AIDS groups. For social relationships, four additional effect sizes were imputed to achieve symmetry in the funnel plot (Fig. 5D). When a mean effect (and its 95% CI) was calculated using the 13 effect sizes plus the four imputed values, the mean effect was d+ − 0.083 (95% CI − 0.262, 0.095). Nevertheless, once again Egger’s test was not statistically significant (p = 0.61).
Funnel plots for the comparison of the AIDS with non-AIDS groups for overall health perception and concern (A), physical and functional health and symptoms (B), psychological health (C), social relationship (D), mental health summary (E) and physical health summary (F) measures. The white circles are the imputed standardized mean changes by means of the Duval and Tweedie's trim and-fill method
For the rest of meta-analyses, the trim-and-fill method did not require imputing new values to the funnel plots (Figs. 4, 5), and the Egger’s tests were not statistically significant. Thus, the results of these meta-analyses are at low risk for publication bias.
Analysis of Moderator Variables
The heterogeneity observed in the standardized mean differences prompted an analysis of moderator variables for outcome measures with at least 10 studies. Tables 4 and 5 show the results of the meta-regressions, and Tables 6 and 7 of the weighted ANOVAs, comparing groups by CD4 counts and AIDS status, respectively.
Of the different potential moderator variables analyzed, only two continuous variables yielded a statistically significant result for the comparison of the CD4 groups (see Table 4): mean age exhibited a statistically significant relationship with the effect size for overall health perception and concern (p = 0.038), accounting for 35.5% of the variance observed. The negative sign of the regression coefficient for this moderator indicated larger standardized mean differences, as the mean age of participants decreased for this outcome measure. On the other hand, the percentage of men showed a significant relationship with the effect sizes of the social relationships measure (p = 0.042), accounting for 30.8% of the variance. Once again, a negative relationship was found, such that the larger the proportion of men, the lower the standardized mean differences for this outcome measure.
Finally, for the comparison of groups with AIDS versus without AIDS, country income level yielded a marginally significant result (p = 0.059) as an effect modifier, explaining a notable percentage of the variance (R2 = 38.21%) for the physical and functional health and symptoms measure (see Table 7). The highest effect was found in the low-income category (d+ − 0.786).
Discussion
We conducted a meta-analysis to assess the influence of AHD on HRQoL and its different domains. We included papers published over the last 25 years of the AIDS pandemic, analyzing possible differences in HRQoL and AHD in different periods of time as well as differences between countries of different income levels. Our main finding was that HRQoL is worse in patients with AHD compared to those without. People with CD4 counts of fewer than 200 cells/µL sustain negative impacts in all domains of HRQoL, especially in overall health perception and concern, followed by physical and functional health and symptoms, and physical health summary, with similar mean effect sizes but large inter-study variability. The outcomes related to psychological health and mental health summary were also negatively affected, though comparisons did not reach statistical significance. In patients with an AIDS diagnosis, the six HRQoL domains showed lower scores than in those without AIDS. The greatest difference was observed for physical health summary. As in patients with low CD4 cells, psychological health scores did not show a significant difference between people with versus without AIDS. This finding is probably related to the negative psychological impact of an HIV diagnosis on mental health in PLHIV, regadless of AIDS diagnosis or number of CD4 cells [96, 97]. PLHIV often experience intersecting types of discrimination (marginalized identities, internalized HIV stigma, limited economic resources, etc.) or suffer from uncertainty about a non-curable and potentially lethal infection. Thus, PLHIV are highly vulnerable to mental health problems [98, 99], independently of their immunovirological status.
In this meta-analysis, we found that overall health perception and concern and physical and functional health and symptoms are the main HRQoL domains affected in PLHIV with AHD. Physical and functional health and symptoms includes different dimensions, including physical functioning, energy, mobility, effects and severity of pain, and level of independence. The symptomatology of advanced stages of HIV infection, the associated comorbidities, and the loss of vitality caused by progression of HIV infection could explain the worse scores in these domains [19,20,21,22].
