Abstract
Germplasm of the calabash tree (Crescentia cujete L.) was collected in five major regions of Colombia, i.e. the Andes, Caribbean, Amazon, Orinoco, and Pacific regions. Collecting this multipurpose tree was guided by the indigenous knowledge of farmers and artisans in each region. Large variation in fruit shapes and sizes was found, of which some forms were typical for certain regions. Overall 56 accessions were collected and roughly classified into 22 types by eight fruit shapes and eight sizes. Molecular markers (Amplified fragment length polymorphisms) were applied to leaf tip tissue originating from vegetatively propagated plants in order to assess the diversity available in the germplasm collected as well as to detect patterns of geographical or morphological similarity. One accession each of C. alata H.B.&K. and C. amazonica Ducke were used as outgroups. Overall, genetic diversity was high (mean Nei and Li’s coefficient of 0.43). No relations could be established between either geographical provenance or fruit morphology and patterns of genetic diversity. Concerning the outgroups, the C. amazonica accession appeared to be a distinct species. The C. alata accession, however, did not seem to be sufficiently distinct from C. cujete to merit species status. The latter material may in fact be a hybrid or serve to challenge the validity of interspecific organization of the genus Crescentia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The calabash (Crescentia cujete L.: Bignoniaceae) is a small tree with multiple uses, originating from tropical America and now widely distributed in the tropics (Burger and Gentry 2000; Widodo 2001). It is a typical component of homegardens not only in Mexico (e.g., Vogl et al. 2002), Central (e.g., Bass 2004) and South America (e.g., Lamont et al. 1999; Gari 2001), but also in Africa and Asia (Widodo 2001). It is also grown as a living fence (Avendaño-Reyes and Acosta-Rosado 2000), for fuelwood, and as an ornamental and shade tree alongside urban streets (Pérez-Arbelaez 1990).
Pérez-Arbelaez (1990) described the ‘totumo’ or ‘jícaro’ as a “popular panacea” with many diverse uses. The bottle-like dry and empty calabash fruits serve as containers, for home-made utensils, and to prepare handicrafts, and the pulp and foliage are used as livestock feed (Cajas-Giron and Sinclair 2001; Bass 2004; Ibrahim et al. 2006). The tree has also known medicinal properties (Gentry 1980; Pérez-Arbelaez 1990; Widodo 2001).
Considerable morphological variation has been observed in C. cujete, particularly in fruit shape and size (Pérez-Arbelaez 1990). Gentry (1980) suggested that two variants of this polymorphic species may in fact deserve taxonomic recognition. Both variants differ from typical C. cujete in possessing smaller, more coriaceous leaves and fruits. Gentry (1973, 1980) also reported that apparent hybrids in Costa Rica had the small fruit characteristic of C. alata H.B.&K. but the simple leaves of C. cujete and only occasional branches bore 3-foliolate leaves typical of C. alata.
The cultivated C. cujete, a native of Mexico and Central America, is often confused with C. alata (Gentry 1973), a wild relative with a more restricted distribution, but often dominant in the dry forest savannas of the Pacific coast from Mexico to Costa Rica (Gentry 1973, 1980; Bridgewater et al. 2002). Whether or not the natural distribution of C. cujete extends to South America has not been established (Gentry 1973; Burger and Gentry 2000).
Although the calabash tree is widely distributed and used in Colombia (Pérez-Arbelaez 1990), little research has been undertaken to underpin the further development of this multipurpose tree. This study was performed within a larger initiative of the non-governmental organization Centro para la Investigación en Sistemas Sostenibles de Producción Agropecuaria (CIPAV) that aims to further develop underutilized multipurpose tree species for their use by smallholders in silvo-pastoral production systems. The aims of this project were to collect germplasm, which represented the morphological and geographic diversity of the species based on indigenous knowledge from farmers and other users; and to assess that diversity with a view to its inclusion in future research.
Materials and methods
Germplasm collection
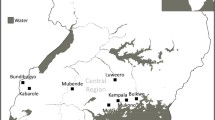
Germplasm and indigenous knowledge of the calabash tree (C. cujete) were collected from five major regions in Colombia (van Wyngaarden and Fandiño-Lozano 2005; Table 1). The collecting strategy was guided by Guarino et al. (1995) and based on information provided by artisans, livestock producers, and/or people applying natural medicines. Specific morphological fruit forms were collected only once from any one region. As the morphological forms were mostly planted as individual trees alongside houses, in backyards, as living fences or scattered in pasture paddocks, a vegetatively propagated sample from such an individual tree represents one accession. One accession each of C. alata and C. amazonica Ducke were collected from a roadside in the town of Cali (Valle del Cauca department) and a gallery forest in the Orinoquian region, respectively, to serve as outgroups.
