Abstract
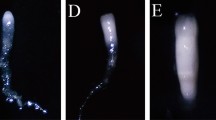
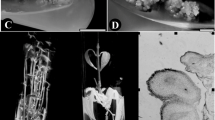
The present study is focussed on development of a high-frequency micro-multiplication system in Calamus thwaitesii, through somatic embryogenesis from immature zygotic embryos cultured in Murashige and Skoog (MS) medium supplemented with 31.68 µM 2,4-dichlorophenoxyacetic acid (2,4-D). The semi-friable calli when cultured in the same medium augmented with 2.22 µM 6-benzylaminopurine (BAP) and 1.07 µM α-naphthalene acetic acid (NAA) induced ~ 12 discrete globular embryoids in 6 weeks. The isolated embryoids in hormone-free media yielded 65% plantlets. Furthermore, embryoids and axenic shoots exhibited maximum shoot induction in medium supplemented with 0.45 µM Thidiazuron (TDZ). The shoot initials after subculture in media supplemented with 1.78 µM BAP and 0.45 µM TDZ produced shoot proliferation followed by elongation in basal medium. The elongated shoots produced roots in media supplemented with 16.11 µM NAA. With this established protocol, ~ 5940 rooted plantlets could be harvested after 40 weeks from a single embryoid. Genetic stability analysis of the plantlets using inter simple sequence repeat markers recorded only 0.05% genetic polymorphism. The plantlets were hardened in a mist house for 8 weeks, and then to 50% shade house for another 16 weeks, and the well-established 6-month-old nursery plants, reintroduced to selected forest segments, exhibited 86% field establishment even after 3 years of observation. Thus, the mass multiplication system developed could be a breakthrough for large-scale multiplication of C. thwaitesii to ensure continuous supply of quality planting material to the cottage industry through the development of agroforestry systems. Furthermore, the in vitro culture system developed here can be replicated for research activities related to the long-term–short-term conservation, micro-multiplication and sustainable utilization of rare, endangered, endemic, monopodial/single stemmed rattan palms.


Similar content being viewed by others
Notes
Annealing temperature of the primers ranges from 50 to 55 °C for the different primers used in this study.
Abbreviations
- BAP:
-
N6-benzylaminopurine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- IBA:
-
Indole-3-butyric acid
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
- PGR:
-
Plant growth regulator
- TDZ:
-
Thidiazuron
References
Aminuddin M, Nur Surpadi, Noor Md, Woon WC (1992) Economics of cultivation of large diameter rattan. In: Wan Mohd., Wan R, Dransfield J, Manokaron N (eds) A guide to the cultivation of rattan. Malayan For.Rec. p 35
Arditti J, Ernst R (1993) Micropropagation of Orchids. Wiley, New York, pp 1–682
Barba RC, Patena LJ, Mercado MM, Lorico L (1985) Tissue culture of rattan (Calamus manillensis. Wendl.) In: Proceedings of the Second National Symposium on Tissue Culture of Rattan, University Partanian Malaysia
Bhatia R, Singh KP, Jhang T, Sharma TR (2008) Assessment of clonal fidelity of micropropagated gerbera plants by ISSR markers. Sci Hortic 119:208–2111
Bingshan Zeng (1997) Tissue culture of Calamus egregius. J. Cent South For Univ 17(4):563–569
Blake J, Eeuwens CJ (1982) Culture of coconut palm tissues with a view to vegetative propagation. In: Rao AN (ed) Proceedings of COSTE\symposium on Tissue Culture of Economically Important Plants, Singapore, pp 145–148
Chengji Zhuang, Jiankui Zhou (1991) Plant regeneration in tissue culture of rattan. Acta Bot Yunnaica 13(1):97–100
Corley RHV, Barrett JN, Jones LH (1976) Vegetative propagation of oil palm via tissue culture. Malaysian International Agricultural Oil Palm Conference, pp 1–8
Cuenca B, Ballester A, Vieitez AM (2000) In vitro adventitious bud regeneration from internode segments of beech. Plant Cell Tiss Org Cult 60:213–220