Our meta-analysis considers numerous effect modifiers (age, gender, treatment with ART, and HIV viral load) that were analyzed for their potential influence on the effect sizes of the HRQoL outcome. We found a moderate impact for age and gender on some HRQoL domains. Age showed an inverse relationship with the effect sizes for overall health perception and concern, such that older age was associated with worse scores, probably due to ageing, comorbidities, and disability associated with AHD [100]. The domain for overall health perception and concern comprises perceptions, distress, concerns, and worries related to general health. Thus, this result is consistent with previous studies showing that HRQoL indexes of physical health are negatively affected in older PLHIV [101]. Furthermore, male sex showed a significant negative relationship with the effect sizes for social relationships. In high-income countries, men with HIV are usually reported as having better scores in social relationships and other related dimensions or predictors than women with HIV [27, 31]. Our results shows that this trend could differ between countries depending on income level, that AHD could affect men’s social relationships more than women’s, or that in low- and medium-income countries women use social support as coping strategy to reduce the stressors on health outcomes [8, 102]. Another possibility is that men are overrepresented in this meta-analysis, especially in studies from low- and middle-income countries.
Regarding the relationship between HRQoL and country income level, we hypothesized that people with AHD from middle- or high-income countries would report higher HRQoL scores than in those living in low-income countries, due to the direct impact of socioeconomic status on HRQoL [8]. However, we did not find differences between the analyzed countries according to this parameter. When we compared the group with versus without AIDS, we found only a borderline significant result for physical and functional health and symptoms, in PLHIV with AIDS from low-income countries. These results could be related with worse conditions in housing, nutrition, health resources, employment difficulties, and other socioeconomic indicators in these settings [88, 95]. In fact, there is evidence supporting the degenerative impact that material deprivation has on HRQoL [103].
Furthermore, given the continuous improvements in the efficacy and safety of ART from 1996 to the present, we hypothesized that HRQoL in PLHIV with AHD in the era of the new ART (2012–2019) would be better than when these drugs were first introduced (1996). We analyzed three 8-year periods: 1996–2003, 2004–2011, and 2012–2019. Surprisingly, we observed no differences between the time periods analyzed. AHD continues being the worst condition in the spectrum of HIV infection, occurring in high-income and low-income countries alike, despite the availability of new antiretroviral treatments, early diagnosis strategies, and easy access to ART. Ghiasvand et al.’s meta-analysis of studies from 2005 to 2016 in low-, middle- and high-income countries likewise found no impact on HRQoL from ART in PLHIV [103]. The present meta-analysis broadens this evidence by also including earlier periods with less effective and more toxic treatments in PLHIV with AHD. These results suggest that improving quality of life for PLHIV may require additional interventions beyond just the provision of ART.
To address the significant impact of AHD on HRQoL, the initial step should be the implementation of prevention programs, early diagnosis, and early treatment to decrease the prevalence of AHD worldwide [44]. Regarding strategies to enhance HRQoL in PLHIV, these should first focus on addressing basic needs such as nutrition, access to healthcare resources, and employment, which have been associated with low HRQoL [6, 95]. This is particularly crucial in low-income countries, where inequalities are often more pronounced and require steadfast policy responses. Indeed, a systemic and comprehensive approach that considers the special individual needs of this population is essential everywhere. This should include bolstering resilience resources, economic empowerment, and self-stigma detection. Moreover, PLHIV with AHD need preventive interventions that focus on both AIDS and non-AIDS events, among other aspects [6]. Other strategies should provide health education along with comprehensive disease management information and training to ensure adherence to ART, thereby empowering patients to effectively participate in controlling their disease [76]. Emotional support services and counseling should be offered to manage stress and other psychological challenges [29]. Encouraging participation in support groups, social activities, and community programs is also crucial for reducing loneliness [74]. Additionally, efforts should be directed towards eradicating the stigma associated with AHD while promoting inclusivity and understanding for PLHIV in society [25]. To achieve all these goals, individuals with AHD should receive multidisciplinary, comprehensive, and personalized care from a team of physicians, nurses, social workers, and psychologists.
Our study has some limitations. First of all, we could only make use of data available from published studies. Second, we were unable to explore the influence of some factors associated with reduced HRQoL: intravenous drug use or substance misuse, socioeconomic inequalities, refugee or migrant status, lower educational level, social support, and internalized stigma [104]. Third, some countries and WHO regions are underrepresented. We did not find data from Latin America (except from Mexico and Guyana), eastern Europe countries, the Western Pacific and Eastern Mediterranean regions (except Pakistan), India, Brazil, or others. The languages used in this meta-analysis (Spanish or English), along with the databases chosen (PubMed and the Web of Science, both dominated by research from Western countries) probably generated some selection bias in the included studies.
