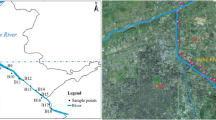
Abstract
Diverse microbial communities play a crucial role in maintaining the proper functioning of river ecosystems and are considered important indicators of river water quality. Although the Seine River being heavily impacted by human activities, little is known about the microbial communities in its surface waters. In order to monitor water quality and promote sustainable use, we studied its microbial diversity using high-throughput sequencing technology, and explored their relationships with physico-chemical properties. The dominant phyla identified were Proteobacteria, Actinobacteriota, Bacteriodota, and Cyanobacteria. The presence of the Alphaproteobacteria and Gammaproteobacteria indicates that the Seine River water nutrient profile is mainly determined by the recalcitrant organic compounds present in WWTP effluents. Bacterial diversity showed significant temporal variability with a highly significant difference in bacterial composition between 2020 and 2021, probably due to variations in water flow favoring Cyanobacteria growth. Summer displayed higher microbial activity and abundance than autumn, attributed to temperature and orthophosphate content. Spatial variation in bacterial composition was observed between sites upstream and downstream of Paris, as well as before and after of the Seine Valenton-WWTP, subject to an accumulation phenomenon and impacted by wastewater treatment. Further assessment of emerging contaminants and other pollutants is required to better understand these variations. These results provide a basic understanding of the microbial community in the Seine River, serving as a reference for assessing the impact of implementing new wastewater disinfection techniques in the near future.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Surface water ecosystems, especially those in rivers, serve as receptor for effluents from wastewater treatment plants (WWTP) and are essential for the biochemical cycling, nutrient recycling, and energy flows (Jin et al. 2018). The rich diversity of microorganisms in these ecosystems, closely linked to physicochemical parameters, plays a crucial role in the functioning and preservation of these processes. The increasing use of surface water for drinking and recreational activities requires microbial analysis to ensure water quality and human health. Various studies have investigated the microbial diversity of surface water (Bryant et al. 2016; Ibekwe et al. 2012). However, the microbial diversity in urban surface water, particularly in the case of the Seine River, remains poorly understood. Only one reference is available in the 2011 activity report (phase 6) of the Piren-Seine program, which investigated the microbial composition and diversity of the Seine River water. The report showed a diverse range of phyla, with Actinobacteria, Proteobacteia, and Bacteroidetes predominating. The study focused on three sites: Marnay, an upstream site, and Bougival and Triel, sites downstream of the Seine River (George et al. 2012), in relation to the Paris conurbation. These sites were situated away from the Paris conurbation where the impact of urbanisation is very significant. Moreover, no correlation was found between microbial and physico-chemical parameters.
The Seine River, which is heavily impacted by human activities, faces challenges in terms of urban wastewater discharges, which are still far from sufficient in relation to the river’s dilution and self-purification capacity. Around 100 km of the river, on the scale of the Parisian conurbation, is subject to significant anthropogenic pressure from treated effluent from WWTPs during dry weather, and treated effluent, combined sewer overflows and urban runoff during wet weather (Flipo et al. 2021). Numerous studies have highlighed the impact of treated, partially treated or untreated effluents on the physicochemical and microbiological quality of surface water, due to the persistent presence of pharmaceutical and cosmetic contaminants (Behera et al. 2011; Luo et al. 2014; Paijens et al. 2022, 2021) and pathogenic microorganisms (Castro-Hermida et al. 2008; Rhymes et al. 2015). Microorganisms, part of the world’s most diverse and abundant biological group, are an essential part of the river biocenosis and play a crucial role in maintaining the functionality of river ecosystem (Findlay 2010). More specifically, the bacterial community within a river contributes significantly to various biogeochemical processes and essential ecosystems services, including pollutant and organic matter degradation (Schmidt 2006), showing sensitivity to environmental factors such as pH, temperature, nutrient levels, and chemical pollutants (Rath et al. 2019; Wang et al. 2023, 2015). Bacterial populations often exhibit rapid-response spatio-temporal variations (Mai et al. 2018; Wang et al. 2015) with bacterial diversity reflecting ecological function within river ecosystems. Consequently, bacterial communities are emerging as promising biological indicators (Hooper et al. 2005; Xie et al. 2016). At the same time, climate change represents a serious threat to the stability and functioning of river ecosystem worldwide, impacting microbial diversity and composition (Weiskopf et al. 2020). Despite extensive research into the ecological impacts of climate change, there remains a notable gap in understanding its specific effects on river microbial communities. Current studies neglect long-term trends and fail to incorporate advanced analytical techniques, preventing us from understanding how climate change affects river microes. This work aims to fill these gaps by providing a comprehensive assessment of the impact of climate change on river microbes. Recent advances in high-throughput, next-generation sequencing methods enable comprehensive characterization of microbial communities in diverse environments, thanks to their capacity to generate large number of sequences. Given this information, local authorities can consider monitoring microbial diversity to assess water quality. The Seine River Bathing plan, launched in 2018 for the Paris 2024 Olympic and Paralympic Games introduces advanced oxydation to further reduce chemical and biological contaminants in the WWTP effluent upstream of Paris to better preserve the river’s water quality, a major concern for local authorities. Understanding microbial diversity and establishing ecological benchmarks before and after treatment are therefore essential to fully characterize new technologies. We therefore studied microbial diversity of the Seine River water to assess the impact of effluents from WWTPs and of tributaries. Excessive volumes of mixed wastewater and stormwater are discharged directly into the river during wet weather, which can have an impact on microbial metabolism and diversity (Drury et al. 2013; Guo et al. 2019). Conversly, during dry weather, high temperatures and low oxygen concentrations also affect microorganisms (Lestel et al. 2021). Consequently, we conducted four sampling campaigns during the two constrating seasons of summer and autumn in consecutive years 2020 and 2021, to get information into microbial communities. Five sampling sites along the Seine River where effluents are discharged from WWTPs, as well as two sites just before the confluence of the Oise and Marne Rivers, were studied to understand the WWTP impact. Microbial abundance, activity and diversity were analyzed using cytometry (BactoSense), enzyme assays and NGS technology, respectively.
