Abstract
Men have higher rates of attrition from antiretroviral therapy (ART) programs than women. In Khayelitsha, a high HIV prevalence area in South Africa, two public sector primary healthcare clinics offer services, including HIV testing and treatment, exclusively to men. We compared attrition from ART care among men initiating ART at these clinics with male attrition in six general primary healthcare clinics in Khayelitsha. We described baseline characteristics of patients initiating ART at the male and general clinics from 1 January 2014 to 31 March 2018. We used exposure propensity scores (generated based on baseline health and age) to match male clinic patients 1:1 to males at other clinics. The association between attrition (death or loss to follow-up, defined as no visits for nine months) and clinic type was estimated using Cox proportional hazards regression. Follow-up time began at ART initiation and ended at attrition, clinic transfer, or dataset closure. Before matching, patients from male clinics (n = 784) were younger than males from general clinics (n = 2726), median age: 31.2 vs 35.5 years. Those initiating at male clinics had higher median CD4 counts at ART initiation [Male Clinic 1: 329 (IQR 210–431), Male Clinic 2: 364 (IQR 260–536), general clinics 258 (IQR 145–398), cells/mm3]. In the matched analysis (1451 person-years, 1568 patients) patients initiating ART at male clinics had lower attrition (HR 0.71; 95% CI 0.60–0.85). In separate analyses for each of the two male clinics, only the more established male clinic showed a protective effect. Male-only clinics reached younger, healthier men, and had lower ART attrition than general services. These findings support clinic-specific adaptations to create more male-friendly environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most African settings, men are less likely to get tested for HIV, link to HIV care or initiate antiretroviral therapy (ART) than women [1,2,3,4,5,6,7]. Those who do are more likely to present to clinics later, with more advanced disease and have worse clinical outcomes [3,4,5, 8,9,10,11,12,13,14,15,16,17]. A meta-analysis found that among 20-year olds starting ART in low and middle-income countries, males on average live 22.9 more years (95% CI 18.4–27.5 years), compared to 33.0 years (95% CI 30.4–35.6 years) for females [18]. Multiple studies have shown men to have higher rates of attrition from HIV care programs than women [5, 8, 19,20,21,22,23,24,25,26,27,28,29,30,31]. In South Africa, ART coverage is substantially lower among adult males (58%) compared with adult females (64%) [32]. In 2017, 36% of the nearly 7 million HIV-positive adults living in South Africa were male, but males accounted for 52% of AIDS deaths [32]. Being on ART not only reduces mortality, but also decreases morbidity and transmission [33,34,35].
Explanations for men’s low attendance and poor outcomes include public health systems that are historically built around maternal and child health, systematic under-funding of men’s services compared to women’s [4, 13, 36, 37], and notions of masculinity that are at odds with both illness [38, 39] and expected patient behavior [40]. Despite higher attrition among males on ART, few strategies have been developed to specifically address poor HIV outcomes for males in low and middle-income countries with generalized epidemics [12, 13, 36].
In 2014, in response to low male engagement and retention in care, the City of Cape Town’s health department, supported by Médecins Sans Frontières started a male clinic, (“Male Clinic 1”) [41] and in 2016 a second male clinic opened Both clinics are located in Khayelitsha, a high HIV prevalence, high-poverty peri-urban area in Cape Town [42, 43]. We evaluate this intervention by comparing attrition from care among men at these two male-only clinics to men attending six general primary healthcare clinics in Khayelitsha.
Methods
Study Design
We conducted a propensity score matched cohort study of adult males receiving ART at primary care clinics in Khayelitsha, comparing attrition among males in male-only clinics to males in general clinics.
Intervention
Male Clinic 1 was opened in 2014 by the City of Cape Town’s health department, supported by Médecins Sans Frontières. The clinic is located near a transport hub and on its own premises, but not far from a larger health center. This service is staffed exclusively by males, is promoted as a males-only space, and offers HIV testing and counselling, ART initiation and dispensing, sexually transmitted infection (STI) diagnosis and treatment, and other primary healthcare services [44]. In July 2016, another clinic following the same model (“Male Clinic 2”) began offering ART services at a small clinic above a transport hub.
Population and Setting
The City of Cape Town’s Health department offers HIV care at 10 primary healthcare clinics in Khayelitsha, including two male-only clinics, six general primary healthcare clinics (with no particular male-targeted programs), and two youth-targeted clinics. Department of Health clinicians provide free services at all facilities, with support from non-governmental organizations who manage all lay adherence counselors at each facility. For the time period considered in this paper, Médecins Sans Frontières paid for and managed the counselor at Male Clinic 1, but not Male Clinic 2.
The study population consisted of adult (18 years and older) males who first initiated ART between 1 January 2014 (the year that Male Clinic 1 opened) and 1 April 2018 at the two male-only clinics or six general primary healthcare clinics. If individuals were not ART naïve or transferred into the clinic (as recorded by clinic) they were excluded. Youth-targeted clinics were excluded from the analysis as these clinics represent a separate novel model of targeted care, and do not provide care to men over the age of 25. Patients who had tuberculosis at ART initiation were excluded because these patients were often referred out of the male clinics for management at the general clinics. We excluded patients known to have initiated ART on a regimen not containing tenofovir (TDF) or efavirenz (EFV) as patients requiring non-tenofovir or non-efavirenz regimens at male clinics were generally referred to a general clinic.