On the other hand, the review also has some important strengths. First, we obtained enough data to demonstrate the highly negative impact of AHD on HRQoL. Second, the long study period enabled a comparison of HRQoL in different periods of the AIDS pandemic and in countries with different income levels. Its results show that despite all the advances in the treatment of HIV infection over the last 25 years, AHD persists as a source of extreme vulnerability for PLHIV.
In conclusion, this meta-analysis shows that AHD has a negative impact on the health and well-being of PLHIV, affecting all HRQoL domains, especially overall self-perceived health, physical health summary, and psychological health. These effects have not changed in the last 25 years and affect all PLHIV with AHD independently of country of residence. HIV clinicians and researchers should focus future studies on improving HRQoL and better understanding the special needs of this vulnerable population.
Data Availability
Dataset is available upon request.
References
Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60.
World Health Organization. Draft global health sector strategies HIV, 2016–2021 [Internet]. 2016. https://apps.who.int/gb/ebwha/pdf_files/WHA69/A69_31-en.pdf?ua=1.
UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014.
UNAIDS. Understanding fast-track targets. Accelerating action to end the AIDS epidemic by 2030. [Internet]. 2015. https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf
Lazarus JV, Van Hout MC, Fuster-Ruizdeapodaca MJ, Brown G, Guaraldi G. A call for health systems to monitor the health-related quality of life of people living with HIV. HIV Med. 2023;24:107–10.
Lazarus JV, Safreed-Harmon K, Kamarulzaman A, Anderson J, Leite RB, Behrens G, et al. Consensus statement on the role of health systems in advancing the long-term well-being of people living with HIV. Nat Commun. 2021;12:4450.
Briongos Figuero L, Bachiller Luque P, Palacios Martín T, González Sagrado M, Eiros BJ. Assessment of factors influencing health-related quality of life in HIV-infected patients: assessment of factors influencing health-related quality of life. HIV Med. 2011;12:22–30.
Degroote S, Vogelaers D, Vandijck DM. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 2014;72:40.
Douab T, Marcellin F, Vilotitch A, Protopopescu C, Préau M, Suzan-Monti M, et al. Health-related quality of life of people living with HIV followed up in hospitals in France: comparing trends and correlates between 2003 and 2011 (ANRS-VESPA and VESPA2 national surveys). AIDS Care. 2014;26:S29-40.
Millar BM, Parsons JT, Redline S, Duncan DT. What’s sleep got to do with it?: Sleep health and sexual risk-taking among men who have sex with men. AIDS Behav. 2019;23:572–9.
Nigusso FT, Mavhandu-Mudzusi AH. Health-related quality of life of people living with HIV/AIDS: the role of social inequalities and disease-related factors. Health Qual Life Outcomes. 2021;19:63.
Rodriguez-Penney AT, Iudicello JE, Riggs PK, Doyle K, Ellis RJ, Letendre SL, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27:5–16.
Turan B, Budhwani H, Fazeli PL, Browning WR, Raper JL, Mugavero MJ, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav. 2017;21:283–91.
Ford N, Meintjes G, Calmy A, Bygrave H, Migone C, Vitoria M, et al. Managing advanced HIV disease in a public health approach. Clin Infect Dis. 2018;66:S106-SS110.
ECDC. HIV/AIDS Surveillance in Europe 2021. [Internet]. 2022. https://www.ecdc.europa.eu/sites/default/files/documents/2022-Annual_HIV_Report_final.pdf
World Health Organization. Key facts HIV 2021 [Internet]. 2022. https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/key-facts-hiv-2021-26july2022.pdf?sfvrsn=8f4e7c93_5
Calmy A, Ford N, Meintjes G. The persistent challenge of advanced HIV disease and AIDS in the era of antiretroviral therapy. Clin Infect Dis. 2018;66:S103-SS105.
Levy I, Maor Y, Mahroum N, Olmer L, Wieder A, Litchevski V, et al. Missed opportunities for earlier diagnosis of HIV in patients who presented with advanced HIV disease: a retrospective cohort study. BMJ Open. 2016;6: e012721.
