Abstract
For adolescent girls (AG) and young women (YW), adherence barriers may limit the effectiveness of daily oral HIV pre-exposure prophylaxis (PrEP). Due to its low-burden and long-lasting product attributes, PrEP implants could remove some of the critical adherence barriers of oral PrEP products for individuals at risk of HIV. To explore stated preferences for a long-acting PrEP implant, we conducted a quantitative survey and discrete choice experiment with AG (ages 15–17), YW (18–34), and female sex workers (FSW; ≥ 18) in Gauteng Province, South Africa. We completed 600 quantitative surveys across the three subgroups of women. Respondents stated preference for an implant that provided longer HIV protection (24 months versus 6 months) and required a single insertion. They stated that they preferred a biodegradable implant that could be removed within 1 month of insertion. Respondents had no preference for a particular insertion location. Overall, 78% of respondents said they would be likely (33%) or very likely (45%) to use a PrEP implant were one available, with the majority (82%) stating preference for a product that would provide dual protection against HIV and unintended pregnancies. To reduce their risk of HIV, AG, YW, and FSW in our survey reported a strong willingness to use long-acting, highly-effective, dissolvable PrEP implants.
Resumen
Las niñas adolescentes (NA) y mujeres jóvenes (MJ), pueden enfrentar barreras de adherencia que limitan la eficacia de la profilaxis oral previa a la exposición al VIH (PrEP). Ya que el implante de PrEP es un producto que requiere de poca intervención de la usuaria y es de larga duración, podría eliminar algunas de las barreras de adherencia más importantes en el uso de los productos orales de PrEP para aquellas personas en riesgo de infección de VIH. Para explorar las preferencias declaradas en cuanto al implante de PrEP de acción prolongada, llevamos a cabo una encuesta cuantitativa y un experimento de elección discreta (DCE) con NA (de 15 a 17 años), MJ (de 18 a 34 años) y mujeres trabajadoras del sexo (MTS; ≥ 18 años) en la provincia de Gauteng, Sudáfrica. Administramos 600 encuestas cuantitativas en los tres subgrupos de mujeres. Los resultados indican la preferencia por un implante que proporciona una protección contra el VIH más prolongada (24 meses a comparación con 6 meses) y que requiere de una única inserción. Las participantes afirmaron que prefieren un implante biodegradable que puede retirarse un mes después de su inserción. Las participantes no tenían preferencia por un sitio específico de inserción. En general, el 78% de las participantes indicaron que probablemente (33%) o muy probablemente (45%) utilizarían un implante de PrEP si estuviera disponible, y la mayoría (82%) manifestó su preferencia por un producto que proporcionaba una doble protección contra el VIH y el embarazo no deseado. Para reducir el riesgo de contraer el VIH, las NA, MJ y MTS participantes se mostraron muy dispuestas a utilizar implantes de PrEP de larga duración, altamente eficaces y disolubles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The HIV epidemic in South Africa disproportionally impacts young women (YW) under the age of 30 [1], who have an HIV prevalence of 17%, approximately 2.5 times higher than that of men in the same age group [2, 3]. Young women are at particularly high risk of contracting HIV, and have the highest HIV incidence amongst all age segments [4, 5]. Among YW, female sex workers (FSW) and those engaged in transactional sex are at particularly high risk for HIV with a prevalence ranging from 48 to 72% [6, 7].
While biomedical HIV prevention options such as oral PrEP are increasingly available in South Africa, women in particular, face barriers to oral PrEP access, uptake, and continuation. Barriers, including frequent visits to health clinics and challenges in taking medication on a daily basis [8,9,10,11], can have a negative impact on adherence of oral PrEP, diminishing the effectiveness against HIV acquisition. In a recently published study, an injectable antiretroviral was more effective (HR 0.34) than oral PrEP in preventing HIV seroconversions in cisgender men and transgender women [12]. Similar results were obtained in cisgender women [13]. A likely explanation for this difference is poorer or inconsistent adherence to the oral PrEP regimen. Furthermore, a number of studies have found that oral PrEP is comparatively less effective among women than among men, especially at lower levels of adherence [14,15,16,17,18,19]. South Africa has a strong enabling environment for increasing young women’s access to biomedical HIV prevention products [20,21,22], including the National Strategic Plan for HIV/TB, which contains PrEP guidance and provision for at-risk youth populations [23,24,25]. Given the barriers to oral PrEP uptake and adherence, however, additional biomedical prevention options are needed to reduce the burden of HIV among women and girls in this context.