Material and methods
Field sites and sample collection
Water samples were collected at seven points along the Seine River, from upstream to downstream of the Parisian conurbation. These sampling sites, which are depicted in the Fig. S1 (supplementary) were chosen to identify the potential impact of both WWTP discharges and tributaries on the quality of the Seine River. In the Ile-de-France region, up to 2.5 million m3/d of effluent during dry weather and up to 5 million m3/d, including a mix of raw wastewater and rainwater, during wet weather, are discharged into the Seine River (Servais et al. 2009).
Seven sampling points were selected, including (i) five points on the Seine River from upstream to downstream of the Paris conurbation, starting at Juvisy-sur-Orge (JSO), followed by Choisy-le-Roi (CLR), Vitry-sur-Seine (VSS), Bougival (BOUG), and Triel-sur-Seine (TSS), (ii) one point on the Marne River at Champigny-sur-Marne (CSM) before the confluence with the Seine River and located just after the discharge of the Marne aval WWTP, and (iii) one point on the Oise River at Conflans-Sainte-Honorine (CSH) before the confluence with the Seine River. GPS coordinates are given in Table 1. Samples were collected from a bridge, using a sampling bucket at the center of the width of the river. Water samples were collected at a depth of approximately 50 cm. All the samples were taken in triplicate and then combined to produce a composite sample. These composite samples were then put in sterile bottles and immediately stored at 6 °C. Afterwards, the samples were then transported to the laboratory for further analysis. The samples were taken in the summer (July) and autumn (November) of two successive years, 2020 and 2021.
Physico-chemical analysis
Parameters including conductivity (EC), pH and temperature were determined on-site after collection of water samples, using a Hach multiprobe portable meter (sensION+, Hach Company, Germany). Ammonium [NH4+], nitrate [NO3−], and nitrite [NO2−] were determined according to NF EN ISO 11732, NF EN ISO 10304-1 and NF EN ISO 10304-1 standards, respectively. Orthophosphate [PO43−] was determined folowing NF EN ISO 6878 standard. The chemical oxygen demand (COD), biochemical oxygen demand (BOD), and total organic carbon (TOC) were analysed using the appropriate analytical methods (ISO 15,705 for COD, NF EN 1899 for BOD, and NF EN 1484 for TOC). The hydrometeorological parameters taken into account include the flow of the Seine River based on records from Vigicrues monitoring stations in France (https://www.vigicrues.gouv.fr/), ambient air temperature and relative humidity data collected from Infoclimat stations (https://www.infoclimat.fr/).
Abundance of microbial cells
The microbial cell count in water samples was determined using the BactoSense automated flow cytometry (FCM) instrument (bNovate, Switzerland). BactoSense is a fully automated flow cytometer using microfluidic detection technology. The sample is injected into the center of a sheath flow containing reagents with fluorescent dyes: SYBR Green I (emitting fluorescence at 535 nm) and propidium iodide (PI, emitting fluorescence at 715 nm). While both dyes are capable of staining DNA, SYBR Green 1 is able to penetrate all cells, whatever the integrity of their cell membrane, whereas PI can only penetrate cells with damaged membranes. Microfluidic flow cytometers use the principle of hydrodynamic focusing to present laser-stained cells through a capillary flow chamber. A single laser diode, emitting light at 488 nm, is used. Fluorescence is measured simultaneously by three detectors: at a wavelength of 525 nm for FL1 (SYBR Green I), at 715 nm for FL2 (PI), and at 488 nm laterally for the Side Scatter (SSC). The results are made available within 20 min.