Morphological and molecular germplasm characterization
A preliminary visual assessment of forms and sizes of mature fruits was performed during collecting. Trees and fruits were also photographically documented. Mature fruits were visually classed into 8 sizes (1/2 = miniature; 3/4 = small; 5/6 = medium; 7/8 = large) and 8 shapes (flattened, oblong, cuneate, elongated, globular, rounded-drop-shaped, oblong-drop-shaped, kidney-shaped). From rooted stakes established in the CIPAV field at Jamundí, Valle del Cauca department, 1–2 fully expanded leaves per accession were obtained from 3 to 4 month old plants to determine leaf shape (lanceolate, oblong, spatulate, oblanceolate, obovate, or elliptic), size (length and width in cm) and form of the apex (acute, acuminate, or obtuse), which were then documented photographically.
After harvesting young leaf tips (300 mg) from one well-established plant per accession in the field at Jamundí, the tissue was immediately stored on ice in a cooling box before freezing the sample at −80°C at the Centro Internacional de Agricultura Tropical (CIAT). DNA was then extracted for use in the molecular analysis. DNA isolation from the leaf material followed the protocol developed by Dellaporta et al. (1983) and modified by González et al. (1995). DNA was quantified with a TKO 100 Hoefer fluorometer (Hoefer Scientific Instruments, San Francisco). An AFLP analysis (Vos et al. 1995) was undertaken using the Analysis System I kit, INVITROGEN® and applying the standard protocol with double restriction (EcoRI and MseI), ligation of adaptors and first PCR amplification. Six different primer combinations were tested on eight randomly selected accessions, with two combinations being chosen for use in this study because of their polymorphism and resolution (Table 2). This selection was based on previous experiences by Roa et al. (1997) and Caicedo (1996, cited by Segura et al. 2002) that two or three primer combinations were sufficient to analyze genetic differences among populations or species by applying AFLPs as long as these provided a high level of polymorphic bands. Electrophoresis and detection of PCR products were carried out on 6% polyacrylamide gel by silver nitrate staining following Bassam et al. (1991) with modifications. Only strong bands were scored visually as present or absent. Eleven accessions had to be excluded from further analysis because they did not amplify.
Data analysis
Passport data (longitude, latitude and altitude) from the collecting points served to generate a map of potential areas of distribution by applying FloraMap® (Jones and Gladkov 2005), a GIS-based program that helps identify areas with similar climates (Jones et al. 1997, 2002). The map was produced on the basis of four principal components that included 97.1% of the data variance. The FloraMap procedure depends on the assumption of continuous distribution of a species in a range of climates that can be described by a single multivariate normal distribution (Jones et al. 1997), however, visual inspection of the first two principal components (data not shown) indicated that this might not be the case for the calabash tree in Colombia. In the subsequent cluster analysis, Ward’s algorithm was used to detect climatic sub-groups (Jones and Gladkov 2005). Climate data from the collecting points were extracted from Worldclim (Version 1.4) (http://www.worldclim.org/) and Bioclim (http://cres.anu.edu.au/outputs/anuclim/doc/bioclim.html) databases (resolution 1 km). The number of dry months per year were defined by precipitation with less than 60 mm, according to Köppen’s (1918) classification.
Descriptive statistics were applied to the morphological data. In order to verify whether regional distribution was reflected in types of particular morphology, a discriminant analysis was performed on different combinations of morphological fruit and leaf variables applying the statistical package SYSTAT version 11. However, overall less than 35% of accessions were correctly assigned to the regions (data not shown).
Similarity measures based on pairwise comparisons of polymorphic AFLP bands were calculated by means of the Nei and Li (1979) coefficient. The resulting similarity matrix was then subjected to cluster analysis by applying the unweighted pair group method with arithmetic means (UPGMA) algorithm (Sneath and Sokal 1973). All computations were done with the procedures from NTSYS-pc, version 2.1 (Rohlf 2001). The robustness of the resulting tree topology was evaluated by bootstrapping (1,000 bootstrap replicates). For the cladistic analysis and the determination of the phylogenetic signal of the data, PAUP version 4.10 was used (Swofford 2003). For ordination, a multiple correspondence analysis was performed using SAS version 8.12. UPGMA was applied on the matrix of Euclidean distances.