Along with long-acting injectables and vaginal rings, long-acting implants are being developed [26]. A biodegradable implantable PrEP (PrEP implant) has the potential to give those at high risk for HIV additional choices for biomedical HIV prevention, and to reduce HIV incidence and clinic visits in high-burden settings [27, 28]. In-depth interviews with healthcare providers in South Africa demonstrate that with adequate training, providers could offer at-risk clients the PrEP implant as an alternative to daily oral PrEP [29]. While promising, initial testing of the PrEP implant, including target end-user stated preferences for implant features, has been limited [30, 31].
To better understand end-user preferences for a potential PrEP implant, we conducted a quantitative survey and discrete choice experiment (DCE) to evaluate perceptions and product preferences for the PrEP implant amongst adolescent girls (AG), YW, and FSW in Gauteng Province, South Africa. This study is the first to focus on gaining insights into the acceptability and stated preferred product attributes of an innovative antiretroviral -releasing pellet implant design (Fig. 1) among different population segments of young women at risk of acquiring HIV in South Africa.
Methods
Study Setting and Population
The survey was conducted in rural and urban settings in Gauteng Province, South Africa. The sites in Gauteng were purposively selected to include geographies with ongoing oral PrEP interventions, and where the target populations were relatively more familiar with oral PrEP [32]. For a diversity of respondents, the primary study site was the Soshanguve township near Pretoria—an area with varied levels of education, income, and access to healthcare [31]. Respondents were eligible for the study if they were: AG ages 15–17, YW aged 18–30, or FSW over the age of 18, were sexually active, currently resided in the study area, and were able/willing to provide informed consent.
Based on accepted approaches to DCE sample sizes, we estimated we would need approximately 200 respondents per population segment for this study [33, 34]. Our sample size of 600 participants was divided across the three study groups (AG, YW, and FSW) in both rural and urban settings. AGYW were randomly sampled from households during a multi-stage household survey. FSW were selected through a respondent driven sampling with seeds identified in bars and shebeens (unlicensed location selling alcohol). More information on the sampling approach is available in the Supplemental Materials.
Quantitative Survey and Discrete Choice Experiment
The quantitative survey collected data on socio-demographic characteristics, awareness and use of analogous products including the contraceptive implant and daily oral PrEP, HIV risk perception, and current HIV prevention approaches (including abstinence, partner reduction, condom use, partner testing, and/or oral PrEP). Willingness to pay for oral PrEP and the PrEP implant was also included, using a contingent evaluation approach.
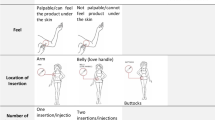
The DCE design was informed by a literature review, input from the product developer, stakeholder engagement, and qualitative formative research with target end-users (Fig. 2). Ngene software was used to develop the experiment design using a D-efficient design approach for analysis of main effects while minimizing attribute level balance, overlap and dependence. The resulting design contained six attributes, with two to four possible levels each (see Fig. 3). To limit cognitive fatigue, each participant was randomized to one block of 8 choice sets during the survey.
Process of determining choice task attributes and levels. Note Schematic of Research Phases 1 and 2 adapted from “Developing attributes and attribute-levels for a discrete choice experiment on micro health insurance in rural Malawi” by Abiiro, G., Leppert, G., Mbera, G. et al. 2014, BMC, copyright by BMC
Study Design and Statistical Analysis
Participants completed the quantitative survey and DCE with a trained interviewer using a tablet. Data were collected and stored in SurveyCTO. During the DCE, participants were presented choice tasks with illustrative graphics. For each choice task, participants selected their preference between the two unlabeled product options and then indicated if they would rather use the option selected or their current HIV prevention approach.
Quantitative survey data were analyzed descriptively using means and frequencies and appropriate tests of association (e.g. t tests and chi-square tests). The probability of living in poverty was estimated using the Probability Poverty Index [35]. Stated preference data were analyzed using fixed effects logistic regression in Stata 15.0 (College Station, TX). Stratification analyses were conducted to determine whether differences in stated preferences existed between end-user groups. Differences in stated preference estimates by strata were evaluated using Chow and Wald tests [36].
