Abstract
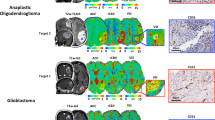
Understanding of structural and functional characteristics of the vascular microenvironment in gliomas and the impact of antiangiogenic treatments is essential for developing better therapeutic strategies. Although a number of methods exist in which this process can be studied experimentally, no single noninvasive test has the capacity to provide information concerning both microvascular function and morphology. The purpose of present study is to demonstrate the feasibility of using a novel three-dimensional ΔR2-based microscopic magnetic resonance angiography (3D ΔR2-μMRA) technique for longitudinal imaging of tumor angiogenesis and monitoring the effects of antiangiogenic treatment in rodent brain tumor models. Using 3D ΔR2-μMRA, a generally consistent early pattern of vascular development in gliomas was revealed, in which a single feeding vessel was visualized first (arteriogenesis), followed by sprouting angiogenesis. Considerable variability of the tumor-associated vasculature was then noted at later stages of tumor evolution. ΔR2-μMRA revealed that anti-vascular endothelial growth factor treatment induced a rapid and significant alteration of the intratumoral angiogenic phenotype. In summary, 3D ΔR2-μMRA enables high-resolution visualization of tumor-associated vessels while simultaneously providing functional information on the tumor microvasculature. It can serve as a useful tool for monitoring both the temporal evolution of tumor angiogenesis and the impact of antiangiogenic therapies.





Similar content being viewed by others
References
Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, Nordquist L, Pfister DG, Piantadosi S, Schiller JH, Smith R, Smith TJ, Strawn JR, Trent D, Giaccone G (2009) American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 27(36):6251–6266. doi:10.1200/JCO.2009.23.5622
Engstrom PF (2008) Systemic therapy for advanced or metastatic colorectal cancer: National Comprehensive Cancer Network guidelines for combining anti-vascular endothelial growth factor and anti-epidermal growth factor receptor monoclonal antibodies with chemotherapy. Pharmacotherapy 28(11 Pt 2):18S–22S. doi:10.1592/phco.28.11-supp.18S
Bellmunt J, Calvo E, Castellano D, Climent MA, Esteban E, Garcia del Muro X, Gonzalez-Larriba JL, Maroto P, Trigo JM (2009) Recommendations from the Spanish Oncology Genitourinary Group for the treatment of metastatic renal cancer. Cancer Chemother Pharmacol 63(Suppl 1):S1–S13. doi:10.1007/s00280-009-0955-3
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13(4):1253–1259. doi:10.1158/1078-0432.CCR-06-2309
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729. doi:10.1200/JCO.2007.12.2440
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF (2006) MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66(8):1258–1260. doi:10.1212/01.wnl.0000208958.29600.87
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11(1):83–95. doi:10.1016/j.ccr.2006.11.021
Tateishi U, Kusumoto M, Nishihara H, Nagashima K, Morikawa T, Moriyama N (2002) Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer 95(4):835–842. doi:10.1002/cncr.10730
Raatschen HJ, Fu Y, Brasch RC, Pietsch H, Shames DM, Yeh BM (2009) In vivo monitoring of angiogenesis inhibitory treatment effects by dynamic contrast-enhanced computed tomography in a xenograft tumor model. Invest Radiol 44(5):265–270. doi:10.1097/RLI.0b013e31819f1b60
Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, Bouma BE (2009) Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med 15(10):1219–1223. doi:10.1038/nm.1971