Results
Ecogeographic diversity
Calabash trees have been encountered in all five main regions of Colombia (Table 1) in a wide range of ecological conditions, from 20 m asl. at the Caribbean coast to almost 1,500 m asl. in the Andes, with mean annual temperatures from 21.6 to 28.3°C, annual rainfall from 1,150 to 7,500 mm, and dry seasons of 0–4 months of length (Table 1). This covers a very wide range of ecosystems in Colombia, from tropical dry forest through subhumid and humid forests of the Caribbean coastal plains and the Mompox depression,Footnote 1 partially flooded rainforests of the Pacific and Amazon regions, hygrophytic forests of the Amazonian piedmont, equatorial forests in the savanna and alluvial plains in the Orinoquian piedmont to premontane forests in the central cordillera of the Andes. The species was not found in montane forests beyond 1,500 m asl., nor in semi-desertic or xerophytic environments, like for example those found in most of the Guajira peninsula, located in northern Colombia and bordering Venezuela. Plants grew in a variety of habitats from gardens through living fences and pastures to fields, being used for various purposes.
The map produced by using climatic parameters at collecting points (Fig. 1) identified a range of probabilities of potential distribution ranging from no climate similarity to high climate similarity within Colombia. While the germplasm collection in this study explored some of the high probability regions, others were not sampled (e.g., the Urabá area in Chocó department, the Sinú and San Jorge valleys (northern Colombia), the Magdalena floodplains (northern and central Colombia), and the Pacific rainforests of Nariño department (southern Colombia). Based on the points of germplasm collection, four groups with distinct climatic sub-groups were identified that essentially corresponded to the regions, (1) Amazon and Orinoco; (2) Pacific; (3) Andes; and (4) Caribbean (Table 3). These climatic groups may stand for ecotypic differences.
Morphological diversity
The rural population reported a variety of uses of the calabash tree, however, the fruit was perceived as the main product, serving predominantly as household utensils. Fruit shape and size were highly variable (Fig. 2), particularly in the Caribbean, Amazon and Orinoco regions (Fig. 3). Mature fruit size ranged in diameter from approximately 4 to 25 cm. In combining both shape and size of fruits, overall 22 types were determined (Table 4) with the least diversity of types from the Andean region. Except for the very small fruits typical for the Caribbean region, none of these types could be associated with a specific geographic region. The shape of the leaf and of the leaf apex were also variable with six and three different forms, respectively (Fig. 4). In the majority of cases, the leaf apex was acuminate. Mean leaf length and width were 15.1 cm (SD = 5.0 cm) and 5.1 cm (SD = 1.7 cm), respectively, with a mean length:width ratio of 3.1 (SD = 0.7). Again, no clear association between region of collecting and leaf or leaf apex shapes were found.
Molecular diversity
The AFLP technique proved a robust tool for detecting genetic diversity within the collection. Among six primer combinations tested, two sets that gave clear, reliable banding patterns were selected for genotyping 47 of the 58 accessions (Table 2). Altogether, a total of 257 markers were amplified, of which 224 (86.8%) were polymorphic. Among the primer combinations tested, primer set EcoRI+ACA/MseI+CAC was the most informative. Sizes of AFLP products ranged from approximately 50 to 500 base pairs (bp). Polymorphic fragments were distributed across the entire size range with the major proportion being between 50 and 300 bp. The number of bands obtained per individual accession ranged from 12 to 44, confirming the high multiplex ratio attained with this type of marker system.
Pairwise comparison of genetic similarity (percentage of matched markers) among C. cujete accessions ranged from 0.22 to 0.82, with an average of 0.43, revealing considerable genetic diversity. This high level of diversity was also reflected in the dendrogram produced by cluster analysis based on Nei and Li’s coefficient of similarity generated from molecular data (Fig. 5). No clear relationships could be established between the molecular analysis and the various morphological characteristics. Except for accession 12, all C. cujete accessions fell into one large group with no specific pattern of diversity. However, despite its isolation based on AFLP analysis, accession 12 (from the Caribbean region) was not morphologically distinct. Both outgroups were separated from other accessions with C. amazonica being the most distinct, and C. alata being shown to be marginally related to several C. cujete accessions. When applying multiple correspondence analysis, this general pattern did not change, although the large group of accessions now split up into three subgroups (Fig. 6). Nevertheless, none of these medium-sized groups was clearly related to either geographical provenance or morphological fruit type.