Ingle SM, May MT, Gill MJ, Mugavero MJ, Lewden C, Abgrall S, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–97.
Pettit AC, Giganti MJ, Ingle SM, May MT, Shepherd BE, Gill MJ, et al. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J Intern AIDS Soc. 2018;21: e25031.
Sobrino-Vegas P, Moreno S, Rubio R, Viciana P, Bernardino JI, Blanco JR, et al. Impact of late presentation of HIV infection on short-, mid- and long-term mortality and causes of death in a multicenter national cohort: 2004–2013. J Infect. 2016;72:587–96.
Zhang S, van Sighem A, Kesselring A, Gras L, Prins J, Hassink E, et al. Risk of non-AIDS-defining events among HIV-infected patients not yet on antiretroviral therapy: Non-AIDS-defining events in untreated patients. HIV Med. 2015;16:265–72.
May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28:1193–202.
Anis AH, Nosyk B, Sun H, Guh DP, Bansback N, Li X, et al. Quality of life of patients with advanced HIV/AIDS: measuring the impact of both AIDS-defining events and non-AIDS serious adverse events. JAIDS. 2009;51:631–9.
Gesesew HA, Tesfay Gebremedhin A, Demissie TD, Kerie MW, Sudhakar M, Mwanri L. Significant association between perceived HIV related stigma and late presentation for HIV/AIDS care in low and middle-income countries: a systematic review and meta-analysis. PLoS ONE. 2017;12: e0173928.
Aden B, Nosyk B, Wittenberg E, Schackman BR. Health-related quality of life in HIV-infected and at-risk women: the impact of illicit drug use and hepatitis C on a community preference weighted measure. Med Decis Making. 2014;34:800–8.
Fuster-RuizdeApodaca MJ, Laguía A, Safreed-Harmon K, Lazarus JV, Cenoz S, del Amo J. Assessing quality of life in people with HIV in Spain: psychometric testing of the Spanish version of WHOQOL-HIV-BREF. Health Qual Life Outcomes. 2019;17:144.
Venturini A, Cenderello G, Di Biagio A, Giannini B, Ameri M, Giacomini M, et al. Quality of life in an Italian cohort of people living with HIV in the era of combined antiretroviral therapy (Evidence from I.A.N.U.A. study-investigation on antiretroviral therapy). AIDS Care. 2017;29:1373–7.
Emuren L, Welles S, Evans AA, Polansky M, Okulicz JF, Macalino G, et al. Health-related quality of life among military HIV patients on antiretroviral therapy. PLoS ONE. 2017;12: e0178953.
Liu C, Johnson L, Ostrow D, Silvestre A, Visscher B, Jacobson LP. Predictors for lower quality of life in the HAART era among HIV-infected men. JAIDS. 2006;42:470–7.
Fumaz CR, Larrañaga-Eguilegor M, Mayordomo-López S, Gómez-Martínez S, González-García M, Ornellas A, et al. Health-related quality of life of people living with HIV infection in Spain: a gender perspective. AIDS Care. 2019;31:1509–17.
Préau M, Marcellin F, Carrieri MP, Lert F, Obadia Y, Spire B. Health-related quality of life in French people living with HIV in 2003: results from the national ANRS-EN12-VESPA Study. AIDS. 2007;21:S19-27.
Preau M, Apostolidis T, Francois C, Raffi F, Spire B. Time perspective and quality of life among HIV-infected patients in the context of HAART. AIDS Care. 2007;19:449–58.
Badia X, Podzamczer D, Garcia M, López-Lavid CC, Consiglio E. A randomized study comparing instruments for measuring health-related quality of life in HIV-infected patients. Spanish MOS-HIV and MQOL-HIV validation group. Medical outcomes study HIV health survey. AIDS. 1999;13:1727–35.
Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050.
Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, et al. The Toxicity of Azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:192–7.
Walker UA, Bäuerle J, Laguno M, Murillas J, Mauss S, Schmutz G, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004;39:311–7.
Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5.
Vogel M, Rockstroh JK. Hepatotoxicity and liver disease in the context of HIV therapy. Curr Opin HIV AIDS. 2007;2:306–13.