For pratical reasons, homogenized water samples (2 ml) were transferred to a plastic screw-capped microtube (bNovate, Switzeland) for analysis. The instrument automatically took an aliquot (260 µl) of the sample, of which 90 µl was used for analysis and 150 µl for instrument cleaning. A cleaning step was performed before and after each measurement. Each sample was measured in triplicate. Vittel bottled water was used as a standard to check the condition of the BactoSense after every five measurements. The collected aliquot is labelled with SYBR Green I and PI, and incubated at 37 °C for 10 min before counting. The process is automated to ensure consistent measurements for all samples.
The raw FCM data files were then analysed using customized software that enabled batch processing of the large datasets generated in this study. In brief, FCM gates were constructed to separate bacterial cell signals (total cell concentration, TCC) from background signals and to differentiate intact cells, stained only by SYBR Green I, from cells with altered membranes, positive to both two fluorochromes, i.e. SYBR Green I and PI.
Microbial activity
The enzymatic activity of the microorganisms was measured using the colorimetric assay technique. This method is based on the hydrolysis of fluorescein diacetate (FDA). The microorganisms produce multiple enzymes (esterases, lipases and proteases), leading to the hydrolysis and consequent release of fluorescein (Adam and Duncan 2001). The absorbance of the released fluorescein is then measured at 490 nm. The higher the intensity, the greater the amount of FDA hydrolysed, resulting in increased microbial activity. The assay is conducted in 96-well microplates using the modified method of (Taylor et al. 2002). A 100 µl water sample was added to each well, followed by 100 µl of Mac Ilvain buffer (0.1 M citric acid and 0.2 M Na2HPO4) and 50 μl of fluorescein diacetate (FDA, 4.16 g/l). The microplates were then incubated under agitation (150 rpm) at 37 °C for 2 h. After the incubation period, the microplates were then centrifuged at 4 °C at 15,000 rpm for 10 min. Then, 100 μl of the supernatant of the centrifuged solution were transferred to a new microplate containing 100 µl of Mac Ilvain buffer. Optical density (OD) was measured using a spectrophotometer (Xenius, Safas, Monaco) at a wavelength of 490 nm. The enzyme activity is expressed in µg of fluorescein per ml and per hour, in accordance with the standard range established beforehand.
Bacterial diversity analysis
DNA extraction and polymerase chain reaction (PCR) amplification
To collect the microorganisms, water samples (500 ml) were filtered through a vacuum pump system with 0.22 µm nitrate-cellulose Whatman filtres (Sartorius, France) in a microbiological safety cabinet (BIOII, ADS Laminaire, France). The vacuum pumping system and the filters were sterilized at 121 °C for 21 min. After filtration, the filters containing the microorganisms were placed in labelled sterile Petri dishes in a laminar flow hood for 30 min to dry. DNA was extracted directly from these filters using the DNeasy PowerWater Kit (QIAGEN) according to the manufacturer’s intructions. The amount of genomic DNA was assessed on 1% agarose gel and quantified with Quant-iT™ PicoGreen dsDNA assay kit according to the manufacturer’s intructions and rapidly stored at − 20 °C for future analysis.
Bacterial 16S rRNA gene libraries were constructed using the “16S metagenomic sequencing library preparation” protocol provided by Illumina for the Illumina MiSeq System. The V3–V4 variable regions of the 16S rRNA gene of the bacterial communities were amplified with the forward primer (5′-TAC GGG AGG CAG CAG-3′) (Turner et al. 1999) and the reverse primer (5′-CCA GGG TAT CTA ATC C-3′). Each primer set contains the Illumina adapter sequence: forward primer target sequence (5′- ACACTCTTTCCCTACACGACGCTCTTCCGATCT-3′) and reverse primer target sequence (5′- GACTGGAGTTCAGACGTGTGCTCTTCCGATCT-3′). PCR conditions were as follows: 94 °C for 5 min, 30 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 45 s, and finally 72 °C for 10 min. DNA amplification was verified by agarose gel electrophoresis (1.5%). DNA purity and concentration were assessed using Nanodrop ND-1000 (Thermo-Fisher Scientific). Amplified DNA was purified using the QIAquick96 PCR purification kit (Qiagen, CA) according to the manufacturers’instructions. Purified amplicons were normalized to 120 ng/µl into a new low-retention microtube prior to sequencing. The amplified DNA was sequenced using 2 × 250 paired-end Illumina MiSeq platform by Eurofins Genomics (Germany) on an Illumina MiSeq plateform according to the standard protocols. The raw sequence data have been deposited in the NCBI Sequence Read Archive under the BioProject PRJNA1016134.