Discussion
Intraspecific diversity
A remarkable level of diversity was assembled in the germplasm collection of the calabash tree in Colombia, suggesting a successful collecting strategy. This is reflected in the overall 22 types with different fruit form and size combinations determined that may assist to establish a preliminary classification scheme for the calabash tree. Nevertheless, there is a clear need not only to determine the existing variability within one individual tree or in a population but also the environmental stability of such morphological forms as the present assessment is based on visual appreciations in a variety of locations. Phenotypic changes in morphological traits of individual accessions of C. cujete under cultivation and irrigation have been observed on Curaçao (Gentry 1980).
The wide geographic distribution of this multipurpose tree (Table 1) in five ecologically distinct regions of Colombia (van Wyngaarden and Fandiño-Lozano 2005) would suggest either extremely wide adaptation of individuals or fairly distinct ecophysiological characteristics of individual plants. The fact that many of the collecting points did not fall inside the high probability distribution areas of C. cujete (Fig. 1) also may hint at a possible differentiation in physiological adaptation of sub-groups of accessions or ecotypes (Jones et al. 2002; Jones and Gladkov 2005). The existence of distinct ecotypes appears highly likely, given the species’ adaptation to semi-arid environments (e.g., Patía area) as well as to seasonally flooded areas (e.g., Pacific region) on the one hand (Table 3), and the altitudinal range of distribution on the other.
This is the first intraspecific molecular study in Crescentia. It revealed considerably higher levels of distinctiveness among all accessions collected (mean Nei and Li’s coefficient of 0.43) from Colombia than similar studies applying AFLPs in tree crop species, such as neem (Azadirachta indica A. Juss. by Singh et al. 1999) and laurel (Laurus L. spp. by Arroyo-García et al. 2001). This variation between individual accessions calls for attention given the predominant vegetative propagation of the species reported by growers (J. Arango-Ulloa 2005, unpublished data). The high degree of diversity also suggests that the Colombian region is part of the species’ original range of distribution.
There is little information available on the ways and distances of species distribution or on its reproductive biology. It is, however, known that flowers are hermaphroditic, and pollination is effected by small bats of the genera Glossophaga and Artibeus that belong to the Phyllostomidae family (Gentry 1973; Janzen 1983, cited by Bass 2004). Bat pollination might lead to extensive exchange of pollen among individual trees scattered over a wide area as bats are known to forage over a range of several kilometers (NAS 1991). Also some long-distance dispersal may happen by fruits floating on water (Gentry 1973). If the Colombian C. cujete is cross-pollinated and vegetatively propagated by landholders as is the case for cassava (Manihot esculenta Crantz) and yams (Dioscorea L. spp.; Zohary 2003), then similarly high levels of heterozygosity in populations of cultivated plants would be expected.
No clear relationships could be established between the different morphological, geographical or genetic characteristics assessed. When applying cluster analysis on Nei and Li’s coefficient of similarity based on molecular data, almost all accessions fell into one group only, however, with a substantial high level of diversity (Fig. 5). The remaining 2 out of 47 accessions not included in the main group were the morphologically inconspicuous accession 12 (fruit shape oblong, size miniature 1) from the Caribbean region and the single accession of C. amazonica. Although, more sub-groups were determined by multiple correspondence analysis of the same molecular data (Fig. 6), no clear pattern of diversity was detected. Hence, the 22 morphological fruit types were neither reflected in molecular diversity nor in geographic origin. This lack of differentiation found amongst the different fruit types by AFLPs is not unusual. Leinonen et al. (2008) showed in a meta-analysis that the putatively neutral markers frequently used in diversity studies are not linked to morphological or adaptive traits.
Taxonomic considerations and pathways of dispersal
Six species of Crescentia have been distinguished by Gentry (1980). In tropical America, they are mostly distributed across Mexico and the West Indies to Central America, while C. cujete and C. amazonica also occur in the northern parts of South America. C. alata is a native plant of the dry forest from Mexico to Guanacaste in Costa Rica (Gentry 1973), whereas C. cujete is considered native at least to Veracruz, Mexico and Belize (Gentry 1982). C. alata is more common in the wild and is, in fact, a characteristic tree of Pacific slope dry forest savannas (Gentry 1973). Its fruit is smaller but similar to that of C. cujete, which farmers of Panama prefer to cultivate over the former (Gentry 1973). Gentry (1973, 1980) and Burger and Gentry (2000) stated that C. alata and C. cujete interbreed. The absence of differentiation between the single accession of C. alata and the C. cujete germplasm accessions (Figs. 5, 6) may either suggest this particular accession to be a hybrid or support the view that this species may not merit its taxonomic rank.