Data Source
All data for this study came from routine patient data collected from patient folders at City of Cape Town clinics in Khayelitsha. Data capturers at each clinic capture data electronically from the patient folder after each patient ART visit. Data includes patient demographics, visits, regimens dispensed, laboratory results, and events such as deaths or transfers.
Measures and Follow-Up Time
The exposure of interest was receiving HIV treatment at one of two male clinics compared with receiving care at a general clinic. Our primary outcome was attrition from HIV care, a measure including all patients who were lost to follow-up or who died. Death was passively reported by families so it was assumed that, as reported in similar contexts, some patients who had died would have been misclassified as lost to follow-up in clinics [45,46,47]. A patient was considered lost to follow-up the first time there was a nine month gap in care, even if he later returned to care. Patient visits are generally 2–4 months apart, so a nine-month gap in care represents approximately six months with no ART. The date of last visit before the gap in care was used as the outcome date [48]. In the exploratory analysis, 6- and 12-month attrition was defined as having the outcome of attrition from HIV care, and having the last visit before attrition less that 6 months (in the case of 6-month attrition) or 12 months (in the case of 12-month attrition)after ART initiation.
The dataset was closed on 30 September 2018. Follow-up time for each person started on the date of ART initiation and ended on the last visit date before dataset closure, regardless of their outcome. Patients who transferred out were censored on their last visit date at their original clinic, even if the transfer was to another clinic represented in this dataset. If a patient’s last attended visit was the same as their ART initiation date, one day of follow-up was added to prevent them from being excluded from Kaplan–Meier and Cox proportional hazards analyses [48]. Baseline CD4 count was defined as the closest available result to the date of ART initiation, between 180 days before and 30 days after initiation.
Analysis
Multiple Imputation
Chained multiple imputation [49,50,51] was performed using the ice procedure in Stata [52, 53] for missing values of baseline WHO disease stage and CD4 count. Missing data were assumed to be missing at random. We created 20 imputed datasets (see Table 4 and 5 in Supplementary file 2).
Descriptive Analyses
Predefined potential confounders included indicators of baseline health, date of ART initiation, and age, variables known to be associated with ART outcomes [17, 19, 20, 54], and plausibly also associated with clinic type. We describe these covariates, stratified by clinic type, before and after propensity score matching. We also describe attrition by 6 and 12 months, stratified by covariates, for observations with at least 9 and 15 months of follow-up time respectively, excluding those who transferred out before 6 and 12 months respectively.
Propensity Score Matching
We used a matched exposure propensity score approach to control for confounding. Propensity scores were generated using logistic regression, with clinic type (male clinics vs general clinics) as the dependent variable. Independent variables were baseline WHO stage, age, year of ART initiation and CD4 count, which were selected from the predefined potential confounders based on observed associations with both attrition and male clinics in the descriptive analyses. This process was repeated for each of the imputed datasets and an average propensity score for each observation was calculated across datasets [55]. The exposed were matched, in random order, 1:1 to the unexposed patient with the closest average propensity score (nearest neighbor matching), without replacement [56].
Time-to-Event Analyses
We performed a Cox proportional hazards regression on the matched data to estimate the hazards ratio (HR) of attrition associated with attending male clinics compared to general clinics. Where appropriate the results of the analyses from the multiple imputed datasets were combined using Stata’s mi estimate feature, which uses Rubin’s methods [57] to adjust coefficients and standard errors for the variability between imputations. To adjust for any residual confounding after matching on propensity score, covariates were individually added to the model to observe their effect on the HR but none were retained as none met the threshold of changing the HR by more than 10% (see Table 2 in Supplementary file 2). To support the Cox regression, and for descriptive purposes, Kaplan–Meier curves of each male clinic cohort and their matched controls, with attrition as the outcome, are also presented.
Goodness of fit tests using Schoenfeld residuals suggested no evidence of violation of the proportional hazards assumption (p = 0.95, see Table 3 in Supplementary file 2). For the survival curves and model diagnostics, only the first imputed dataset was used, because after propensity score generation and matching clinic type, outcome and outcome date were identical across datasets.