Bartlett JA, Fath MJ, DeMasi R, Hermes A, Quinn J, Mondou E, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–64.
Ryom L, Lundgren JD, El-Sadr W, Reiss P, Kirk O, Law M, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A: D international prospective multicohort study. The Lancet HIV. 2018;5:e291-300.
Scherzer R, Estrella M, Li Y, Choi AI, Deeks SG, Grunfeld C, et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–75.
Sax PE, Arribas JR, Orkin C, Lazzarin A, Pozniak A, DeJesus E, et al. Bictegravir/emtricitabine/tenofovir alafenamide as initial treatment for HIV-1: five-year follow-up from two randomized trials. ClinicalMedicine. 2023;59: 101991.
US Public Health Service. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents [Internet]. 2011. www.aidsinfo.nih.gov.
US Public Health Service. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. [Internet]. 2006. www.aidsinfo.nih.gov/guidelines/default_db2.asp?id=50.
Vitoria M, Hill A, Ford N, Doherty M, Clayden P, Venter F, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS. 2018;32:1551–61.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–12.
Wells, G. A., Shea, B., O’Connell, D. A., Peterson, J., Welch, V., Losos, M., & Tugwell, P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2020. http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso/Perfezionamento/EBM_Faggiano/NOS_oxford.pdf
Hedges LV, Olkin I. Statistical methods for meta-analysis. Saint Louis: Academic Press; 1985.
Cohen J. Statistical power analysis for the behavioral sciences. Oxfordshire: Routledge; 1988.
Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to meta-analysis. 2nd ed. Hoboken: Wiley; 2021.
Sánchez-Meca J, Marín-Martínez F, Chacón-Moscoso S. Effect-size indices for dichotomized outcomes in meta-analysis. Psychol Methods. 2003;8:448–67.
Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 3rd edition. New York: Russell Sage Foundation; 2019
Hartung J. An alternative method for meta-analysis. Biom J. 1999;41:901–16.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
Rothstein HR. Publication bias as a threat to the validity of meta-analytic results. J Exp Criminol. 2008;4:61–81.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Aguinis H, Gottfredson RK, Wright TA. Best-practice recommendations for estimating interaction effects using meta-analysis: interaction effects in meta-analysis. J Organiz Behav. 2011;32:1033–43.
Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710.
Rubio-Aparicio M, López-López JA, Viechtbauer W, Marín-Martínez F, Botella J, Sánchez-Meca J. Testing categorical moderators in mixed-effects meta-analysis in the presence of heteroscedasticity. J Exp Educ. 2020;88:288–310.
López-López JA, Marín-Martínez F, Sánchez-Meca J, Van den Noortgate W, Viechtbauer W. Estimation of the predictive power of the model in mixed-effects meta-regression: a simulation study. Br J Math Stat Psychol. 2014;67:30–48.
Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft. 2010. https://doi.org/10.18637/jss.v036.i03.
Ahmed A, Saqlain M, Akhtar N, Hashmi F, Blebil A, Dujaili J, et al. Translation and cross-cultural adaptation of WHOQOL-HIV Bref among people living with HIV/AIDS in Pakistan. Health Qual Life Outcomes. 2021;19:48.
Ahmed A, Saqlain M, Bashir N, Dujaili J, Hashmi F, Mazhar F, et al. Health-related quality of life and its predictors among adults living with HIV/AIDS and receiving antiretroviral therapy in Pakistan. Qual Life Res. 2021;30:1653–64.
Amara PS, Naveed Z, Wichman CS, Fox HS, Baccaglini L. Neurocognitive impairment and health-related quality of life among people living with Human Immunodeficiency Virus (HIV). PLoS ONE. 2021;16: e0248802.
Armon C, Lichtenstein K. The associations among coping, nadir CD4+ T-cell count, and non-HIV-related variables with health-related quality of life among an ambulatory HIV-positive patient population. Qual Life Res. 2012;21:993–1003.
Bekele T, Rourke SB, Tucker R, Greene S, Sobota M, Koornstra J, et al. Direct and indirect effects of perceived social support on health-related quality of life in persons living with HIV/AIDS. AIDS Care. 2013;25:337–46.