Gentry (1980) suggested that the South American C. amazonica may not be a separate species despite it possessing a very different distribution to all other species, which are confined to Central America and the West Indies. Rather, he suggested that C. amazonica might prove no more than a small-fruited wild form of the widely cultivated C. cujete (Gentry 1980). However, he reported that the earliest European explorers did record small-fruited plants of C. amazonica’s appearance, which suggests that, if the plant is not originally native to its present area of distribution in South America, it at least must have been introduced in pre-Colombian times (Gentry 1980; Clement 1999). During the sample collecting for this research, it proved difficult to locate C. amazonica in the Orinoquian region, but one accession was collected from a gallery forest near Puerto Gaitán, Meta department. The results from AFLPs support C. amazonica as a separate species not closely related to C. cujete, although this view is based on one single accession.
Gentry (1973, 1980) and Burger and Gentry (2000) also maintained that there was still doubt about whether or not C. cujete had been spread from its native range in Mexico and Central America to South America, including Colombia. If the species was spread by man, it must have occurred in pre-Colombian times as it was already present in South America at the advent of the Spanish conquerors (Gentry 1980; Clement 1999). Therefore, the original range of distribution of C. cujete is difficult to trace because of its extensive cultivation through most of tropical America (Gentry 1973, 1980; Pérez-Arbelaez 1990; Burger and Gentry 2000; Widodo 2001). Even trees apparently growing in the wild may be descendants from cultivated plants (Gentry 1980). During collecting for the present study, scattered populations of C. cujete were found in pastures in the area of Patía (Andean region), which is characterized by a marked arid climate, and the Caribbean region, where considerable populations were located as part of the vegetation in grazed savannas or as living fences (J. Arango-Ulloa 2005, unpublished data). These plants might have been dispersed by large animals (Gentry 1973), particularly through livestock dung as suggested by Bass (2004) in Honduras. On the other hand, no such distribution in grasslands has been observed in the Orinoquian region (J. Arango-Ulloa 2004, unpublished data). Among Colombian farmers and artisans, however, the calabash tree is usually distributed vegetatively by stakes from selected trees (J. Arango-Ulloa 2004, unpublished data), whereas in Honduras it appears to be planted from seeds (Bass 2004).
Prospects
To understand the pathways of distribution of plants, further collecting is required both in Colombia as well as in the most likely center of origin, in Central America. Additional accessions from C. alata and C. amazonica as well as from the other Crescentia species described should be collected and included in such studies. Based on molecular data with further primers or additional co-dominant markers, the specific status of C. amazonica and C. alata should be revised.
The ecogeographic mapping by FloraMap indicates further potential areas of high probability not only for collecting but also for future cultivation in Colombia. This mapping indicated that there might be important, as yet uncollected regions around Urabá, in the Sinú, San Jorge and Magdalena river valleys, and the Pacific rainforests of Nariño department (Fig. 1). Nevertheless, caution needs to be applied by such predictions that are only based on climatic similarities and disregard other ecologically important determinants for species distribution (Dormann 2007).
In Colombia, the calabash tree is particularly appreciated for its hardiness and resistance to drought (Cajas-Giron and Sinclair 2001) and fire (Bridgewater et al. 2002) as well as its ease of propagation. The present germplasm collection should offer ample opportunity to select for agro-ecological adaptation of particular genotypes, hence, providing new/improved genotypes to farmers and other users, a fact that should assist the species’ conservation through its increased utilization in agroforestry systems (e.g., Cajas-Giron and Sinclair 2001; Ibrahim et al. 2006). While its traditional use as a container will most likely disappear due to more practical alternatives (Bass 2004), the creativity of artisans appears to have opened new avenues for the continued utilization of the calabash tree (e.g., Summit and Widess 1998; J. Arango-Ulloa 2005, unpublished data).
