Abstract
Background
Mitochondrial fission has been identified as the pathogenesis underlying the development of cardiac microvascular ischemia reperfusion (IR) injury, although the regulatory signaling upstream from fission is far from clear. Bax inhibitor is a novel anti-apoptotic factor, and, however, its role of cardiac microvascular IR injury and mitochondrial homeostasis remains unclear.
Methods
The cardiac microvascular IR injury was performed in WT mice and BI1 transgenic (BITG) mice. The alterations of microvascular structure and function were detected via electron microscope, immunohistochemistry and immunofluorescence in vivo. Cardiac microvascular endothelial cells were isolated form WT and BITG mice and underwent hypoxia/reoxygenation injury in vitro. Cellular viability and apoptosis were analyzed via MTT assay and caspase-3 activity. Mitochondrial function, morphology and apoptosis were detected. Signaling pathways were analyzed via inhibitor, siRNA and mutant plasmid.
Results
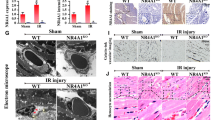
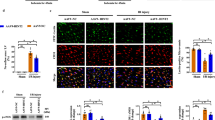
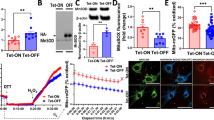
Herein, we demonstrated that Bax inhibitor 1 (BI1) was downregulated following cardiac microvascular IR injury, and its expression correlated negatively with microvascular collapse, endothelial cell apoptosis and mitochondrial damage. However, compared to wild-type mice, BI1 transgenic mice were actually protected from the acute microvascular injury and mitochondrial dysfunction. Functional studies illustrated that reintroduced BI1 directly interacted with and inhibited the Syk pathway, leading to the inactivation of Nox2. Subsequently, less Nox2 was associated with ROS downregulation, inhibiting Drp1 phosphorylated activation. Through repression of the Syk–Nox2–Drp1 signaling axis, BI1 strongly disrupted mitochondrial fission, abolishing mitochondrial apoptosis and thus sustaining endothelial cell viability.
Conclusions
In summary, our report illustrates that BI1 functions as a novel microvascular guardian in cardiac IR injury that operates via inhibition of the Syk–Nox2–Drp1-mitochondrial fission signaling axis. Thus, novel therapeutic strategies to regulate the balance between BI1 and mitochondrial fission could provide a survival advantage to microvasculature following IR stress.







Similar content being viewed by others
Change history
30 June 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10456-023-09882-9
References
Zhou SS, Tian F, Chen YD, Wang J, Sun ZJ, Guo J, Jin QH (2015) Combination therapy reduces the incidence of no-reflow after primary per-cutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction. J Geriatr Cardiol 12(2):135–142. https://doi.org/10.11909/j.issn.1671-5411.2015.02.003
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018) Protective role of melatonin in cardiac ischemia–reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. https://doi.org/10.1111/jpi.12471
Chen WR, Tian F, Chen YD, Wang J, Yang JJ, Wang ZF, Da Wang J, Ning QX (2016) Effects of liraglutide on no-reflow in patients with acute ST-segment elevation myocardial infarction. Int J Cardiol 208:109–114. https://doi.org/10.1016/j.ijcard.2015.12.009
Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, Yang JJ, Wang ZF, Tian F, Ning QX (2015) Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 170(5):845–854. https://doi.org/10.1016/j.ahj.2015.07.014
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. https://doi.org/10.1038/s41418-018-0086-7
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res. https://doi.org/10.1111/jpi.12438
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507. https://doi.org/10.1016/j.redox.2017.07.007
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission–VDAC1–HK2–mPTP–mitophagy axis. J Pineal Res. https://doi.org/10.1111/jpi.12413