Damasco OP, Graham GC, Henry RJ, Adkins SW, Smith MK, Godwin ID (1996) Random amplified polymorphic DNA (RAPD) detection of dwarf off-types in micropropagated Cavendish (Musa spp. AAA) bananas. Plant Cell Rep 16:118–123
Debnath SC (2005) A two-step procedure for adventitious shoot regeneration from in vitro-derived lingonberry leaves: shoot induction with TDZ and shoot elongation using zeatin. Hortic Sci 40:189–192
Eeuwens CJ (1976) Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera) and cultured in vitro. Physiol Plant 36:23–28
Eeuwens CJ (1978) Effects of organic nutrients and hormones on growth and development of tissue explants from coconut (Cocos nucifera) and date palms (Phoenix dactylifera) cultured in vitro. Physiol Plant 42:173–178
Eshraghi P, Zarghami R, Mirabdulbaghi M (2005) Somatic embryogenesis in two Iranian date palm cultivars. Afr J Biotechnol 4(11):1309–1312. http://www.academicjournals.org/AJB, ISSN 1684–5315
Fangqiu Zhang (1993) A study on Rattan tissue culture. J For Res 6(5):486–492
Fasolo F, Zimmerman RH, Fordham I (1989) Adventitious shoot formation on excised leaves of in vitro grown shoots of apple cultivars. Plant Cell Tiss Org Cult 16:75–87
Goto S, Thakur RC, Ishii K (1998) Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl. using RAPD markers. Plant Cell Rep 18:193–197
Hazarika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85:1704–1712
Hemanthakumar AS (2010) Studies on embryo and tissue culture of three economically important rattan sps. Thesis submitted to Kerala University
Hemanthakumar AS, Preetha TS, Krishnan PN, Seeni S (2013) Utilization of zygotic embryos of an economic rattan palm Calamus thwaitesii Becc. (Arecaceae) for somaplant regeneration and cryobanking. 3 Biotech 3:195–203
Joshi P, Dhawan V (2007) Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol Plant 51(1):22–26
Kundu M, Sett R (1999) Regeneration through organogenesis in rattan. Plant Cell Tissue Org Cult 59:219–222
Lopez-Villalobos A, Dodds PF, Hornung R (2001) Changes in fatty acid composition during development of tissues of coconut (Cocos nucifera L.) embryos in the intact nut and in vitro. J Exp Bot 52:933–942
Lyyra S, Lima A, Merkle S (2006) In vitro regeneration of Salix nigra from adventitious shoots. Tree Physiol 26:969–975
Magioli C, Rocha APM, de Oliveria DE, Mansur E (1998) Efficient shoot organogenesis of eggplant (Solanum melongena L.) induced by thidiazuron. Plant Cell Rep 17:661–663
Martin JP, Rabechault H (1976) Procede de multiplication vegetative de vegetaux et plants ainsi obtenus, French Patent No. 7628361
Martin KP, Pachathundkandi SK, Zhang CL, Slater A (2006) RAPD analysis of a variant of Banana (Musa sp.) cv. Grande Naine and its propagation via shoot tip culture. In Vitro Cell Dev Biol 42:188–192
Merillon JM, Rideau M, Chenieux JC (1984) Influence of sucrose on levels of ajmalicine, serpentine and triptamine in Catharanthas roseus cells in vitro. Plant Med 50:497–501
Mojarabi M, Nasr SMH, Jalilvand H, Kooch Y (2011) Effect of activated charcoal, growth supplements and storage on removing dormancy, germination indices and vigour of Ash (Fraxinus excelsior L.). Ann Biol Res 2(5):203–212
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murray HG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucl Acids Res 8:4321–4325
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro morphogenesis. In Vitro Cell Dev Biol 34:267–275
Nair LG, Seeni S (2002) Rapid clonal multiplication of Morinda umbellate Linn. (Rubiaceae), a medicinal liana, through cultures of nodes and shoot tips from mature plant. Phytomorphology 52:77–81
Padmanabhan D, Ilangovan R (1993) Surgical induction of multiple shoots in embryo cultures of Calamus gamblei Becc. RIC Bull 12:8–12
Ramanayake SMSD (1999) Viability of excised embryos, shoot proliferation and in vitro flowering in a species of rattan Calamus thwaitesii Becc. J Hortic Sci Biotechnol 74(5):594–601
Reynolds JF, Murashige T (1979) Asexual embryogenesis in callus cultures of palms. In Vitro 15:383–387