Notes
Mompox tectonic depression, a low floodplain in the lower reaches of the Magdalena River.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- GIS:
-
Geographic information systems
- PCR:
-
Polymerase chain reaction
References
Arroyo-García R, Martínez-Zapater JM, Fernández Prieto JA, Álvarez-Arbessú R (2001) AFLP evaluation of genetic similarity among laurel populations (Laurus L.). Euphytica 122:155–164. doi:10.1023/A:1012654514381
Avendaño-Reyes S, Acosta-Rosado I (2000) Plantas utilizadas como cercas vivas en el estado de Veracruz. Madera Bosques 6(1):55–71
Bass J (2004) Incidental agroforestry in Honduras: the jícaro tree (Crescentia spp.) and pasture land use. J Lat Am Geogr 3(1):67–80. doi:10.1353/lag.2005.0002
Bassam BJ, Caetano-Anollés G, Gresshoff PM (1991) Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196(1):80–83. doi:10.1016/0003-2697(91)90120-I
Bridgewater S, Ibáñez A, Ratter JA, Furley P (2002) Vegetation classification and floristics of the savannas and associated wetlands of the Rio Bravo conservation and management area, Belize. Edingb J Bot 59(3):421–442
Burger W, Gentry AH (2000) Crescentia Linnaeus. In: Burger W (ed), Flora Costaricensis. Fieldiana, vol 41, pp 118–121
Cajas-Giron YS, Sinclair FL (2001) Characterization of multistrata silvopastoral systems on seasonally dry pastures in the Caribbean region of Colombia. Agrofor Syst 53:215–225. doi:10.1023/A:1013384706085
Clement CR (1999) 1492 and the loss of Amazonian crop genetic resources. II. Crop biogeography at contact. Econ Bot 53:203–216. doi:10.1007/BF02866499
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation, version II. Plant Mol Biol Rep 1:19–21. doi:10.1007/BF02712670
Dormann C (2007) Promising the future? Global change projections of species distributions. Basic Appl Ecol 8(5):387–397. doi:10.1016/j.baae.2006.11.001
Gari JA (2001) Biodiversity and indigenous agroecology in Amazonia: the indigenous peoples of Pastaza. Etnoecologica 5(7):21–37
Gentry AH (1973) Crescentia. In: Flora of Panama—family 172. Bignoniaceae. Ann Miss Bot Garden, vol 60, pp 829–833
Gentry AH (1980) Crescentia. In: Bignoniaceae—part I (Crescentieae and Tourretieae). Fl Neotrop Monogr, vol 25, pp 82–96
Gentry AH (1982) Crescentia. In: Bignoniaceae. Flora de Veracruz. Instituto de Ecología AC, Xalapa, Veracruz, Mexico, Fasc 24, pp 87–94
González DO, Palacios N, Gallego G, Tohme J (1995) Protocolos para marcadores moleculares. Centro Internacional de Agricultura Tropical (CIAT), Cali, pp 36–40
Guarino L, Ramanatha Rao V, Reid R (eds) (1995) Collecting plant genetic diversity: technical guidelines. CAB International, Wallingford 748 pp
Ibrahim M, Villanueva C, Mora J (2006) Traditional and improved silvopastoral systems and their importance in sustainability of livestock farms. In: Mosquera-Losada MR, McAdam J, Rigueiro-Rodriguez A (eds) Silvopastoralism and sustainable land management. CABI Publishing, Wallingford, pp 13–16
Jones PG, Gladkov A (2005) FloraMap: a computer tool for predicting the distribution of plants and other organisms in the wild, version 1.03. Centro Internacional de Agricultura Tropical (CIAT), Cali
Jones PG, Galwey NW, Beebe SE, Tohme J (1997) The use of geographical information systems in biodiversity exploration and conservation. Biodivers Conserv 6:947–958
Jones PG, Guarino L, Jarvis A (2002) Computer tools for spatial analysis of plant genetic resources data: 2. FloraMap. Plant Genet Resour Newsl 130:6–10
Köppen W (1918) Klassifikation der Klimate nach Temperatur, Niederschlag und Jahreslauf. Petermanns Geogr Mitt 64:193–203
Lamont SR, Eshbaugh WH, Greenberg AM (1999) Species composition, diversity, and use of homegardens among three Amazonian villages. Econ Bot 53(3):312–326. doi:10.1007/BF02866644
Leinonen T, O’Hara RB, Cano JM, Merilä J (2008) Comparative studies of quantitative trait and neutral marker divergence: a meta-analysis. J Evol Biol 21:1–17. doi:10.1111/j.1420-9101.2007.01445.x