Ethics
The University of the Witwatersrand Human Research Ethics Committee (Medical) and the Population Services International Research Ethics Board (PSI REB) granted the necessary ethical and regulatory approval and each respondent provided written informed consent prior to their participation (FSW provided verbal consent to participate).
Results
Between March 7–23, 2020, we recruited 600 participants, including 201 AG, 200 YW, and 199 FSW. Half of the study population lived in urban settings and the remaining half resided in rural, peri urban and urban areas of Gauteng province (Table 1). More than half (52.4%) of the sample reported ever having been pregnant, though this varied from 7% among AG to 70% among YW and 81.4% among FSW (Pearson’s \(\chi^{2}\) p < 0.001). YW had significantly higher levels of education than FSW or AG, with 68.8% having completed Metric education or above (beyond secondary education), versus 13.9% of AG and 36.8% of FSW (Pearson’s \(\chi^{2}\) p < 0.001).
Quantitative Survey
Experience with Analogous Products
While most respondents (68%) had heard of contraceptive implants, only 18% of the study sample reported having ever used a contraceptive implant. Experience with the implant was higher among FSW (29%) than YW (20%) or AG (5%, Pearson’s \(\chi^{2}\) p < 0.001). Implant experience did not vary significantly between urban (19%) and rural/peri urban (17%) settings (Pearson’s \(\chi^{2}\) p = 0.387). Awareness of oral PrEP was low amongst respondents (33%). This was especially true for AG (18%) and YW (32%), though awareness was higher among FSW (51%, Pearson’s \(\chi^{2}\) p < 0.001). The respondents who were aware of oral PrEP had a higher average perceived risk of acquiring HIV (5.9 vs. 3.8, t test p < 0.001). Even amongst those aware of oral PrEP, use of the product remained low, at just 20%. This ranged from 24% among PrEP-aware FSW to just 20% among AG and 14% among YW (Pearson’s \(\chi^{2}\) p = 0.330).
Service Delivery Stated Preferences
While 66% of respondents said they would be “likely” or “very likely” to take oral PrEP in the future, only 34% of respondents had knowledge of where to access oral PrEP. As with oral PrEP awareness, knowledge of where to access oral PrEP was higher amongst FSW (47%) than YW (34%) or AG (21%, Pearson's \(\chi^{2}\) p < 0.001). Across the respondent sub-groups, hospitals and clinics were the stated preferred channels for accessing oral PrEP.
After hearing about the PrEP implant, the majority of respondents said they would be “likely” (33%) or “very likely” (45%) to use it were it available. The likelihood of using the PrEP implant was particularly high for both FSW (86%) and YW (81%) compared to AG (67%, Pearson’s \(\chi^{2}\) p = 0.002). Most respondents (55%) desired to receive the PrEP implant from a family planning (FP) provider in comparison to an HIV care provider, a preference that did not differ significantly by sub-group (Pearson’s \(\chi^{2}\) p = 0.411). The majority of respondents (82%) preferred a product that would provide dual protection against HIV and unintended pregnancies. Data show that respondents on balance would prefer a product that led to fewer and more short-lived side effects. Adjusting for other product features, respondents had 2 (95% CI 1.72–2.22) times the odds of choosing a product that caused temporary pain at the injection site rather than one that caused pain at the injection site, muscle pain, and headaches lasting for weeks.
PrEP Implant Product Stated Preferences
Across the sub-groups, respondents stated a preference for a long-acting, dissolvable implant product. Importantly, 72% of respondents said they would prefer a product that dissolved and didn’t require surgical removal. Respondents also preferred longer-acting PrEP implants over shorter-acting PrEP injectable products: 83% of respondents preferred 12-month implants to 3-month injectables (Table 2).
Discrete Choice Experiment
Among all respondents, product effectiveness, side effects, duration of protection, number of insertions, and product removability were all significantly associated with PrEP implant product choice (Fig. 4). Only location of insertion was not significantly associated with product preference after adjusting for other attributes. Respondents were more likely to select a product with higher prevention efficacy (Fig. 4). Compared to a product providing 6 months of HIV protection, respondents had 1.4 (95% CI 1.2–1.6) times the odds of selecting a product that provided 24 months of protection and 1.1 (95% CI 0.9–1.2) times the odds of selecting a 12-month protection product. Respondents also stated preference for products that required one insertion vs. one that required two separate insertion sites [Utility Ratio (UR) 1.5, 95% CI 1.3–1.6]. While dissolvability was preferred over a product that needed to be surgically removed, this was only significant for dissolvable products that had a window of removability (UR 1.2, 95% CI 1.05–1.4) that lasted up to 1-month post insertion.