Sequence analysis and data processing
The raw sequences (.fastq) for all of the samples from Eurofins were imported into the FROGS pipeline Find Rapidly OTU with Galaxy Solution) implemented on a galaxy instance (v.2.3.0) (http://sigenae-workbench.toulouse.inra.fr/galaxy/). Pair-end reads were merged using the SoftWare FLASH (v1.2.6) with an average merging rate of 98% (Magoč and Salzberg 2011). Barcode and primer sequences were removed with cutadapt (Martin 2011). Sequences were then clustered into operational taxonomic units (OTU) with SWARM (Mahé et al. 2014), which generates the OTU abundance table with an OTU being defined at the 97% sequence similarity level. Chimeras were removed using the VSEARCH tool (Rognes et al. 2016). The taxonomic assignation of each OTU was performed using the BLAST tool against with the database SILVA-132 16S rRNA (Quast et al. 2013). The Shannon index and the Chao1 index were calculated to estimate alphadiversity (Magurran 2004). The Shannon index was used to estimate species richness and evenness, while the Chao1 index used to estimate the number of species in a community, taking into account both the singletons and doubletons species (Chao 1984).
Statistical analysis
Before performing the various analyses to test for significant differences between the treatments, the normality of the data matrices was tested using the Kolmogorov–Smirnov test implemented in R-3.6.1 software (R Core Team 2020).
To compare the variation between sites and seasons, Bartlett’s Test was conducted to assess the homoscedasticity of the dataset. When the variances were found to be equal, the Anova test was selected (Zar 2014). Differences between the physical and chemical parameters, the relative abundance of microbial cells, the enzyme activity, and the diversity indexes were analyzed by one-way ANOVA (Fisher’s post hoc test) with a 95% confidence interval.
Further analyses, principal component analysis (PCA), non-metric multidimensional scaling analysis (NMDS), redundancy analysis (RDA), and correlation tests, were performed using R software (R Core Team 2020). To visualize the spatio-temporal effect on physico-chemical characteristics, a PCA was performed by with the ade4 package (Dray and Dufour 2007). A permutation test was used to determine whether the differences observed between the modality were large enough to reject the null hypothesis. Values were considered different if the probability of a null hypothesis was less than 0.05. NMDS and RDA were performed using the “vegan” package in R (Oksanen et al. 2022) to explore the correlations between bacteria at the phylum level and environment variables. For the correlation test between microbial activity and abundance, depending on the results of the normality test, we selected either the Pearson or Spearman test. If both variables exhibited a normal distribution, Pearson’s test was chosen; otherwise, Spearman’s test was used.
Result
Physico-chemical parameters
The results for the physico-chemical parameters of the surface water of the Seine River during summer and autumn 2020 and 2021 are presented in Table S1. The pH ranged between 7.8 and 8.2, no variability was observed between sampling sites nor sampling periods (Table 2). Besides, N-NO3− exhibited the highest concentration compared to N-NO2− and N-NH4+ for all samples, ranging from 16.1 to 29.3 mg/L. The results of the Two-ways Anova analysis revealed a significant spatial variability in NO2−, NO3−, and NH4+ concentrations, while no seasonal variability existed between the sampling periods (Table 2). The total organic carbon (TOC) content varied significantly among sampling sites from 1.8 to 5.1 mgC/L (Table 2). The TSS site, situated downstream of the Parisian conurbation, recorded the highest TOC value; while the lowest value was observed at the CSM (Champigny-sur-Marne) site, located on the Marne River, upstream Paris. COD ranged between 5.8 mg/L (TSS in summer 2021) and 15.1 mgO2/L (CSM in summer 2020). BOD ranged from 0.5 mgO2/L (CLR in summer 2021) to 5.1 mgO2/L (TSS in summer 2021). Both BOD and COD maximum were observed during summer 2021 at TSS site. The results of the two-way Anova analysis releaved that there were no significant differences in COD and BOD concentrations along the spatial axis, while a significant seasonal variability was noted (Table 2). P-PO43− concentrations ranged from 0.2 mg/L (JSO in summer 2021) to 0.39 mg/L (TSS in autumn 2021) and varied significantivly between seasons, whereas there was no significant difference between the sampling sites. The highest [PO43−] values were recorded during autum 2021 for all sites. The water temperature (T °C) did not differ between summer and autumn in 2020, however it was significantly different between the seasons in 2021. Indeed, the average water temperature was approximately 20.5 °C during summer and 9.8 °C in autumn (Table S1).
A principal component analysis (PCA) was conducted on the physico-chemical data of all parameters for the 7 sampling sites and the 2 seasons (summer and autumn) in 2020 and 2021 (Fig. 1). The PCA revealed that there was no significant disparity between the Seine River and its tributaries, i.e., Marne and Oise Rivers (Fig. 1A, B, C). When compared to the other sites, TSS exhibited significantly higher concentrations of [NO3−], [NO2−], [NH4+], and TOC (Fig. 1D), and appeared separated from the other sites. All the samples, regardless both the site and the year, collected during summer displayed higher concentrations of PO43− and COD compared to those collected during autumn. The constrast in seasonal changes (summer vs. autumn) during 2021 was clearly apparent; whereas this distinction was less noticeable during 2020.