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca2+]c/VDAC-[Ca2+]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23(1):101–113. https://doi.org/10.1007/s12192-017-0827-4
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F, Chen Y (2016) Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2 +)-XO-ROS axis via activation of the GLP-1R/PI3 K/Akt/survivin pathways. Free Radic Biol Med 95:278–292. https://doi.org/10.1016/j.freeradbiomed.2016.03.035
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.005328
Zhou H, Yue Y, Wang J, Ma Q, Chen Y (2018) Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal. https://doi.org/10.1016/j.cellsig.2018.03.012
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, Li Y, Zhou H, Chen Y (2018) Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 16:157–168. https://doi.org/10.1016/j.redox.2018.02.019
Jeong H, Park J, Jun Y, Lee C (2017) Crystal structures of Mmm1 and Mdm12–Mmm1 reveal mechanistic insight into phospholipid trafficking at ER-mitochondria contact sites. Proc Natl Acad Sci USA 114(45):E9502–E9511. https://doi.org/10.1073/pnas.1715592114
Ruberti C, Lai Y, Brandizzi F (2018) Recovery from temporary endoplasmic reticulum stress in plants relies on the tissue-specific and largely independent roles of bZIP28 and bZIP60, as well as an antagonizing function of BAX-Inhibitor 1 upon the pro-adaptive signaling mediated by bZIP28. Plant J 93(1):155–165. https://doi.org/10.1111/tpj.13768
Carrara G, Parsons M, Saraiva N, Smith GL (2017) Golgi anti-apoptotic protein: a tale of camels, calcium, channels and cancer. Open Biol. https://doi.org/10.1098/rsob.170045
Castillo K, Rojas-Rivera D, Lisbona F, Caballero B, Nassif M, Court FA, Schuck S, Ibar C, Walter P, Sierralta J, Glavic A, Hetz C (2017) BAX inhibitor-1 regulates autophagy by controlling the IRE1alpha branch of the unfolded protein response. EMBO J 36(11):1640. https://doi.org/10.15252/embj.201797020
Lee GH, Lee HY, Li B, Kim HR, Chae HJ (2014) Bax inhibitor-1-mediated inhibition of mitochondrial Ca2+ intake regulates mitochondrial permeability transition pore opening and cell death. Sci Rep 4:5194. https://doi.org/10.1038/srep05194
Lee GH, Yan C, Shin SJ, Hong SC, Ahn T, Moon A, Park SJ, Lee YC, Yoo WH, Kim HT, Kim DS, Chae SW, Kim HR, Chae HJ (2016) BAX inhibitor-1 enhances cancer metastasis by altering glucose metabolism and activating the sodium-hydrogen exchanger: the alteration of mitochondrial function. Oncogene 35(6):803–804. https://doi.org/10.1038/onc.2015.421
Li B, Yadav RK, Jeong GS, Kim HR, Chae HJ (2014) The characteristics of Bax inhibitor-1 and its related diseases. Curr Mol Med 14(5):603–615
Fuhrmann DC, Brune B (2017) Mitochondrial composition and function under the control of hypoxia. Redox Biol 12:208–215. https://doi.org/10.1016/j.redox.2017.02.012
Eelen G, de Zeeuw P, Simons M, Carmeliet P (2015) Endothelial cell metabolism in normal and diseased vasculature. Circ Res 116(7):1231–1244. https://doi.org/10.1161/CIRCRESAHA.116.302855
Kozlov AV, Lancaster JR Jr, Meszaros AT, Weidinger A (2017) Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol 13:170–181. https://doi.org/10.1016/j.redox.2017.05.017
Griffiths HR, Gao D, Pararasa C (2017) Redox regulation in metabolic programming and inflammation. Redox Biol 12:50–57. https://doi.org/10.1016/j.redox.2017.01.023
Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X, Xu R, Fu F, Wang X, Pei J (2018) Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res. https://doi.org/10.1111/jpi.12447
Xu S, Pi H, Zhang L, Zhang N, Li Y, Zhang H, Tang J, Li H, Feng M, Deng P, Guo P, Tian L, Xie J, He M, Lu Y, Zhong M, Zhang Y, Wang W, Reiter RJ, Yu Z, Zhou Z (2016) Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium-dependent translocation of Drp1 to the mitochondria. J Pineal Res 60(3):291–302. https://doi.org/10.1111/jpi.12310