Stated preferences were generally similar across the three study sub-groups, especially between YW and FSW where no significant differences in attribute preferences were observed (Fig. 4). Some significant differences emerged when comparing preferences between AG and YW, where AG showed stronger stated preferences for a product with a 90% effectiveness relative to one with 70% effectiveness against HIV (UR 15.3 vs. 11.9, Wald \(\chi^{2}\) p = 0.048) and a 24-month product vs. a 6-month product (UR 1.7 vs. 1.3, Wald \(\chi^{2}\) p = 0.022). AG were less driven by preferences for one insertion versus two insertions relative to their older YW counterparts (UR 1.1 vs. 1.6, Wald \(\chi^{2}\) p < 0.001).
While preferences across the study population groups were generally consistent, significant differences in preferences were observed between urban and rural/peri-urban respondents. AG, YW and FWS in urban areas had stronger stated preferences for implants that lasted for 24 months versus the 12-month option than respondents in rural and peri-urban settings (UR 0.49 vs. 0.22, Wald \(\chi^{2}\) p = 0.0151). Respondents in urban settings also had stronger preferences for one insertion versus two insertions to respondents in rural and peri-urban settings (UR 0.43 vs. 0.29, Wald \(\chi^{2}\) p = 0.0195). In contrast, respondents in rural and peri-urban settings had stronger stated preferences for implants that are dissolvable but removable within 1 month versus their urban counterparts. (UR 0.27 vs. 0.08, Wald \(\chi^{2}\) p = 0.0094).
Discussion
This research was intended to primarily inform product development, while providing additional market segmentation information. Findings from this study, as well as an expanded DCE study currently in progress, will be integrated into the target product profile to guide final product development. While there is a growing body of research on oral PrEP DCEs conducted in South Africa [9, 36,37,38,39], this is among the first studies to evaluate priorities and preferences of implantable PrEP across different female population segments [8, 29, 37]. While AG in South Africa are frequently targeted with HIV prevention social and behavior change campaigns (SBC) [40,41,42], it is not common for adolescents to be the target population for DCEs [35, 43]. To better explore barriers to youth uptake of HIV prevention products, a number of studies have called for the increased incorporation of adolescent populations into DCEs [20, 31, 44, 45]. This study highlighted the importance of understanding adolescents’ stated preferences as end-users of HIV biomedical prevention products. Despite the availability of oral PrEP in South Africa, consistent with other studies determining oral PrEP awareness, AG and YW were significantly less aware of the product than FSW respondents [46,47,48]. While South Africa has policies to improve AG and YW access to oral PrEP, the product was not initially marketed to those priority groups [24]. Oral PrEP in South Africa was originally targeted primarily at FSW, and our results are consistent with other studies that have found a higher awareness of oral PrEP among FSW compared to other segments [49].
Notwithstanding the low awareness of oral PrEP, respondents expressed interest and a high likelihood of using a PrEP implant once the product concept was explained to them, especially among the older age groups. YW have demonstrated a willingness to use daily oral PrEP across sub-Saharan Africa, and more recent research has also shown the acceptability of other biomedical prevention options including monthly PrEP vaginal rings and long-acting injectable products [50, 51]. While stated acceptability of oral PrEP products is high among YW, studies in South Africa have demonstrated that this group is more likely to adopt HIV prevention products that blend into their lifestyles [51]. While YW may be willing to use oral PrEP, the daily dosage requirement is often viewed as inconvenient and not-discreet, which can limit uptake and continuation [10]. Similarly, due to frequent visits to providers and concerns about male partner reactions, the monthly PrEP vaginal ring has also been perceived by some women as inconvenient [52]. Consistent with other studies on biomedical HIV prevention products in South Africa, this study demonstrated that young women prioritize products that are: (i) highly effective in preventing HIV, (ii), long-lasting and (iii) discreet [51]. The findings show that the PrEP implant has the potential to address the barriers to oral and vaginal ring PrEP products due to its assumed high effectiveness, convenience, long-lasting protection, and discreet nature.