Sensitivity Analyses
We performed several sensitivity analyses of the Cox regression analysis. First, the analysis, including propensity score matching procedures, was performed separately for each male clinic, to see if there was a difference in effect between the two clinics. We did an additional analysis which excluded Male Clinic 1 patients and follow-up time after the first two years of the clinic’s ART services, to see if any differences between the two male clinics could be explained by Male Clinic 1 being a more established clinic. Second, we changed the outcome definition to reflect the patient’s status at dataset closure. The clinic data system automatically classified patients as lost to follow-up if a patient was three months late for a scheduled visit with no subsequent visit. If a patient returned to care before dataset closure they were no longer considered lost to follow-up. Third, we considered the effects of ‘silent transfers’ [58, 59], where patients switched to another facility but were not captured as transfer-outs. We manually searched for results from patients who were lost to follow-up on the National Health Laboratory System, which contains laboratory results from all patients in the public sector, regardless of where they attend care. Patients were reclassified as transfers if they had a viral load (a routine HIV monitoring test) result within one year of their last known visit, at any primary healthcare facility. We excluded blood tests that were performed at tertiary care facilities as these were likely done because the patient presented with an illness, and did not necessarily indicate that the patient was in ART care.
Quantitative Bias Analysis
A hypothesized cluster of behavioural characteristics, including mental health and attitudes towards healthcare and female healthcare workers, may be associated with male clinic attendance and attrition [60,61,62]. Considering this cluster of characteristics as a single confounder, we conducted a quantitative bias analysis. We hypothesized plausible distributions of (1) confounder prevalence in general clinics (triangular distribution 0–20%, mode = 10%); (2) association between the confounder and male clinic attendance (triangular distribution 1.5–2.5, mode = 2), and (3) association between the confounder and attrition at any time (triangular distribution 0.5 to 0.9, mode = 0.7). Sampling from these distributions, we ran 100,000 simulated analyses where HRs were adjusted for the unmeasured confounder. The resulting HRs show the effect of male clinics on attrition that incorporate hypothesized systematic and random error [63]. The median bias-adjusted HR is presented, along with a 95% interval (2.5–97.5 percentile) (See Supplementary file 1 for additional details).
Ethics
Ethics approval was granted by the University of Cape Town’s Human Research Ethics Committee (HREC395/2005), who waived consent as the analyses used de-identified data collected as part of routine patient care.
Results
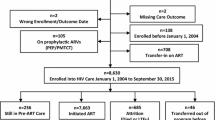
We had data for 31 578 patients ever on ART in male-targeted or general primary healthcare clinics run by City Health in Khayelitsha. After exclusions, the analytic dataset included 3510 observations: 462 observations from Male Clinic 1, 322 from Male Clinic 2, and 2726 eligible matched comparisons from other clinics (Fig. 1).
Patients at Male Clinic 2 were most likely to have initiated ART recently, with 89% of patients initiating after ART eligibility criteria were removed in 2016, compared to 39% in the general clinics (Table 1). Men initiating ART at male clinics were younger than those in the general clinics [31.2 (IQR 26.9–36) compared to 35.5 (IQR 30.3–42.2) years old]. General clinics had a higher proportion of patients initiating ART at WHO disease stages 2–4 (31%) compared to Male Clinic 1 (7%) and Male Clinic 2 (2%). Men initiating treatment in the male clinics had a higher median CD4 count than general clinics [Male Clinic 1: 329 (IQR 210–431), Male Clinic 2: 364 (IQR 260–536) vs general clinics 258 (IQR 145–398) cells/mm3]. General clinics had a higher proportion of missing data on disease stage at ART initiation and CD4 count (Table 1).
Descriptive analyses before imputation or propensity score matching revealed that Male Clinic 2 had a higher risk of 12-month attrition (35%) compared to Male Clinic 1 (23%) or general clinics (32%) (Table 2). Risk of attrition by six and 12 months was associated with more recent ART initiation and younger age. Baseline WHO stage was not associated with attrition, but missing information was associated with higher attrition and those healthier at baseline were at a slightly lower risk of attrition. We observed 31% attrition at 12 months for those with a CD4 count below 200 cells/mm3, compared to 33% for those with CD4 count above 500 cells/mm3. For survival estimates at months 6 and 12 by covariates see Table 1 in Supplementary file 2.
Propensity Score Estimation and Matching
For males clinics patients, the median propensity score predicting the probability of attending a male clinic was 0.35 (IQR 0.23–0.46), compared to 0.16 (IQR 0.06–0.28) among general patients (Supplementary file 2, Fig. 1). After matching, the median propensity score was 0.35 for both male clinics (IQR 0.23–0.46) and general clinics (IQR 0.23–0.43).
Propensity score matching reduced the association between exposure and covariates, but there were still small differences in terms of ART start date, age, disease stage and CD4 count at initiation (Table 1 and Supplementary file 2, Fig. 2).
Compared to males that were not matched to a male clinic patient, matched general clinic patients initiated ART at later dates, younger age, higher CD4 counts and less advanced disease stage (see also Fig. 2 in Supplementary file 2).
Analysis of Matched Cohort
The matched cohort (N = 1568) represented a total of 1568.9 person-years, 807.6 of which were in the general clinics and 761.3 of which were in the male clinics.
Kaplan–Meier curves for the matched cohort show greater attrition (loss to follow-up or death) among men at general clinics, compared to male clinics (Fig. 2, see Supplementary file 2, Fig. 3 for Kaplan–Meier curves by clinic).