Rout GR, Das P, Goel S, Raina SN (1998) Genetic stability of micropropagated plants of ginger using random amplified polymorphic DNA (RAPD) markers. Bot Bull Acad Sin 39:23–27
Sáenz L, Azpeitia A, Chuc-Armendariz B, Chan JL, Verdeil JL, Hocher V, Oropeza C (2006) Morphological and histological changes during somatic embryo formation from coconut plumule explants. In Vitro Cell Dev Biol 42:19–25. https://doi.org/10.1079/ivp2005728
Sane D, Aberlenc-Bertossi F, Gassama-Dia YK, Sagna M, Trouslot MF, Duval Y, Borgel A (2006) Histocytological analysis of callogenesis and somatic embryogenesis from cell suspensions of date palm (Phoenix dactylifera). Ann Bot 98(2):301–308
Satheeshkumar K, Seeni S (2000) In vitro multiplication of Nothapodites foetida (Wight.) Sleumer through seedling explant cultures. Indian J Exp Biol 38:273–277
Scherwinski-Pereira JE, da Guedes RS, Fermino PCP Jr, Silva TL, Costa FHS (2010) Somatic embryogenesis and plant regeneration in oil palm using the thin cell layer technique. In Vitro Cell Dev Biol 46:378–385. https://doi.org/10.1007/s11627-010-9279-6
Seeni S, Latha PG (2000) In vitro multiplication and ecorehabilitation of the endangered Blue Vanda. Plant Cell Tiss Org Cult 61:1–8
Sett R, Kundu M, Sharma P (2002) Regeneration of plantlets through organogenesis in Calamus tenuis. Indian J Plant Physiol 7:358–361
Singha S, Bhatia SK (1988) Shoot proliferation of pear cultures on medium containing thidiazuron and benzylamino purine. Hortic Sci 23:803
SPSS 9 (version 10.0) (1999) Manuals SPSS Inc., Chicago
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26(6):618–631
Tisserat B (1983) Tissue culture of date palms: a new method to propagate an ancient crop-and a short discussion of the california date industry. Principes 27(3):105–117
Victório CP, Lage CLS, Sato A (2012) Tissue culture techniques in the proliferation of shoots and roots of Calendula officinalis. Rev Ciênc Agron 43(3):539–545
Vieitez AM, San-Jose MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell Dev Biol 32:140–147
Wainwright H, Scrace J (1989) Influence of in vitro preconditioning with carbohydrates during the rooting of microcuttings on in vivo establishment. Sci Hortic 38:261–267
Wang H-C, Chen J-T, Wu S-P, Lin M-C, Chang W-C (2003) Plant regeneration through shoot formation from callus of Areca catechu. Plant Cell Tiss Org Cult 75:95–98
Yang H, Tabei Y, Kamada H, Kayamo T, Takaiwa F (1999) Detection of somaclonal variation in cultured rice cells using digoxygenin-based random amplified polymorphic DNA. Plant Cell Rep 18:520–526
Yap IV, Nelson RJ (1996) Win Boot: a program for performing bootstrap analysis of binary data to determine the confidence limits of UPGMA-based dendrograms. IRRI Discussion Paper Series No. 14, International Rice Research Institute, Manila, Philippines
Yusoff AM (1989) Shoot formation in Calamus manan under in vitro. In: Rao AN, Aziah Mohd. Yusoff (eds) Proceedings of the seminar on Tissue Culture of Forest Species, Forest Research Institute Malaysia and International Development Research Centre, Singapore, pp 45–49
Acknowledgements
We thankfully acknowledge the Department of Biotechnology (DBT), Government of India, for providing the financial assistance through the research grant (No BT/R&D/08/04/95), the Director, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI) for providing laboratory facilities and the Department of Forests, Government of Kerala extended support for eco-restoration activities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hemanthakumar, A.S., Preetha, T.S., Pillai, P.P. et al. Embryogenesis followed by enhanced micro-multiplication and eco-restoration of Calamus thwaitesii Becc.: an economic non-wood forest produce for strengthening agroforestry system. Agroforest Syst 93, 1093–1105 (2019). https://doi.org/10.1007/s10457-018-0207-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-018-0207-9