NAS (National Academy of Sciences) (1991) Structure of genetic variation. In: Managing global genetic resources: forest trees. The National Academy Press, Washington DC, pp 51–72
Nei M, Li W (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273. doi:10.1073/pnas.76.10.5269
Pérez-Arbelaez E (1990) Totumo. In: Plantas útiles de Colombia. Editorial Victor Hugo, Medellín, Colombia, pp 320–321
Roa AC, Maya MM, Duque MC, Tohme J, Allem AC, Bonierbale MW (1997) AFLP analysis of relationships among cassava and other Manihot species. Theor Appl Genet 95:741–750. doi:10.1007/s001220050620
Rohlf FJ (2001) NTSYS-pc: numerical taxonomy and multivariate analysis system. Version 2.1. Exeter Software. Setauket, New York
Segura S, d’Eeckenbrugge CG, Bohorquez A, Ollitrault P, Thome J (2002) An AFLP diversity study of the genus Passiflora focusing on subgenus Tacsonia. Genet Resour Crop Evol 49:111–123. doi:10.1023/A:1014731922490
Singh A, Negi MS, Rajagopal J, Bhatia S, Tomar UK, Srivastava PS, Lakshmikumaran M (1999) Assessment of genetic diversity in Azadirachta indica using AFLP markers. Theor Appl Genet 99:272–279. doi:10.1007/s001220051232
Sneath PHA, Sokal RR (1973) Numerical taxonomy: the principles and practise of numerical classification. Freeman, San Francisco
Summit G, Widess J (1998) The complete book of gourd craft: 22 projects, 55 decorative techniques, 300 inspirational designs. Lark Books, Asheville 144 pp
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4.10. Sinauer Associates, Sunderland
van Wyngaarden W, Fandiño-Lozano M (2005) Mapping the actual and original distribution of the ecosystems and the chorological types for conservation planning in Colombia. Divers Distrib 11(5):461–473. doi:10.1111/j.1366-9516.2005.00163.x
Vogl CR, Vogl-Lukasser B, Caballero J (2002) Homegardens of Maya migrants in the district of Patenque (Chiapas/Mexico): implications for sustainable rural development. In: Stepp JR, Wyndham FS, Zarger RK (eds) Ethnobiology and biocultural diversity. University of Georgia Press, Athens, pp 631–647
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23(21):4407–4414. doi:10.1093/nar/23.21.4407
Widodo SH (2001) Crescentia cujete L. In: Bunyapraphatsara N, van Valkenburg JLCH (eds) Plant resources of south-east Asia (PROSEA), no. 12(2), medicinal and poisonous plants. Pudoc, Wageningen, pp 193–194
Zohary D (2003) Unconscious selection in plants under domestication. In: Knüpffer H, Ochsmann J (eds) Rudolf Mansfeld and plant genetic resources. Proceedings of a symposium dedicated to the 100th birthday of Rudolf Mansfeld, Gatersleben, Germany, 8–9 October 2001. Schriften zu Genetischen Ressourcen, vol 22, Informationszentrum Biologische Vielfalt (IBV) der ZADI, Bonn, Germany, pp 121–128
Acknowledgments
Special thanks to all the respondent people in Colombia for their cooperation and ready help during the field survey and collecting. The support provided by members and staff of CIPAV, particularly E. Murgueitio, and the Agrobiodiversity and Biotechnology Project at CIAT under Dr. J. Tohme’s leadership, both Cali, Colombia is gratefully acknowledged. L. Collet at CIAT helped with climate data. Dr. M. Kessler from Albrecht von Haller Institute of Plant Sciences, University of Göttingen provided helpful discussions during the preparation of the manuscript. Dr. B. C. Pengelly is thanked for critical comments and language editing. This study has been financially supported by the Ginés-Mera Memorial Fellowship Fund for Postgraduate Studies in Biodiversity administered through CIAT, DAAD (German Academic Exchange Service), and STUBE (Studienbegleitprogramm für ausländische Studierende an niedersächsischen Hochschulen).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Arango-Ulloa, J., Bohorquez, A., Duque, M.C. et al. Diversity of the calabash tree (Crescentia cujete L.) in Colombia. Agroforest Syst 76, 543–553 (2009). https://doi.org/10.1007/s10457-009-9207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-009-9207-0