There was a small association between type of clinic and attrition [HR 0.71 (95% CI 0.60–0.85) for male clinic compared to matched patients at general clinics] (Table 3).
Sensitivity Analyses
In separate analyses of male clinics compared to a newly matched cohort of general clinic patients, Male Clinic 1 showed a similar protective effect against attrition (HR 0.67; 95% CI 0.53–0.85), but no protective effect was observed for Male Clinic 2 (HR 0.99; 95% CI 0.75–1.31) (Table 3, for Kaplan–Meier curves see Fig. 4 in Supplementary file 2). Using the full matched cohort (both male clinics), a weaker protective effect was observed when changing the definition of loss to follow-up to a missed appointment with no return to the clinic by the end of follow-up period. Similar results were found reclassifying failures who had a viral load result within one year of their last visit.
Quantitative Bias Analysis
Incorporating total error, the median adjusted HR was 0.74 (95% interval: 0.61–0.89) (Table 3). Adjusting for the hypothesized distributions of confounder prevalence in general clinics, confounder-male clinic associations, and confounder-attrition associations, there was still an small protective effect of male clinics on attrition (Supplementary file 1).
Discussion
This study compared attrition from care in two male-targeted clinics and six general primary healthcare clinics in a low-resource, high HIV prevalence area. Males at the male-targeted clinics had a somewhat lower (HR 0.71; 95% CI 0.60–0.85) attrition than those in general clinics, adjusting for baseline clinical and demographic characteristics using a matched propensity score approach. Separate analyses of the two male clinics showed a protective effect only for Male Clinic 1 when compared to general clinics.
Men initiating ART at the male clinics were younger, with less advanced HIV than those initiating in the general primary healthcare clinics. This may be partly because, while not quantified in this paper, a large proportion of male clinic ART patients initially presented with STIs [44], which men may not be willing to reveal to female healthcare providers [64]. STI treatment is an important opportunity for HIV diagnosis and treatment initiation for males, who otherwise have fewer opportunities to interact with the healthcare system than women [65, 66], thereby making them less likely to be diagnosed and treated with HIV earlier in disease progression. Male clinics might also be more successful at linking newly diagnosed patients to ART care earlier in HIV disease progression. Earlier ART initiation also reduces the risk of transmission [35].
Despite being younger and healthier at ART initiation, male clinic patients (particularly those at Male Clinic 2) had a higher risk of 6- and 12-month attrition than general clinic patients in a crude analysis (Table 2). This is consistent with other literature showing that being younger and having fewer comorbidities makes patients more likely to drop out of care, possibly because they do not see an immediate benefit of ART [67, 68]. This is an important consideration when evaluating programs that improve uptake of testing or earlier ART initiation: attrition may appear to worsen because of changing baseline characteristics.
Through propensity score matching, we created a general clinic cohort that was more comparable to the male clinic group in terms of baseline health and demographic characteristics. In this analysis, we found a lower attrition from male clinics than from general clinics. When defining loss to follow-up as a gap in care with no return to clinic in the study period, the observed effect was less protective. This suggests that male clinics may be particularly protective against shorter disengagements from care, which is a growing concern with increasing ART duration [69,70,71]. It is possible that creating a male-only environment can make clinics more acceptable to men, possibly through helping them to overcome social and cultural barriers to care [38,39,40], including complicated gender dynamics with female healthcare providers [72], and leading to improved retention in care. Research has also suggested that men’s healthcare experiences are influenced by peers and social networks [73], so it is possible that the benefits of the service are enhanced through these relationships.
In an analysis of Male Clinic 2 compared to general clinics, no protective effect was observed. Although Male Clinic 2 only began ART services in mid-2016, Male Clinic 1’s lower attrition cannot be explained by the fact that the older clinic has had longer to establish itself, as protective effects were also observed in the first two years of Male Clinic 1’s ART services. It is possible that Male Clinic 2 attracted a more transient population as it is located at a transport hub, where men may have attended when convenient, as opposed to selecting a clinic near their homes. Most attrition happened soon after ART initiation. In a context where people have precarious livelihoods, with 38% unemployment in Khayelitsha, time and transport costs may present early barriers to continuing ART care [74, 75]. It is also possible that support from Médecins Sans Frontières via the lay adherence counselor provided at Male Clinic 1 differed from the standard of care at Male Clinic 2 and other clinics, where counselors were provided by another organisation and paid less than the Male Clinic 1 counselor. Nonetheless, Male Clinic 2 was successful in initiating younger, healthier men, with comparable retention to general clinics, when controlling for these factors. Despite favorable results compared to general clinics, there was 23% attrition by 12 months at Male Clinic 1. This likely reflects the myriad documented challenges to continued engagement in ART care including competing responsibilities, social stigma, mental health issues, migration and substance abuse [76,77,78,79,80,81,82].
There is a limited amount of literature on successful strategies to identify men living with HIV and link them to care [13]. Strategies include index testing, targeted community-based testing [83], self-testing for HIV [84], and incentives for ART initiation and retention [85]. This study adds important evidence about the type of clinical setting that can contribute to retaining males in HIV care.