PCA analysis of physico-chemical parameters of the surface water of the Seine River for the two seasons (summer and autumn) 2020 and 2021. A Correlation circle. Ordination of the samples in the plane defined by axes 1 and 2 of the PCA highlighting, B the similarity of the 3 rivers, C the differences between the samples, D the differences between the sampling sites, E the differences between two seasons, and F the differences between the season and the year. N = 7 sites for all parameters and 2 seasons of 2020 and 2021. TOC total organic carbon, BOD biological oxygen demand, COD chemical oxygen demand, T°C water temperature. JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine, CSM Champigny-sur-Marne, BOUG Bougival, CSH Conflans-Sainte-Honorine, TSS Triel-sur-Seine
The mean flow of the Seine River in 2021 (> 200 m3/s) was higher than that in 2020 (< 200 m3/s). This was the case regardless of the location from April to December (Fig. 2A, B).
Abundance of intact/damaged cells
Total microbial cells (TTC) and damaged microbial cells (DMC) were quantified and are presented in Fig. 3A for TTC and Fig. 3B for DMC. TTC ranged between 1 × 106 and 6 × 106 cells/mL, DMC represents an average of 6% of TTC. CSH samples had the highest TTC, observed in autumn 2020 at 5 × 106 cells/mL. Conversely, the lowest TTC, 1 × 106 cells/ml, was recorded at JSO in summer 2021. The two-way Anova results showed that no significant differences were observed for TTC and DMC between sampling sites and seasons.
Microbial activity
The values of microbial activity ranged between 12 and 30 µg fluorescein/ml/h (Fig. 4). The FDA activity in the samples collected during summer was significantly higher than that for sample collected during autumn. These findings were confirmed by the two-way Anova analysis (***p < 0.01) (Table 1). However, no significant difference between the sampling sites and no interaction between the sampling sites and seasons were observed as shown Table 1. Besides, Fig. S2 shows that there is a strong correlation between microbial activity and the number of microbial cells.
FDA activity (µg fluorescein/ml/h) of the Seine River for the 7 sampling sites in summer and autumn 2020 and 2021. N = 12 for each site and each season. JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine, CSM Champigny-sur-Marne, BOUG Bougival, CSH Conflans-Sainte-Honorine, TSS Triel-sur-Seine
Bacterial community composition
After eliminating low-quality sequences and chimeras, a total of 6 million high-quality 16S rRNA sequences were obtained. Subsequently, all samples were then randomly subsampled to 25,700 sequences, which were subjected to further statistical analysis. The mean number of Operational Taxonomic Units (OTU) in CSM samples (approximately 5,500 OTUs) was significantly lower than that found in the other sites (approximately 7,800 OTUs), regardless of the season or year. Additionaly, the average number of OTUs in all samples during both summer and autumn of 2020 was significantly higher than that of 2021. The classified OTUs belonged to 40, 43, 34, and 25 phyla in the autumn and summer of 2020 and 2021, respectively. It has been observed that changes in the relative abundance of phyla between years (2020 and 2021) were much more significant than those between seasons (summer and autumn) and sites (Fig. 5). Whatever the site, there was a significant difference in the number of classified OTUs between summer and autumn 2021 (**p < 0.05), between summer 2020 and 2021 (***p < 0.001), and between autumn 2020 and 2021 (***p < 0.001), as determined by a two ways Anova analysis. Moreover, the OTU count in the downstream samples was significantly higher than that in the upstream samples (Fig. 6). Figure 5 illustrates the predominant groups present in each sample, with abundances exceeding 1%, based on their relative abundance. The dominant phyla observed were Proteobacteria, Actinobacteriota, Bacteroidota and Cyanobacteria, which accounted for approximately 90–95% of the total abundance. Other phyla, comprising Chloroflexi, Firmicutes, Verrumomicrobiota, Campilobacterota, Bdellovibrionota, and Patescibacteria, constituted 5–10% of the abundance.