As in other studies [29, 38], women and girls in our survey significantly valued high product effectiveness and long duration of protection. In this study population, these two attributes have not been associated with oral PrEP. In our study, however, other modifiable product characteristics emerged as important to target end-users. Dissolvability, relative to a product that required surgical removal (analogous to the currently available contraceptive implants) appealed to end-users. This is important because all contraceptive implants and most PrEP implants in development are non-biodegradable and require removal [29]. However, this was only true for degradable products that additionally had a short initial window of removability. This finding was further supported by the results of qualitative formative research [53], which found that end-users had concerns about a fully non-removable product, primarily due to concern over being “stuck” with a product causing undesirable side-effects.
This study’s results are consistent with the finding that young women are willing to tolerate some side effects of a prevention product if they are counselled about them ahead of time, and develop a plan to address them [54]. With regards to oral PrEP, studies have found that gastrointestinal side effects only last for a brief time and with consumer-centered counseling prior to starting the product, clients are able to tolerate and manage the side-effects [55,56,57]. This DCE has reaffirmed the need to clarify and improve messaging around potential side effects with users up-front.
These DCE findings add to the increasing documented need to integrate FP and HIV prevention service delivery for AGYW in South Africa [58], and further research should be conducted into HIV-FP biomedical integration products to better examine family planning needs of HIV at-risk women and girls. Women in the survey stated a preference for a dual protection product for the prevention of HIV and unintended pregnancy over single independent products. This finding has been also discussed in previous surveys [59]. A recent study also demonstrated young women and men having sex with men also stated preference for PrEP injectable products [60]. However, we did not explore trade-offs (e.g. effectiveness, duration of protection, removability, etc.) between dual- and mono-protection options, and further research is needed to facilitate the next generation of multipurpose prevention technologies (MPT) products to address both HIV prevention and contraception in one product for target populations in Sub-Saharan Africa.
The study has contributed to the evidence that HIV at-risk women in South Africa desire a choice of highly-effective, discreet, convenient, and long-lasting biomedical HIV prevention products. Respondents in this study expressed a preference for a PrEP implant providing a year of protection over PrEP injectable products that are effective for 3 months. Globally, the findings demonstrate that a PrEP implant could resolve certain barriers of existing biomedical HIV prevention product options, potentially offering an avenue to improving AGYW’s uptake and adherence to a biomedical HIV prevention product and ultimately reducing HIV incidence in this high-risk group.
Limitations
This study has several important limitations. First, PrEP implants remain in research and development and, for HIV prevention, they represented a new concept to all respondents. While the product was described using visual aids and a standard script, it is possible not all respondents understood the product or its characteristics in the same way. Additionally, though oral PrEP and the contraceptive implant are available in South Africa, awareness of—and more importantly—direct experience with these products were relatively low, especially among AG. As with all discrete choice experiments and other stated preference methods, there is a potential that the preferences expressed during the choice tasks may not reflect actual preferences or future observed behavior.
Conclusion
The survey and DCE results demonstrate the importance of understanding at-risk end-user perspectives in designing, developing and scaling biomedical HIV prevention products. Providing AGYW with their preferred options and a range of product choices to control their HIV risk is a crucial step in the pathways to reduce HIV incidence in this priority population. All study groups expressed strong interest in adopting the theoretical PrEP implant, highlighting the need for continued investment in the design, development, testing, and scale-up of diverse biomedical HIV prevention products, especially long-acting ones, in high-incidence settings.
Data Availability
The individual data is not published due to ethical constraints, however data from the study is available from the corresponding author with sponsor approval on reasonable request.
Code Availability
The custom code generated to analyze data from the study is available from the corresponding author with sponsor approval on reasonable request.
References
Weinrib R, Minnis A, Agot K, Ahmed K, Owino F, Manenzhe K, et al. HHS public access. J Int AIDS Soc. 2018;22(1):139–48. https://doi.org/10.1016/j.jval.2017.04.007.
Mabaso M, Sokhela Z, Mohlabane N, Chibi B, Zuma K, Simbayi L. Determinants of HIV infection among adolescent girls and young women aged 15–24 years in South Africa: a 2012 population-based national household survey. BMC Public Health. 2018;18(1):1–7.
Govender K, Beckett SE, George G, Lewis L, Cawood C, Khanyile D, et al. Factors associated with HIV in younger and older adult men in South Africa: findings from a cross-sectional survey. BMJ Open. 2019;9(12):1–11.