Data for this analysis was limited to laboratory records and clinical data from selected clinics, and may have failed to detect deaths which actually occurred. In addition, data quality may differ between clinics, leading to differential misclassification of loss to follow-up. For multiple imputation, we assumed missing data was missing at random. However, the sensitivity analysis incorporating laboratory data to approximate “silent transfers” did not substantially change our results. A second limitation is the potential for unmeasured confounding, stemming from self-selection of males into the male clinics based on factors that were not measured in our limited set of potentially confounding covariates. Our quantitative bias analysis showed that even if there were reasonably strong confounder-exposure and confounder-attrition associations, there would still be a protective effect of male clinics on attrition. The hypothesized confounding characteristics in the male clinic patients may also increase the risk of attrition. For example, characteristics that are associated with a strong aversion to female healthcare workers may also be associated with other health behaviors such as avoidance of clinics in general, and non-disclosure of their HIV status, which is associated with poorer retention [86, 87]. If this is the case, then the observed effect sizes are underestimates and the true effect of male clinics may be more protective.
Conclusions
In an environment where engaging health services may be perceived as a sign of vulnerability [88], contrary to hegemonic masculine ideals [89], these clinics aim to create explicitly male spaces to make men feel more comfortable attending the clinic and reduce attrition from care. Our results suggest this has been successful at Male Clinic 1. Both clinics successfully attracted younger, healthier males than the general clinics, reaching men sooner after HIV infection. Stand-alone male-only clinics may not always be feasible, particularly in lower-resource, or lower population density settings. However these findings support smaller-scale interventions such as increasing the proportion of male nurses and lay counselors in public clinics, or strengthening male-targeted STI treatment as an entry-point for males. Further research could explore other ways of designating male services such as separate spaces, rooms, programs, queues, entrances, or operating hours for men, which may also help to improve confidentiality and counter the perception of healthcare facilities as female spaces.
Data Availability
The data used for this analysis is routine clinical data that cannot be passed on to third parties without prior research approval from the relevant health authorities (the City of Cape Town: https://www.capetown.gov.za/City-Connect/Access-information/Submit-a-research-request or Western Cape Provincial Department of Health: http://nhrd.hst.org.za/). The data is stored and managed by the Provincial Health Data Center1.
Code Availability
Stata code for all analyses are available here: https://github.com/talicassidy/males.
Abbreviations
- ART:
-
Antiretroviral therapy
- CD4:
-
Cluster of differentiation 4
- CI:
-
Confidence interval
- HIV:
-
Human immodeficiency virus
- HR:
-
Hazards ratio
- IQR:
-
Interquartile range
- STI:
-
Sexually transmitted infection
- WHO:
-
World Health Organization
References
Staveteig S, Wang S, Head SK, Bradley SEK, Nybro E. Demographic patterns of HIV testing uptake in sub-Saharan Africa. DHS Comparative Reports No. 30. Demogr Patterns HIV Test Uptake Sub-Saharan Africa. 2013; 81. http://dhsprogram.com/pubs/pdf/CR30/CR30.pdf
Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: successes and challenges. AIDS. 2015;29(11):1401–9.
Lippman SA, El Ayadi AM, Grignon JS, Puren A, Liegler T, Venter WDF, et al. Improvements in the South African HIV care cascade: findings on 90–90–90 targets from successive population-representative surveys in North West Province. J Int AIDS Soc. 2019;22(6):e25295. https://doi.org/10.1002/jia2.25295.
Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS. 2013;27(3):417–25.
Mills EJ, Bakanda C, Birungi J, Chan K, Hogg RS, Ford N, et al. Male gender predicts mortality in a large cohort of patients receiving antiretroviral therapy in Uganda. J Int AIDS Soc. 2011;14(1):52.
Braitstein P, Boulle A, Nash D, Brinkhof MWG, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Women’s Health. 2008;17(1):47–55.
Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17(12):1509–20.
Johnson LF, Keiser O, Fox MP, Tanser F, Cornell M, Hoffmann CJ, et al. Life expectancy trends in adults on antiretroviral treatment in South Africa. AIDS. 2016;30(16):2545–50.
Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. https://doi.org/10.1371/journal.pmed.1001418.
Osler M, Cornell M, Ford N, Hilderbrand K, Goemaere E, Boulle A. Population-wide differentials in HIV service access and outcomes in the Western Cape for men as compared to women, South Africa: 2008 to 2018: a cohort analysis. J Int AIDS Soc. 2020;23(S2):5–14.
Maman D, Pujades-Rodriguez M, Subtil F, Pinoges L, McGuire M, Ecochard R, et al. Gender differences in immune reconstitution: a multicentric cohort analysis in sub-Saharan Africa. PLoS ONE. 2012;7(2):1–8.
Cornell M, Cox V, Wilkinson L. Public health blindness towards men in HIV programmes in Africa. Trop Med Int Health. 2015;20(12):1634–5.
Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health. 2011;16(7):828–9. https://doi.org/10.1111/j.1365-3156.2011.02767.x.
Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, et al. Sex differences in antiretroviral treatment outcomes among HIV-infected adults in an urban Tanzanian setting. AIDS. 2011;25(9):1189–97.
Beckham SW, Beyrer C, Luckow P, Doherty M, Negussie EK, Baral SD. Marked sex differences in all-cause mortality on antiretroviral therapy in low-and middle-income countries: a systematic review and meta-analysis. J Int AIDS Soc. 2016;19(1):21106.
Geng EH, Odeny TA, Lyamuya RE, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2(3):e107–16.
DeSilva MB, Merry SP, Fischer PR, Rohrer JE, Isichei CO, Cha SS. Youth, unemployment, and male gender predict mortality in AIDS patients started on HAART in Nigeria. AIDS Care. 2009;21(1):70–7.
Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256–66.
Gosset A, Protopopescu C, Larmarange J. Retention in care trajectories of HIV-positive individuals participating in a universal test-and-treat program in rural South Africa (ANRS 12249 TasP Trial). J Acquir Immune Defic Syndr. 2019;80(4):375–85.
Charurat M, Oyegunle M, Benjamin R, Habib A, Eze E, Ele P, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS ONE. 2010;5(5):e10584.
Janssen S, Wieten RW, Stolp S, Cremers AL, Rossatanga EG, Klipstein-Grobusch K, et al. Factors associated with retention to care in an HIV clinic in Gabon, central Africa. PLoS ONE. 2015;10(10):1–14.
Mutasa-Apollo T, Shiraishi RW, Takarinda KC, Dzangare J, Mugurungi O, Murungu J, et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe’s National Antiretroviral Therapy Programme, 2007–2010. PLoS ONE. 2014;9(1):e86305.
Ochieng-Ooko V, Ochieng D, Sidle JE, Holdsworth M, Wools-Kaloustian K, Siika A, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88(9):681–8.
Cornell M, Myer L, Kaplan R, Bekker L-G, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14(7):722–31.
Cornell M, Schomaker M, Garone DB, Giddy J, Hoffmann CJ, Lessells R, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med. 2012;9(9):1–12.
Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. https://doi.org/10.1371/journal.pmed.1001718.
Mugisha V, Teasdale CA, Wang C, Lahuerta M, Nuwagaba-Biribonwoha H, Tayebwa E, et al. Determinants of mortality and loss to follow-up among adults enrolled in HIV care services in Rwanda. PLoS ONE. 2014;9(1):1–9.
Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, de Wit TFR, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health. 2015;15(1):478. https://doi.org/10.1186/s12889-015-1814-2.
Bucciardini R, Fragola V, Abegaz T, Lucattini S, Halifom A, Tadesse E, et al. Retention in care of adult HIV patients initiating antiretroviral therapy in Tigray, Ethiopia: a prospective observational cohort study. PLoS ONE. 2015;10(9):1–12.
Kerschberger B, Schomaker M, Ciglenecki I, Pasipamire L, Mabhena E, Telnov A, et al. Programmatic outcomes and impact of rapid public sector antiretroviral therapy expansion in adults prior to introduction of the WHO treat-all approach in rural Eswatini. Trop Med Int Health. 2019;24(6):701–14.
Mee P, Rice B, Lemsalu L, Sambu V, Harklerode R, Somi G, et al. Changes in patterns of retention in HIV care and antiretroviral treatment in Tanzania between 2008 and 2016: an analysis of routinely collected national programme data. J Glob Health. 2019;9(1):1–11.
Johnson LF, May MT, Dorrington RE, Cornell M, Boulle A, Egger M, et al. Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: a mathematical modelling study. PLoS Med. 2017;14(12):1–17.
Ford N, Meintjes G, Calmy A, Bygrave H, Migone C, Vitoria M, et al. Managing advanced HIV disease in a public health approach. Clin Infect Dis. 2018;66(Suppl 2):S106–10.
Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis. 2018;66:S118–25.
Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–71. https://doi.org/10.1126/science.1228160.
Cornell M. Gender inequality: bad for men’s health. S Afr J HIV Med. 2013;14(1):12–4.
Varga CA. The forgotten fifty per cent: a review of sexual and reproductive health research and programs focused on boys and young men in sub-Saharan Africa. Afr J Reprod Health. 2001;5(3):175.
Fitzgerald M, Collumbien M, Hosegood V. “No one can ask me ‘why do you take that stuff?’”: men’s experiences of antiretroviral treatment in South Africa. AIDS Care. 2010;22(3):355–60.
Nyamhanga TM, Muhondwa EPY, Shayo R. Masculine attitudes of superiority deter men from accessing antiretroviral therapy in Dar es Salaam, Tanzania. Glob Health Action. 2013;6:21812.
Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men’ s use of HIV services in Zimbabwe. Glob Health. 2011;7:1–14.
Garone DB, Hilderbrand K, Boulle AM, Coetzee D, Goemaere E, Van CG, et al. Review khayelitsha 2001–2011: 10 years of primary care HIV. S Afr J HIV Med. 2011;12:4.