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. https://doi.org/10.1111/jpi.12450
Kim JH, Lee ER, Jeon K, Choi HY, Lim H, Kim SJ, Chae HJ, Park SH, Kim S, Seo YR, Kim JH, Cho SG (2012) Role of BI-1 (TEGT)-mediated ERK1/2 activation in mitochondria-mediated apoptosis and splenomegaly in BI-1 transgenic mice. Biochim Biophys Acta 1823(4):876–888. https://doi.org/10.1016/j.bbamcr.2012.01.016
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Zhou H, Wang J, Zhu P, Hu S, Ren J (2018) Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal 45:12–22. https://doi.org/10.1016/j.cellsig.2018.01.020
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2017) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. https://doi.org/10.1016/j.redox.2017.12.019
Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res. https://doi.org/10.1111/jpi.12404
Kitsati N, Mantzaris MD, Galaris D (2016) Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol 10:233–242. https://doi.org/10.1016/j.redox.2016.10.006
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S, Zhou S, Zhang T, Zhang Y, Han T, Chen Y (2014) Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3 K/Akt-Sfrp2 pathways. Free Radic Biol Med 77:363–375. https://doi.org/10.1016/j.freeradbiomed.2014.09.033
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3 K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res. https://doi.org/10.1111/jpi.12380
Xu J, Wu Y, Lu G, Xie S, Ma Z, Chen Z, Shen HM, Xia D (2017) Importance of ROS-mediated autophagy in determining apoptotic cell death induced by physapubescin B. Redox Biol 12:198–207. https://doi.org/10.1016/j.redox.2017.02.017
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P, Yang YC, Chen WY, Yang SF, Hsiao M, Chien MH (2016) Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt–MAPKs pathway and NF-kappaB DNA-binding activity. J Pineal Res 60(3):277–290. https://doi.org/10.1111/jpi.12308
King AL, Mantena SK, Andringa KK, Millender-Swain T, Dunham-Snary KJ, Oliva CR, Griguer CE, Bailey SM (2016) The methyl donor S-adenosylmethionine prevents liver hypoxia and dysregulation of mitochondrial bioenergetic function in a rat model of alcohol-induced fatty liver disease. Redox Biol 9:188–197. https://doi.org/10.1016/j.redox.2016.08.005
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H (2018) Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol 14:59–71. https://doi.org/10.1016/j.redox.2017.08.013
Carloni S, Favrais G, Saliba E, Albertini MC, Chalon S, Longini M, Gressens P, Buonocore G, Balduini W (2016) Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J Pineal Res 61(3):370–380. https://doi.org/10.1111/jpi.12354
Rizzo NR, Hank NC, Zhang J (2016) Detecting presence of cardiovascular disease through mitochondria respiration as depicted through biophotonic emission. Redox Biol 8:11–17. https://doi.org/10.1016/j.redox.2015.11.014
Bhatia M, McGrath KL, Di Trapani G, Charoentong P, Shah F, King MM, Clarke FM, Tonissen KF (2016) The thioredoxin system in breast cancer cell invasion and migration. Redox Biol 8:68–78. https://doi.org/10.1016/j.redox.2015.12.004
Agorastos A, Linthorst AC (2016) Potential pleiotropic beneficial effects of adjuvant melatonergic treatment in posttraumatic stress disorder. J Pineal Res 61(1):3–26. https://doi.org/10.1111/jpi.12330
Lee K, Back K (2017) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res. https://doi.org/10.1111/jpi.12392
de Souza P, Guarido KL, Scheschowitsch K, da Silva LM, Werner MF, Assreuy J, da Silva-Santos JE (2016) Impaired vascular function in sepsis-surviving rats mediated by oxidative stress and Rho-Kinase pathway. Redox Biol 10:140–147. https://doi.org/10.1016/j.redox.2016.09.016