Comparisons of OTU number—Richness Index (A), and diversity indexes including Chao1 (B), Shannon index (C) and Shannon Equitability (D) were made between seasons (three figures above) and localizations (three figures below) at five sampling sites along the Seine River during autumn and summer 2020 and 2021. Upstream sites: JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine and downstream sites: BOUG Bougival, CSH Conflans-Sainte-Honorine, TSS Triel-sur-Seine
Regardless of site, the most dominant phylum Proteobacteria was significantly less abundant in summer and autumn 2020 (75.5% in summer and 75.4% in autumn) when compared to 2021 (37.8% in summer and 40.5% in autumn) (Fig. 7A). The second most abundant phylum, Actinobacteriota, accounted for 10–30% of the total abundance. The occurence of this phylum was significantly higher in 2020 than in 2021 for both seasons, in contrast to the results obtained for Proteobacteia (***p < 0.001). There were no significant differences observed for Bacteroidota, between seasons and years, and they accounted for approximately 18% of the total abundance. The Cyanobacteria phylum was detected at a relative abundance of around 10% in the samples taken throughout 2020. This level of abundance has been consistently decreased over the seasons, while its presence in samples taken during 2021 was insignificant, at less than 1%. A significant difference in the abundance of Cyanobacteria between seasons was observed (*p < 0.05). The other phyla, including Chloroflexi, Firmicutes, Verrumomicrobiota and Campilobacterota were detected only in the samples collected in 2020, with a minimal relative abundance of less than 1%. For the comparison between sites, we noted a higher relative abundance of the phyla Bacteroidota and Patescibacteria in the downstream sites (i.e., BOU and TSS) compared to the upstream samples (i.e., JSO, CLR, and VSS). Conversely, the relative abundance of Firmicutes and Cyanobacteria was lower in the dowstream sites than upstream (Fig. 8).
Comparisons of the relative abundance of the microbial community at phylum (A) and class (B) levels between five sampling locations along the Seine River during autumn and summer 2020 and 2021. Upstream sites: JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine, and downstream sites: BOUG Bougival, CSH Conflans-Sainte-Honorine, TSS Triel-sur-Seine
To better undrstand the microbial composition in the Seine River water at different locations and seasons, we examined the relative abundance and classification of OTUs from the four most prevalent phyla including Proteobacteria, Bacteroidia, Actinobacteriota and Cyanobacteria at the class level (Fig. 7B). The analysis carried out to detect spatial discrepancies (between seasons and years) revealed the existence of two classes, Alphaproteobacteria and Gammaproteobacteria, both of which belong to the Proteobacteria phylum, in the Seine River. The abundance of Gammaproteobacteria was higher than that of Alphaproteobacteria in all samples. Meanwhile, there were no significant seasonal differences in the relative abundance of Alphaproteobacteria. However, in 2021 samples, the relative abundance of Gammaproteobacteria was significantly higher than that for 2020 samples, whatever the season. Furthermore, the relative abundance of other classes such as Cyanobacteriia (members of Cyanobacteria) and Acidimicrobiia (members of Actinobacteria) was significantly greater in the 2020 samples than in the 2021 samples. When comparing the relative abundance between the studied sites at phylum level, it is worth mentioning that there was no difference in the relative abundance of the Proteobacteria phylum between downstream and upstream samples. However, when comparing the classes α- and γ-Proteobacteria (two important members of Proteobacteria), the downstream samples showed a higher abundance of alpha Proteobacteria than the upstream samples, and conversely, a lower abundance of gamma (Fig. 7B). Hence, no significant difference was detected at the phylum level. Within the Actinobacteriota phylum, the abundance of the class Actinobacteria was higher in downstream samples than upstream; and conversely Acidimicrobiia exhibited a lower abundance in upstream samples. Moreover, the downstream samples showed a higher abundance of classes such as Bacteroidia, Clostridia, and Saccharimonadia compared to the upstream samples (Fig. 8).
In addition, we analyzed the OTUs classification and relative abundance at the genus level (Fig. 9). The dominant genus abundance varied both for year and season, with Microtrichales_CL500-29 marine group, Sporochthyaceae_hgcI clade, unclassified Actinobacteriota, Cyanobium PCC-6307, and unclassified-Cyanobacteriia being dominant in samples from 2020 in both seasons. Conversely, Methylophilaceae_OM43clade, Methylotenera, and Pseudomonas were dominant in 2021 samples. During 2021 summer of, Sphingorhabidus and Massilia were the two most common genera. Flaviobacterium was present throughout all seasons and particularly plentiful at Bougival as illustrated Fig. 9. It is worth noting that Methylophilus accounted for more than 30% of the Bougival samples during the summer of 2021. Additionally, the aquatic_group Burkholderiales_MWH-UniP1 was detected in all samples and was more widespread in the 2021 samples than in the 2020 ones.
Relative abundance of the dominant genera in the Seine River during summer and autumn 2020 and 2021 at different sampling sites from upstream to downstream of the Parisian conurbation. JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine, CSM Champigny-sur-Marne, BOUG Bougival, CSH Conflans-Sainte-Honorine, TSS Triel-sur-Seine
General bacterial community characteristics were assessed at phylum level in all samples collected at different sites and seasons in 2020 and 2021, using non-metric multidimensional scaling analysis (NMDS). Figure 10A shows that axis 1 separated four sampling sites along the Seine River (excluding TSS, which is the furthest site downstream from Paris) from CSM, situated on the Marne River and CSH located on the Oise River. Thus, each river has its own distinct microbial community. The NMDS analysis outcomes demonstrated a clear differentiation between bacterial communities in summer and autumn (Fig. 10B). This was further confirmed by the analysis of data from individual sites (Fig. 10C). However, when the year variable (2020 and 2021) was added to the data (Fig. 10D), samples from both years were segregated along axis 1, irrespective of the seasons. This suggests that the effect from one year to the next, as demonstrated in our findings between 2020 and 2021, carries more weight than the impact of seasonal variations.