Phelanyane F. Prevention of mother-to-child-transmission (PMTCT) of HIV in Khayelitsha, South Africa: a contemporary review of the service 20 years later. In: AIDS. San Francisco; 2020. https://cattendee.abstractsonline.com/meeting/9289/Presentation/188
StatsSA. StatsSA. City Cape T—2011 Census Suburb Khayelitsha. 2011;(July):1–7. https://resource.capetown.gov.za/documentcentre/Documents/Maps%20and%20statistics/2011_Census_CT_Suburb_Khayelitsha_Profile.pdf
Medecins Sans Frontieres. HIV Care for Men: Lessons learnt from Medecins Sans Frontieres experiences in rural and peri-urban South Africa. 2019. https://samumsf.org/sites/default/files/2019-07/HIVCareforMen.pdf
Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE. 2009;4(6):e5790. https://doi.org/10.1371/journal.pone.0005790.
Brinkhof MWG, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Boulle A, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS ONE. 2010;5(11):3–8.
Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–13.
Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66(9):1006–13. https://doi.org/10.1016/j.jclinepi.2013.03.013.
StataCorp. Stata Multiple-Imputation Reference Manual (release 13) [Internet]. TX: StataCorp LLC; 2013. p. 137. https://www.stata.com/manuals13/mi.pdf
StataCorp. Stata Statistical Software: Release 14. TX: StataCorp LP. 2015.
Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9.
Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–41.
Royston JP. Multiple imputation of missing values: Further update of ice, with an emphasis on categorical variables. Stata J. 2009;9(3):466–77.
Ugoji C, Okere N, Dakum P, Ake-Uzoigwe R, Igboelina D, Ndembi N, et al. Correlates of patient retention in HIV care and treatment programs in Nigeria. Curr HIV Res. 2015;13(4):300–7.
Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Stat Methods Med Res. 2016;25(1):188–204. https://doi.org/10.1177/0962280212445945.
Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057–69.
Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987.
Hennessey C, Smith M, Mshweshwe Z, Boulle A, Stinson K. Lost to found: the silent transfer of antiretroviral therapy patients in Khayelitsha, South Africa. Boston: CROI; 2016.
Fox M, Bor J, MacLeod W, Maskew M, Brennan A, Stevens W, et al. Is retention on ART underestimated due to patient transfers? Estimating system-wide retention using a national labs database in South Africa. In: 21st International AIDS Conference. Durban; 2016.
Rooks-Peck CR, Adegbite AH, Wichser ME, Ramshaw R, Mullins MM, Higa D, et al. Mental health and retention in HIV care: a systematic review and meta-analysis. Health Psychol. 2018;37(6):574–85.
Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16(8):2119–43.
Nakimuli-Mpungu E, Bass JK, Alexandre P, Mills EJ, Musisi S, Ram M, et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16(8):2101–8.
Lash TL, Fox MP, Fink AK. Applying quantitative bias analysis to epidemiologic data. New York: Springer; 2011.
Leichliter J, Paz-Bailey G, Friedman A, Habel M, Vezi A, Sello M, et al. “Clinics aren’t meant for men”: Sexual health care access and seeking behaviours among men in Gauteng province, South Africa. J Soc Asp HIV/AIDS. 2012;8(2):1–7.
Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, Okamura M, Alvim MF, Fernandes R, et al. Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PLoS ONE. 2012;7(5):1–10.
Ndawinz JDA, Chaix B, Koulla-Shiro S, Delaporte E, Okouda B, Abanda A, et al. Factors associated with late antiretroviral therapy initiation in cameroon: a representative multilevel analysis. J Antimicrob Chemother. 2013;68(6):1388–99.
Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. https://doi.org/10.1310/hct1005-299.
Crawford TN. Examining the relationship between multiple comorbidities and retention in HIV medical care: a retrospective analysis. AIDS Care. 2015;27(7):892–9. https://doi.org/10.1080/09540121.2015.1009361.
Kaplan SR, Oosthuizen C, Stinson K, Little F, Euvrard J, Schomaker M, et al. Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: a cohort study. PLOS Med. 2017;14(11):e1002407. https://doi.org/10.1371/journal.pmed.1002407.
Eshun-Wilson I, Rohwer A, Hendricks L, Oliver S, Garner P. Being HIV positive and staying on antiretroviral therapy in Africa: a qualitative systematic review and theoretical model. PLoS ONE. 2019;14(1): e0210408.
Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):1–7.
Knight J, Wachira J, Kafu C, Braitstein P, Wilson IB, Harrison A. The role of gender in patient–provider relationships : a qualitative analysis of HIV care providers in Western Kenya with implications for retention in care. AIDS Behav. 2019;23(2):395–405. https://doi.org/10.1007/s10461-018-2265-4.
Yamanis TJ, Dervisevic E, Mulawa M, Conserve DF. Social network influence on HIV testing among urban men in Tanzania. AIDS Behav. 2017;21(4):1171–82.