van Vliet M, van Dijke CF, Wielopolski PA, ten Hagen TL, Veenland JF, Preda A, Loeve AJ, Eggermont AM, Krestin GP (2005) MR angiography of tumor-related vasculature: from the clinic to the micro-environment. Radiographics 25(Suppl 1):S85–S97; discussion S97–S88. doi:10.1148/rg.25si055512
Valable S, Lemasson B, Farion R, Beaumont M, Segebarth C, Remy C, Barbier EL (2008) Assessment of blood volume, vessel size, and the expression of angiogenic factors in two rat glioma models: a longitudinal in vivo and ex vivo study. NMR Biomed 21(10):1043–1056. doi:10.1002/nbm.1278
Lemasson B, Valable S, Farion R, Krainik A, Remy C, Barbier EL (2012) In vivo imaging of vessel diameter, size, and density: a comparative study between MRI and histology. Magn Reson Med. doi:10.1002/mrm.24218
Peng SL, Chen CF, Liu HL, Lui CC, Huang YJ, Lee TH, Chang CC, Wang FN (2012) Analysis of parametric histogram from dynamic contrast-enhanced MRI: application in evaluating brain tumor response to radiotherapy. NMR Biomed. doi:10.1002/nbm.2882
Coenegrachts K, Bols A, Haspeslagh M, Rigauts H (2012) Prediction and monitoring of treatment effect using T1-weighted dynamic contrast-enhanced magnetic resonance imaging in colorectal liver metastases: potential of whole tumour ROI and selective ROI analysis. Eur J Radiol. doi:10.1016/j.ejrad.2012.07.022
Shih TT, Hou HA, Liu CY, Chen BB, Tang JL, Chen HY, Wei SY, Yao M, Huang SY, Chou WC, Hsu SC, Tsay W, Yu CW, Hsu CY, Tien HF, Yang PC (2009) Bone marrow angiogenesis magnetic resonance imaging in patients with acute myeloid leukemia: peak enhancement ratio is an independent predictor for overall survival. Blood 113(14):3161–3167. doi:10.1182/blood-2008-08-173104
Bullitt E, Zeng D, Gerig G, Aylward S, Joshi S, Smith JK, Lin W, Ewend MG (2005) Vessel tortuosity and brain tumor malignancy: a blinded study. Acad Radiol 12(10):1232–1240. doi:10.1016/j.acra.2005.05.027
Nishimura S, Hirai T, Shigematsu Y, Kitajima M, Morioka M, Kai Y, Minoda R, Uetani H, Murakami R, Yamashita Y (2012) Evaluation of brain and head and neck tumors with 4D contrast-enhanced MR angiography at 3T. AJNR Am J Neuroradiol 33(3):445–448. doi:10.3174/ajnr.A2819
Zou Z, Ma L, Cheng L, Cai Y, Meng X (2008) Time-resolved contrast-enhanced MR angiography of intracranial lesions. J Magn Reson Imaging (JMRI) 27(4):692–699. doi:10.1002/jmri.21303
Pipe JG (2001) Limits of time-of-flight magnetic resonance angiography. Top Magn Reson Imaging (TMRI) 12(3):163–174
Lin CY, Lin MH, Cheung WM, Lin TN, Chen JH, Chang C (2009) In vivo cerebromicrovasculatural visualization using 3D DeltaR2-based microscopy of magnetic resonance angiography (3DDeltaR2-mMRA). NeuroImage 45(3):824–831. doi:10.1016/j.neuroimage.2008.12.030
Jang T, Litofsky NS, Smith TW, Ross AH, Recht LD (2004) Aberrant nestin expression during ethylnitrosourea-(ENU)-induced neurocarcinogenesis. Neurobiol Dis 15(3):544–552. doi:10.1016/j.nbd.2003.11.016
Lee CV, Liang WC, Dennis MS, Eigenbrot C, Sidhu SS, Fuh G (2004) High-affinity human antibodies from phage-displayed synthetic Fab libraries with a single framework scaffold. J Mol Biol 340(5):1073–1093. doi:10.1016/j.jmb.2004.05.051
Fuh G, Wu P, Liang WC, Ultsch M, Lee CV, Moffat B, Wiesmann C (2006) Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J Biol Chem 281(10):6625–6631. doi:10.1074/jbc.M507783200
Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, Fu L, Malik AK, Gerber HP, Ferrara N, Fuh G (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 281(2):951–961. doi:10.1074/jbc.M508199200
Bagri A, Berry L, Gunter B, Singh M, Kasman I, Damico LA, Xiang H, Schmidt M, Fuh G, Hollister B, Rosen O, Plowman GD (2010) Effects of anti-VEGF treatment duration on tumor growth, tumor regrowth, and treatment efficacy. Clin Cancer Res 16(15):3887–3900. doi:10.1158/1078-0432.CCR-09-3100