Ramjee G, Sartorius B, Morris N, Wand H, Reddy T, Yssel JD, et al. A decade of sustained geographic spread of HIV infections among women in Durban, South Africa. BMC Infect Dis. 2019;19(1):1–9.
Bekker LG, Johnson L, Cowan F, Overs C, Besada D, Hillier S, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet. 2015;385(9962):72–87.
SANAC. Let Our Actions Count South Africa ’ S National Strategic Plan for; 2017. p. 1–32. http://sanac.org.za/2017/05/11/download-the-full-version-of-the-national-strategic-plan-for-hiv-tb-and-stis-2017-2022/.
Twahirwa Rwema JO, Baral S, Ketende S, Phaswana-Mafuya N, Lambert A, Kose Z, et al. Evaluating the vertical HIV transmission risks among South African female sex workers; Have we forgotten PMTCT in their HIV programming? BMC Public Health. 2019;19(Suppl 1):1–9.
Van Der Straten A, Shapley-Quinn MK, Reddy K, Cheng H, Etima J, Woeber K, et al. Favoring “peace of Mind”: a qualitative study of African women’s HIV prevention product formulation preferences from the MTN-020/ASPIRE trial. AIDS Patient Care STDS. 2017;31(7):305–14.
van Vliet MM, Hendrickson C, Nichols BE, Boucher CAB, Peters RPH, van de Vijver DAMC. Epidemiological impact and cost-effectiveness of providing long-acting pre-exposure prophylaxis to injectable contraceptive users for HIV prevention in South Africa: a modelling study. J Int AIDS Soc. 2019;22(12):1–9.
Van Der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118.
Ministry of Health—Republic of South Africa. South African national HIV prevalence, incidence, behaviour and communication survey, 2017: Presentation for July 2018 launch. vol. 2017. 2018. p. 5–8.
Landovitz RJ, Donnell D, Clement ME, et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608.
Delany-Moretlwe S. Long acting injectable cabotegravir is safe and effective in preventing HIV infection in cisgender women: interim results from HPTN 084. In: HIV R4P Virtual Conference. 2021.
Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;112(3):11re24.
Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25.
Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32(1):32–43.
Velloza J, Baeten JM, Haberer J, Irungu E, Mugo NR, Celum C, et al. Effect of depression on adherence to oral PrEP among men and women in East Africa. J Acquir Immune Defic Syndr. 2018;79(3):330–8.
Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. 2016;11(1):18–26.
Mehrotra ML, Westreich D, McMahan VM, Glymour MM, Geng E, Grant RM, et al. Baseline characteristics explain differences in effectiveness of randomization to daily oral TDF/FTC PrEP between transgender women and cisgender men who have sex with men in the iPrEx trial. J Acquir Immune Defic Syndr. 2019;81(3):e94–8.
Badawi MM, SalahEldin MA, Idris AB, Hasabo EA, Osman ZH, Osman WM. Knowledge gaps of STIs in Africa; Systematic review. PLoS ONE. 2019;14:1–33. https://doi.org/10.1371/journal.pone.0213224.
de Bekker-Grob EW, Swait JD, Kassahun HT, Bliemer MCJ, Jonker MF, Veldwijk J, et al. Are healthcare choices predictable? The impact of discrete choice experiment designs and models. Value Heal. 2019;22(9):1050–62. https://doi.org/10.1016/j.jval.2019.04.1924.
Corneli A, Perry B, Agot K, Ahmed K, Malamatsho F, Van Damme L. Facilitators of adherence to the study pill in the FEM-PrEP clinical trial. PLoS ONE. 2015;10(4):1–18.
Taggart T, Bond KT, Ritchwood TD, Smith JC. Getting youth PrEPared: adolescent consent laws and implications for the availability of PrEP among youth in countries outside of the United States. J Int AIDS Soc. 2019;22(7):1–9.
Sinead DM, Hosek S, Celum C, Wilson CM, Kapogiannis B, Bekker LG. Preventing HIV among adolescents with oral PrEP: observations and challenges in the United States and South Africa. J Int AIDS Soc. 2016;19(Suppl 6):1–7.
Buchbinder SP, Liu AY. CROI 2016: hot spots in HIV infection and advances in HIV prevention. Top Antivir Med. 2016;24(1):10–28.
Krovi SA, Johnson LM, Luecke E, van der Straten A. Advances in long-acting injectables, implants, and vaginal rings for contraception and HIV prevention. Adv Drug Deliv Rev. 2021;176:13849.