Stern E, Colvin C, Gxabagxaba N, Schutz C, Burton R, Meintjes G. Conceptions of agency and constraint for HIV-positive patients and healthcare workers to support long-term engagement with antiretroviral therapy care in Khayelitsha, South Africa. Afr J AIDS Res. 2017;16(1):19–29.
Strategic Development Information and GIS Department (SDI&GIS). City of Cape Town—2011 Census Suburb Khayelitsha. 2013. http://resource.capetown.gov.za/documentcentre/Documents/Mapsandstatistics/2011_Census_CT_Suburb_Khayelitsha_Profile.pdf
Geng EH, Odeny TA, Lyamuya R, Nakiwogga-Muwanga A, Diero L, Bwana M, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis. 2016;62(7):935–44.
Varni SE, Miller CT, McCuin T, Solomon S. Disengagement and engagement coping with HIV/AIDS stigma and psychological well-being of people with HIV/AIDS. J Soc Clin Psychol. 2012;31(2):123–50. https://doi.org/10.1521/jscp.2012.31.2.123.
Shabalala FS, Vernooij E, Pell C, Simelane N, Masilela N, Spiegelman D, et al. Understanding reasons for discontinued antiretroviral treatment among clients in test and treat: a qualitative study in Swaziland. J Int AIDS Soc. 2018;21:e25120. https://doi.org/10.1002/jia2.25120.
Tayler-Smith K, Zachariah R, Massaquoi M, Manzi M, Pasulani O, van den Akker T, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104(5):313–9. https://doi.org/10.1016/j.trstmh.2010.01.007.
Gwadz M, de Guzman R, Freeman R, Kutnick A, Silverman E, Leonard NR, et al. Exploring how substance use impedes engagement along the HIV care continuum: a qualitative study. Front Public Health. 2016;4:62.
Cichowitz C, Maraba N, Hamilton R, Charalambous S, Hoffmann CJ. Depression and alcohol use disorder at antiretroviral therapy initiation led to disengagement from care in South Africa. PLoS ONE. 2017;12(12):1–11.
Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2015;20(3):255–65. https://doi.org/10.1080/13548506.2014.945601.
Mwango LK, Stafford KA, Blanco NC, Lavoie MC, Mujansi M, Nyirongo N, et al. Index and targeted community-based testing to optimize HIV case finding and ART linkage among men in Zambia. J Int AIDS Soc. 2020;23(S2):51–61.
Shapiro AE, van Heerden A, Krows M, Sausi K, Sithole N, Schaafsma TT, et al. An implementation study of oral and blood-based HIV self-testing and linkage to care among men in rural and peri-urban KwaZulu-Natal, South Africa. J Int AIDS Soc. 2020;23(S2):35–42.
Barnabas RV, van Heerden A, McConnell M, Szpiro AA, Krows ML, Schaafsma TT, et al. Lottery incentives have short-term impact on ART initiation among men: results from a randomized pilot study. J Int AIDS Soc. 2020;23(S2):e25519.
Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Predictors of loss to follow-up among children on long-term antiretroviral therapy in Zambia (2003–2015). BMC Public Health. 2019;19(1):1–13.
Umeokonkwo CD, Onoka CA, Agu PA, Ossai EN, Balogun MS, Ogbonnaya LU. Retention in care and adherence to HIV and AIDS treatment in Anambra State Nigeria. BMC Infect Dis. 2019;19(1):1–11.
Jewkes R, Morrell R. Gender and sexuality: emerging perspectives from the heterosexual epidemic in South Africa and implications for HIV risk and prevention. J Int AIDS Soc. 2010;13:6.
Walsh S, Mitchell C. “I’m too young to die”: HIV, masculinity, danger and desire in urban South Africa. Gend Dev. 2006;14(1):57–68.
Acknowledgements
The authors would like to acknowledge clinic staff and Médecins Sans Frontières staff who ran and supported the male clinics. 1. Boulle A, Heekes A, Tiffin N, et al. Data Centre Profile: The Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Popul Data Sci. 2019;4(2). https://doi.org/10.23889/ijpds.v4i2.1143
Funding
Dr. Fox and Dr. Horsburgh are supported by the Providence/Boston Center for AIDS Research (P30AI042853). Dr Cornell is supported by NIH grant number U01AI069924.
Author information
Authors and Affiliations
Contributions
VdA, LTD and BM were involved with developing and implementing the clinics. TC and MPF designed the research study. TC analysed the data and drafted the manuscript, with support from MPF and MC. CRH provided input on the study design, analysis and discussion. All authors have reviewed, provided input on, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Ethics approval was granted by the University of Cape Town’s Human Research Ethics Committee (HREC395/2005), who waived consent as the analyses used de-identified data collected as part of routine patient care.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cassidy, T., Cornell, M., Makeleni, B. et al. Attrition from Care Among Men Initiating ART in Male-Only Clinics Compared with Men in General Primary Healthcare Clinics in Khayelitsha, South Africa: A Matched Propensity Score Analysis. AIDS Behav 27, 358–369 (2023). https://doi.org/10.1007/s10461-022-03772-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-022-03772-9