Belay YB, Ali EE, Sander B, Gebretekle GB. Health-related quality of life of patients with HIV/AIDS at a tertiary care teaching hospital in Ethiopia. Health Qual Life Outcomes. 2021;19:24.
Burgoyne RW, Saunders DS. Quality of life among urban Canadian HIV/AIDS clinic outpatients. Int J STD AIDS. 2001;12:505–12.
Call SA, Klapow JC, Stewart KE, Westfall AO, Mallinger AP, DeMasi RA, et al. Health-related Quality of Life and Virologic outcomes in an HIV clinic. Qual Life Res. 2000;9:977–85.
Degroote S, Vogelaers DP, Vermeir P, Mariman A, De Rick A, Van Der Gucht B, et al. Socio-economic, behavioural, (neuro)psychological and clinical determinants of HRQoL in people living with HIV in Belgium: a pilot study. J Int AIDS Soc. 2013;16:18643.
Garcia-Ordoñez MA, Mansilla Francisco JJ, Nieto Aragon E, Cereto MR, Salas Samper F, Vallejo Diaz M, et al. Quality of life associated with the health of patients with HIV infection measured with the Health Questionnaire SF-36. An Med Interna. 2001;18:74–9.
Gibson K, Rueda S, Rourke SB, Bekele T, Gardner S, Fenta H, et al. Mastery and coping moderate the negative effect of acute and chronic stressors on mental health-related quality of life in HIV. AIDS Patient Care STDS. 2011;25:371–81.
Hailu T, Yitayal M, Yazachew L. Health-related quality of life and associated factors among adult HIV mono-infected and TB/HIV co-infected patients in public health facilities in northeast Ethiopia: a comparative cross-sectional study. PPA. 2020;14:1873–87.
Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, et al. Health-related quality of life in patients with human immunodeficiency virus infection in the United States: results from the HIV cost and services utilization study. Am J Med. 2000;108:714–22.
Igumbor J, Stewart A, Holzemer W. Comparison of the health-related quality of life, CD4 count and viral load of AIDS patients and people with HIV who have been on treatment for 12 months in rural South Africa. SAHARA-J J Soc Aspects HIV/AIDS. 2013. https://doi.org/10.1080/17290376.2013.807070.
Imam MH, Flora MS, Moni MA, Shameem RK, Haque MA, Mamun SA. Health related quality of life with HIV/AIDS in different stages of HIV infection. Mymensingh Med J. 2012;21:509–15.
Kanu NE, Tobin-West CI. Health-related quality of life of HIV patients with and without tuberculosis registered in a Tertiary Hospital in Port Harcourt. Nigeria Hivar. 2018;17:210–7.
Liping M, Peng X, Haijiang L, Lahong J, Fan L. Quality of life of people living with HIV/AIDS: a cross-sectional study in Zhejiang Province, China. PLoS ONE. 2015;10: e0135705.
Mafirakureva N, Dzingirai B, Postma MJ, van Hulst M, Khoza S. Health-related quality of life in HIV/AIDS patients on antiretroviral therapy at a tertiary care facility in Zimbabwe. AIDS Care. 2016;28:904–12.
Meemon N, Paek SC, Yenchai D, Wan TTH. Application of the WHOQOL-HIV-BREF questionnaire in HIV-infected Thai patients: reliability and validity of the instrument. J Assoc Nurses AIDS Care. 2016;27:698–708.
Melaku T, Mamo G, Chelkeba L, Chanie T. Health-related quality of life among people living with human immunodeficiency virus on highly active antiretroviral therapy in Ethiopia: PROQOL-HIV based survey. PROM. 2020;11:73–86.
Murri R, Fantoni M, Del Borgo C, Visona R, Barracco A, Zambelli A, et al. Determinants of health-related quality of life in HIV-infected patients. AIDS Care. 2003;15:581–90.
Patel AR, Lester RT, Marra CA, van der Kop ML, Ritvo P, Engel L, et al. The validity of the SF-12 and SF-6D instruments in people living with HIV/AIDS in Kenya. Health Qual Life Outcomes. 2017;15:143.
Peltzer K, Phaswana-Mafuya N. Health-related quality of life in a sample of HIV-infected South Africans. Afr J AIDS Res. 2008;7:209–18.