Minnis AM, Browne EN, Boeri M, Agot K, Van Der Straten A, Ahmed K, et al. Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. 2019;80(4):394–403.
Fernández-romero JA, Deal C, Herold BC, Schiller J, Zydowsky T, Romano J, et al. Protection. 2016;23(7):429–36.
Krogstad EA, Montgomery ET, Atujuna M, Minnis AM, O’Rourke S, Ahmed K, et al. Design of an implant for long-acting HIV pre-exposure prophylaxis: input from South African Health Care Providers. AIDS Patient Care STDS. 2019;33(4):157–66.
Ngure K, Mugo NR, Bukusi EA, et al. Pills, injections, rings or implants? PrEP formulation preferences of PrEP-experienced African women for HIV prevention. J Acquir Immune Defic Syndr. 2021;10(1097):e30.
Krogstad EA, Atujuna M, Montgomery ET, Minnis A, Ndwayana S, Malapane T, et al. Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc. 2018;21(8):1–10.
Quaife M, Eakle R, Cabrera M, Vickerman P, Tsepe M, Cianci F, et al. Preferences for ARV-based HIV prevention methods among men and women, adolescent girls and female sex workers in Gauteng Province, South Africa: a protocol for a discrete choice experiment. BMJ Open. 2016;6(6):1–8.
Fields A, Bryan K. Indiana University Bloomington I U C A T Bloomington. 2020.
Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Heal. 2013;16(1):3–13.
Quaife M, Eakle R, Cabrera Escobar MA, Vickerman P, Kilbourne-Brook M, Mvundura M, et al. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Mak. 2018;38(1):120–33.
Vickerman P, Quaife M, Kilbourne-Brook M, Mvundura M, Eakle R, Terris-Prestholt F. HIV prevention is not all about HIV—using a discrete choice experiment among women to model how the uptake and effectiveness of HIV prevention products may also rely on pregnancy and STI protection. BMC Infect Dis. 2020;20(1):1–11.
Luecke EH, Cheng H, Woeber K, Nakyanzi T, Mudekunye-Mahaka IC, Van Der Straten A. Stated product formulation preferences for HIV pre-exposure prophylaxis among women in the VOICE-D (MTN-003D) study. J Int AIDS Soc. 2016;19(1):1–9.
Quaife M, Terris-Prestholt F, Eakle R, Escobar MAC, Kilbourne-Brook M, Mvundura M, et al. The cost-effectiveness of multi-purpose HIV and pregnancy prevention technologies in South Africa. J Int AIDS Soc. 2018;21(3):e25064.
Mohammadi T, Bansback N, Marra F, Khakban A, Campbell JR, FitzGerald JM, et al. Testing the external validity of a discrete choice experiment method: an application to latent tuberculosis infection treatment. Value Heal. 2017;20(7):969–75. https://doi.org/10.1016/j.jval.2017.04.007.
Saul J, Bachman G, Allen S, Toiv NF, Cooney C, Beamon T. The DREAMS core package of interventions: a comprehensive approach to preventing HIV among adolescent girls and young women. PLoS ONE. 2018;13(12):1–18.
Girls AA, Woman Y. HIV prevention among adolescent and Among Adolescent Girls. 2016.
Nel A, Bekker LG, Bukusi E, Hellström E, Kotze P, Louw C, et al. Safety, acceptability and adherence of dapivirine vaginal ring in a microbicide clinical trial conducted in multiple countries in sub-Saharan Africa. PLoS ONE. 2016;11(3):1–19.
Mavedzenge SN, Luecke E, Ross DA. Effective approaches for programming to reduce adolescent vulnerability to HIV infection, HIV risk, and HIV-related morbidity and mortality: a systematic review of systematic reviews. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):154–69.
Celum CL, Delany-Moretlwe S, McConnell M, Van Rooyen H, Bekker LG, Kurth A, et al. Rethinking HIV prevention to prepare for oral PrEP implementation for young African women. J Int AIDS Soc. 2015;18(Suppl 3):1–10.
Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The science of being a study participant: FEM-PrEP participants’ explanations for overreporting adherence to the study pills and for the whereabouts of unused pills. J Acquir Immune Defic Syndr. 2015;68(5):578–84.