Nonmetric multidimensional scaling plot (MDS) analysis of bacterial community at the phylum level in water for all sampling by comparison between sites (A), between seasons according to the sites (B), between seasons according to the year (C) and between seasons including summer and autumn (D). JSO Juvisy-sur-Orge, CLR Choisy-le-Roi, VSS Vitry-sur-Seine, BOUG Bougival, TSS Triel-sur-Seine (located on the Seine River), CSM Champigny-sur-Marne (located on the Marne River), CSH Conflans-Sainte-Honorine (located on the Oise River)
Diversity indices
Diversity indices, including Chao1, Shannon, and Shannon Equitability were calculated using the OTU count and their presence or absence. A higher richness index was found in the 2020 samples compared to those from 2021 (Fig. 6A). The 2021 samples for both seasons exhibited a significantly higher Chao1 than those of 2020 (**p < 0.05), as depicted in Fig. 6B. The Shannon index of the 2021 samples has decreased compared to that for 2020 samples (*p < 0.01) both for both summer (*p < 0.01) and autumn (**p < 0.05) (Fig. 6C). The Shannon equitability (Fig. 6D) showed a higher value for the 2020 samples compared to that for the 2021 samples, regardless of the seasons.
From the comparison between the upstream (i.e., JSO, CLR, VSS) and downstream (i.e., BOU and TSS) sites along the Seine River, it clearly appeared that the richness index values of the downstream samples were higher than those of the upstream samples. In addition, the Shannon index and Shannon equitability values in the upstream samples were higher than in the downstream samples.
Multivariate correlation analysis between water bacterial communities at the phylum level and environmental variables
RDA (Redundancy analysis) was performed to assess the correlation between environmental factors and the family-level composition of bacterial communities in water samples from seven sampling sites during summer and autumn 2020 and 2021. Unsurprisingly, different bacterial phyla responded differently to nutrients, temperature, and pH at different sampling sites, rivers, and seasons according to the year (Fig S3). RDA analysis has revealed that the relative abundance of four phyla, namely Proteobacteria, Cyanobacteria, Bacteroidota and Actinobacteriota, varies across the sampling sites, based on different parameters, including N-NO3−, temperature, BOD and P-PO43−, respectively. Proteobacteria and Bacteroidota were found to be dominant phyla in the Seine and Oise samples, due to various nutrient parameters such as N-NO3−, N-NH4+, N-NO2−, TOC, DOC and temperature. At last, Actinobacteria and Cyanobacteria were the predominant phyla identified in the 2020 samples, despite seasonal variations. However, Proteobacteria was the predominant phylum in 2021. No particular environmental factor was fully identified to provide a satisfactory explanation for this variability.
Discussion
Microorganisms are highly sensitive to environmental conditions such as pH, oxygen, nutrients, contaminants, and temperature (Savio et al. 2015; Wang et al. 2023, 2018, 2012). These factors act as selective pressures, shaping the microbial communities within ecosystems. We first present the results of physico-chemical parameters of the water in the Seine River. In previous works (Bagagnan et al. 2024), we noted the stability of the surface water physico-chemical properties over the last 10 years. Comparing with global river data reveals similar pH levels in the Seine River (approximately 8.0) and the Qin and Yongding Rivers in China (7.8 and 8.3, respectively), despite high human impacts (Li et al. 2022; Zhao et al. 2022). Nutrient parameters (N-NO3− at 22.8 mg/L, N-NH4+ at 0.12 mg/L and P-PO43− at 0.13 mg/L) fall within ranges observed in other rivers like the Yellow Delta River, and Maozhou River (Lv et al. 2016; Ouyang et al. 2020). The shift from one season to another and from one year to the next results in remarkable alterations of the physico-chemical properties of the water. However, no changes between the sampling sites was observed when each site was analysed separately. Seasonal variation in water temperature in the Seine River and its tributaries is a natural occurrence typical of temperate climates, closely tied to atmospheric temperatures. Meanwhile, nitrogen-based nutrients and other parameters remain relatively stable between seasons, with disparities observed between upstream and downstream samples. Downstream sites, particularly TSS, exhibit a high nutrient concentrations (NO2−, NO3−, NH4+, and TOC), due to upstream accumulation, similar to observations in the Maozhou River (Ouyang et al. 2020). In addition, nitrifying biofilters are increasingly adopted in dense urban areas, such as the Paris conurbation, due to their increased nitrogen treatment capacity with limited footprint, but the trade-off is a higher nitrite input to watercourses (Raimonet et al. 2017; Rocher et al. 2015). Seasonal orthophosphate fluctuations attributed partially to reduced river flows during summer (Liu et al. 2013) underscore the important of understanding nutrient dynamics in aquatic ecosystems. Reduction in river flows during the summer is a common phenomenon in many regions, with significant implications for nutrient dynamics in rivers. When flow rates are low, the residence time of water in the river increases. As a result, nutrients like orthophosphates may become more concentrated in the water. Changes in environmental factors can significantly impact the abundance and composition/diversity of microorgnisms.