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou Y, Hu S, Tian F, Wang J, Chen Y (2015) Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep 5:12898. https://doi.org/10.1038/srep12898
Robinson KS, Clements A, Williams AC, Berger CN, Frankel G (2011) Bax inhibitor 1 in apoptosis and disease. Oncogene 30(21):2391–2400. https://doi.org/10.1038/onc.2010.636
Wang D, Luo P, Wang Y, Li W, Wang C, Sun D, Zhang R, Su T, Ma X, Zeng C, Wang H, Ren J, Cao F (2013) Glucagon-like peptide-1 protects against cardiac microvascular injury in diabetes via a cAMP/PKA/Rho-dependent mechanism. Diabetes 62(5):1697–1708. https://doi.org/10.2337/db12-1025
Delaney MK, Kim K, Estevez B, Xu Z, Stojanovic-Terpo A, Shen B, Ushio-Fukai M, Cho J, Du X (2016) Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler Thromb Vasc Biol 36(5):846–854. https://doi.org/10.1161/ATVBAHA.116.307308
Weir RA, Murphy CA, Petrie CJ, Martin TN, Balmain S, Clements S, Steedman T, Wagner GS, Dargie HJ, McMurray JJ (2010) Microvascular obstruction remains a portent of adverse remodeling in optimally treated patients with left ventricular systolic dysfunction after acute myocardial infarction. Circ Cardiovasc Imaging 3(4):360–367. https://doi.org/10.1161/CIRCIMAGING.109.897439
Chen WR, Shen XQ, Zhang Y, Chen YD, Hu SY, Qian G, Wang J, Yang JJ, Wang ZF, Tian F (2016) Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine 52(3):516–526. https://doi.org/10.1007/s12020-015-0798-0
Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, Huang K, Xie Z, Zou MH (2017) Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation 136(23):2248–2266. https://doi.org/10.1161/CIRCULATIONAHA.117.030235
Thornton C, Jones A, Nair S, Aabdien A, Mallard C, Hagberg H (2017) Mitochondrial dynamics, mitophagy and biogenesis in neonatal hypoxic-ischaemic brain injury. FEBS Lett. https://doi.org/10.1002/1873-3468.12943
Kraus F, Ryan MT (2017) The constriction and scission machineries involved in mitochondrial fission. J Cell Sci 130(18):2953–2960. https://doi.org/10.1242/jcs.199562
Caja S, Enriquez JA (2017) Mitochondria in endothelial cells: sensors and integrators of environmental cues. Redox Biol 12:821–827. https://doi.org/10.1016/j.redox.2017.04.021
Mocsai A, Ruland J, Tybulewicz VL (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10(6):387–402. https://doi.org/10.1038/nri2765
Ryan J, Kanellis J, Blease K, Ma FY, Nikolic-Paterson DJ (2016) Spleen tyrosine kinase signaling promotes myeloid cell recruitment and kidney damage after renal ischemia/reperfusion injury. Am J Pathol 186(8):2032–2042. https://doi.org/10.1016/j.ajpath.2016.04.007
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81770237). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HZ and YDC involved in conception and design, performance of experiments, data analysis and interpretation, and manuscript writing; HZ, CS and JR involved in the development of methodology, SYH and JR involved in the data acquisition, and HZ and YDC involved in financial support, study supervision and final approval of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that they have no conflicts of interest.
Data access
The authors are available to share their data.
Additional information
The original online version of this article was revised: Figures 1R, 3A and 3O unfortunately contained mistakes due to the software incompatibility in the production process. The correct images are updated.
Rights and permissions
About this article
Cite this article
Zhou, H., Shi, C., Hu, S. et al. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk–Nox2–Drp1-mitochondrial fission pathways. Angiogenesis 21, 599–615 (2018). https://doi.org/10.1007/s10456-018-9611-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-018-9611-z