Ajayi AI, Ismail KO, Adeniyi OV, Akpan W. Awareness and use of pre-exposure and postexposure prophylaxes among Nigerian university students. Medecine. 2018;97(36):1–2.
Misra K, Udeagu CC. Disparities in awareness of HIV postexposure and preexposure prophylaxis among notified partners of HIV-positive individuals, New York City 2015–2017. J Acquir Immune Defic Syndr. 2017;76(2):132–40.
Desai M, Field N, Grant R, McCormack S. State of the art review: Recent advances in pre-exposure prophylaxis for HIV. BMJ. 2017;359:j5011.
Eakle R, Bourne A, Mbogua J, Mutanha N, Rees H. Exploring acceptability of oral PrEP prior to implementation among female sex workers in South Africa. J Int AIDS Soc. 2018;21(2):e25081.
Weinrib R, Minnis A, Agot K, Ahmed K, Owino F, Manenzhe K, et al. End-users’ product preference across three multipurpose prevention technology delivery forms: baseline results from young women in Kenya and South Africa. AIDS Behav. 2018;22(1):133–45.
Laher F, Salami T, Hornschuh S, Makhale LM, Khunwane M, Andrasik MP, et al. Willingness to use HIV prevention methods among vaccine efficacy trial participants in Soweto, South Africa: discretion is important. BMC Public Health. 2020;20(1):1–9.
van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, et al. The tablets, ring, injections as options (TRIO) study: what young african women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. 2018;21(3):1–9.
K.Little, H.Hanif, S. Anderson, M. Clark GFD. Preferences for implantable pre-exposure prophylaxis products among girls, young women , and female sex workers in South Africa. https://programme.hivr4p.org/Abstract/Abstract/533.
Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;5(367):399–410.
Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;5(367):423–34.
Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;27(363):2587–99.
Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396(10246):239–54.
Dulli L, Field S, Masaba R, Ndiritu J. Addressing broader reproductive health needs of female sex workers through integrated family planning/ HIV prevention services: a non-randomized trial of a health-services intervention designed to improve uptake of family planning services in Kenya. PLoS ONE. 2019;14(7):1–14.
IPSOS. Assessing the potential of MPTS in Uganda, Nigeria and South Africa. Bill & Melinda Gates Foundation. 2014. https://theimpt.org/documents/BMGF-AssessingAcceptabilityMPTs-QuantReport-FinalSummaries.pdf.
Minnis AM, Atujuna M, Browne EN, Ndwayana S, Hartmann M, Sindelo S, et al. Preferences for long-acting Pre-Exposure Prophylaxis (PrEP) for HIV prevention among South African youth: results of a discrete choice experiment. J Int AIDS Soc. 2020;23(6):1–10.
Acknowledgements
This research and manuscript are made possible by the generous support of the American People through the United States Agency for International Development (USAID) with funds from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) under a Cooperative Agreement (AID-OAA-A-17-00014) with CONRAD/Eastern Virginia Medical School). The contents of this manuscript are the sole responsibility of the authors and do not necessarily reflect the views of USAID, PEPFAR or the United States Government.
Funding
This study was funded by the United States Agency for International Development (USAID) with funds from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) provided through a cooperative agreement (AID-OAA-A-17-00014) between USAID and CONRAD/Eastern Virginia Medical School. The contents of this manuscript are the sole responsibility of the authors and do not necessarily reflect the views of USAID, PEPFAR or the United States Government.
Author information
Authors and Affiliations
Contributions
KML co-designed the study, conducted all statistical analyses and wrote the results section. LF wrote the background, methods and discussions sections. HH, SMA, AT, MRC and GFD co-designed the study and significantly contributed content to each section. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
The University of the Witwatersrand Human Research Ethics Committee (Medical) in South Africa (M180861) and the Population Services International Research Ethics Board (54.2018) granted the necessary ethical and regulatory approval for this study.
Consent to Participate
Each adolescent girl (AG) and young woman (YW) respondent provided written informed consent prior to their participation. Each female sex worker (FWS) respondent provided verbal informed consent prior to their participation.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Little, K.M., Flomen, L., Hanif, H. et al. HIV Pre-exposure Prophylaxis Implant Stated Preferences and Priorities: Results of a Discrete Choice Experiment Among Women and Adolescent Girls in Gauteng Province, South Africa. AIDS Behav 26, 3099–3109 (2022). https://doi.org/10.1007/s10461-022-03658-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-022-03658-w