Remor E. Reliability and validity of the Spanish version of the MOS-SF-30 to assess the health related quality of life in people infected by HIV. Aten Primaria. 2003;32:15–22.
Rueda S, Raboud J, Mustard C, Bayoumi A, Lavis JN, Rourke SB. Employment status is associated with both physical and mental health quality of life in people living with HIV. AIDS Care. 2011;23:435–43.
Schnall R, Liu J, Cho H, Hirshfield S, Siegel K, Olender S. A health-related quality-of-life measure for use in patients with HIV: a validation study. AIDS Patient Care STDS. 2017;31:43–8.
Stasinopoulou PG, Tzavara C, Dimitrakaki C, Georgiou O, Baraboutis IG, Skoutelis A, et al. Reliability and validity of the Greek translation of the MOS-HIV health survey in HIV-infected individuals. Qual Life Res. 2010;19:199–205.
Torres TS, Harrison LJ, La Rosa AM, Lavenberg JA, Zheng L, Safren SA, et al. Quality of life among HIV-infected individuals failing first-line antiretroviral therapy in resource-limited settings. AIDS Care. 2018;30:954–62.
Tran B, Ohinmaa A, Nguyen L. Quality of life profile and psychometric properties of the EQ-5D-5L in HIV/AIDS patients. Health Qual Life Outcomes. 2012;10:132.
Tran B. Quality of life outcomes of antiretroviral treatment for HIV/AIDS patients in Vietnam. PLoS ONE. 2012;7: e41062.
Uchechukwu D, Iorfa SK, Ugwu DI. Negative centralisation of HIV/AIDS trauma and health-related quality of life: do post-traumatic stress symptoms explain the link? Afr J AIDS Res. 2020;19:206–13.
Worthington C, Krentz HB. Socio-economic factors and health-related quality of life in adults living with HIV. Int J STD AIDS. 2005;16:608–14.
de Oliveira Gomes M, Castro R, Correa da Mota J, De Boni RB. Association of syndemic conditions and quality of life among people living with HIV/AIDS. AIDS Care. 2022. https://doi.org/10.1080/09540121.2022.2080801.
Schönnesson LN. Psychological and existential issues and quality of life in people living with HIV infection. AIDS Care. 2002;14:399–404.
Collins PY, Velloza J, Concepcion T, Oseso L, Chwastiak L, Kemp CG, et al. Intervening for HIV prevention and mental health: a review of global literature. J Int AIDS Soc. 2021. https://doi.org/10.1002/jia2.25710.
Rasoolinajad M, Abedinia N, Noorbala AA, Mohraz M, Badie BM, Hamad A, et al. Relationship among HIV-related stigma, mental health and quality of life for HIV-positive patients in Tehran. AIDS Behav. 2018;22:3773–82.
Cahill S, Valadéz R. Growing older with HIV/AIDS: new public health challenges. Am J Public Health. 2013;103:e7-15.
Hsieh E, Polo R, Qian H-Z, Fuster-RuizdeApodaca MJ, Del Amo J. Intersectionality of stigmas and health-related quality of life in people ageing with HIV in China, Europe, and Latin America. Lancet Healthy Longev. 2022;3:e206–15.
Cobb S. Social support as a moderator of life stress. Psychosom Med. 1976;38:300–14.
Ghiasvand H, Higgs P, Noroozi M, Ghaedamini Harouni G, Hemmat M, Ahounbar E, et al. Social and demographical determinants of quality of life in people who live with HIV/AIDS infection: evidence from a meta-analysis. Biodemography Soc Biol. 2020;65:57–72.
Portilla-Tamarit J, Reus S, Portilla I, Fuster Ruiz-de-Apodaca MJ, Portilla J. Impact of advanced HIV disease on quality of life and mortality in the era of combined antiretroviral treatment. JCM. 2021;10:716.
Acknowledgements
We acknowledge people living with HIV who have contributed to each study included.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Portilla-Tamarit, I., Rubio-Aparicio, M., Fuster-RuizdeApodaca, M.J. et al. Health-Related Quality of Life in People with Advanced HIV Disease, from 1996 to 2021: Systematic Review and Meta-analysis. AIDS Behav 28, 1978–1998 (2024). https://doi.org/10.1007/s10461-024-04298-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-024-04298-y