Wu EX, Wong KK, Andrassy M, Tang H (2003) High-resolution in vivo CBV mapping with MRI in wild-type mice. Magn Reson Med 49(4):765–770. doi:10.1002/mrm.10425
Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. Am J Neuroradiol (AJNR) 25(5):746–755
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol (AJNR) 24(10):1989–1998
Shin JH, Lee HK, Kwun BD, Kim JS, Kang W, Choi CG, Suh DC (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. Am J Roentgenol (AJR) 179(3):783–789
Ludemann L, Grieger W, Wurm R, Budzisch M, Hamm B, Zimmer C (2001) Comparison of dynamic contrast-enhanced MRI with WHO tumor grading for gliomas. Eur Radiol 11(7):1231–1241
Siegal T, Rubinstein R, Tzuk-Shina T, Gomori JM (1997) Utility of relative cerebral blood volume mapping derived from perfusion magnetic resonance imaging in the routine follow up of brain tumors. J Neurosurg 86(1):22–27. doi:10.3171/jns.1997.86.1.0022
Sugahara T, Korogi Y, Tomiguchi S, Shigematsu Y, Ikushima I, Kira T, Liang L, Ushio Y, Takahashi M (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. Am J Neuroradiol (AJNR) 21(5):901–909
Cha S, Knopp EA, Johnson G, Litt A, Glass J, Gruber ML, Lu S, Zagzag D (2000) Dynamic contrast-enhanced T2-weighted MR imaging of recurrent malignant gliomas treated with thalidomide and carboplatin. Am J Neuroradiol (AJNR) 21(5):881–890
Akella NS, Twieg DB, Mikkelsen T, Hochberg FH, Grossman S, Cloud GA, Nabors LB (2004) Assessment of brain tumor angiogenesis inhibitors using perfusion magnetic resonance imaging: quality and analysis results of a phase I trial. J Magn Reson Imaging (JMRI) 20(6):913–922. doi:10.1002/jmri.20202
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62. doi:10.1126/science.1104819
Constable RT, Anderson AW, Zhong J, Gore JC (1992) Factors influencing contrast in fast spin-echo MR imaging. Magn Reson Imaging 10(4):497–511
Constable RT, Gore JC (1992) The loss of small objects in variable TE imaging: implications for FSE, RARE, and EPI. Magn Reson Med 28(1):9–24
Busse RF, Hariharan H, Vu A, Brittain JH (2006) Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn Reson Med 55(5):1030–1037. doi:10.1002/mrm.20863
Lebel RM, Wilman AH (2009) Time-efficient fast spin echo imaging at 4.7 T with low refocusing angles. Magn Reson Med 62(1):96–105. doi:10.1002/mrm.21999
Boxerman JL, Hamberg LM, Rosen BR, Weisskoff RM (1995) MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 34(4):555–566
Buschmann I, Schaper W (1999) Arteriogenesis versus angiogenesis: two mechanisms of vessel growth. News Physiol Sci 14:121–125
Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W (2006) Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10(1):45–55
Jang T, Sathy B, Hsu YH, Merchant M, Recht B, Chang C, Recht L (2008) A distinct phenotypic change in gliomas at the time of magnetic resonance imaging detection. J Neurosurg 108(4):782–790. doi:10.3171/JNS/2008/108/4/0782
Acknowledgments
This research was supported by the National Research Program for Genomic Medicine, National Science Council, Taiwan, Republic of China (Grant Number: NSC100-2319-B-001-003).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All animal procedures were approved by the Academia Sinica Institutional Animal Care and Utilization Committee, Taipei, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chien-Yuan Lin and Tiing Yee Siow contributioned equally to this work.
Rights and permissions
About this article
Cite this article
Lin, CY., Siow, T.Y., Lin, MH. et al. Visualization of rodent brain tumor angiogenesis and effects of antiangiogenic treatment using 3D ΔR2-μMRA. Angiogenesis 16, 785–793 (2013). https://doi.org/10.1007/s10456-013-9355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9355-8