According to the results obtained from high-throughput sequencing technology, the total number of phyla varied from 25 to 43, a range commonly reported in similar studies analyzing bacterial communities in rivers worldwide. Comparable numbers of phyla have previously been identified on the Danube in Europe (Savio et al. 2015), the Apies in South Africa (Abia et al. 2018), the Mississipisi in the USA (Korajkic et al. 2015; Staley et al. 2013), the Kalamas in Greece (Meziti et al. 2016), the Yangtze in Japan (Liu et al. 2018), and the Maozhou in China (Ouyang et al. 2020). The dominant phyla identified included Proteobacteria, Actinobacteriota, Bacteroidota, and Cyanobacteria, consistent with previous findings (George et al. 2012). These phyla were predominant at three sites of the previously cited French PIREN-Seine study, at Marnay-sur-Seine upstream Paris, and Bougival and Triel-sur-Seine downstream Paris. Their presence is persistent with observations made in other urban rivers (Mai et al. 2018; Ouyang et al. 2020; Savio et al. 2015; Wang et al. 2023), indicating commonalities in microbial communities under urban pressure. Each phylum likely contributes uniquely to the river ecosystem’s overall ecological functioning. Proteobacteria are metabolically versatile bacteria involved in nitrogen cycling, organic matter decomposition, and pollutant degradation (Goetghebuer 2019; Sun et al. 2017). Actinobacteria contribute to nutrient recycling by decomposing complex organic compounds, including plant material and recalcitrant organic matter (Mikhailov et al. 2019). Bacteroidota degrade complex carbohydrates (i.e., glycanes) and play a role in organic matter breakdown, influencing nutrient cycling (Kruczyńska et al. 2023). Cyanobacteria are photosynthetic bacteria that act as primary producers, contributing to oxygen production, but can also form toxic algal blooms under specific conditions, negatively impacting ecosystem (Soto Ramos et al. 2023). Regarding microbial abundance, the Seine River water contained approximately 106 cells/ml, consistent with findings from the PIREN-Seine program reported by (George et al. 2012). This abundance level is similar to that reported for the Wensum River in England (Albaggar 2014), ranging between 0.2 and 5.3 × 106 cells/ml, depending on the sampling sites and higher to that measured on the Lancang River in China (Luo et al. 2019), lying between 0.3 and 0.5 × 106 cells/ml, depending on the seasons.
Spatial and temporal changes of bacterial communities in water
Comparing bacterial communities across seasons revealed significant differences overall. However, there was substantial variation inter-year, overshadowing seasonal intra-year variations. Regardless of the seasons, we noted fascinating results as we compared the bacterial compositions between those years. The finding that bacterial communities in the water of the Seine River were more diverse and evenly distributed in 2020 compared to 2021 is very interesting. The hypothesis, that the COVID-19 lockdown in 2020 may have played a role in this disparity, warrants further investigation. During the lockdown period, numerous human activities were suspended, resulting in a decrease in wastewater production and fewer contaminants being discharged into the Seine River. Another explanation could be linked to the lower water flow in the Seine River in 2020 compared to 2021. Unlike the even distribution between the phyla in 2020, in 2021, Proteobacteria constituted more than 60% of the relative abundance. This dominance was primarily driven by genera such as Methylotenera, Methylophilaceae-OM43clade, Pseudomonas, and Massilia, which are recognized as opportunistic pathogens, with their presence potentially linked to insufficient removal at the WWTP level, as suggested by multiple authors (Pandey et al. 2014; Shao et al. 2019). Conversely, in 2020, there was a significantly higher relative abundance of the genera Cyanobacteriia_unclassifier and Cyanobium from the phylum Cyanobacteria. The presence of Cyanobacteria in 2020 can be explained by the low river flow conditions as Cyanobacteria are often associated with slower-moving or stagnant water bodies where they can form blooms (Shang et al. 2023). High water flow prevents stratification and and favors the mixing of suspended matter, which can hinder the acquisition of nutrients by Cyanobacteria and their optimal positioning in the water column for light absorption. These conditions led to a change in microbial composition, marked by a high abundance of Actinobacteria. Interestingly, (Tu et al. 2022) demonstrated that a Cyanobacteria bloom accelerates the conversion of organic phosphorus to soluble inorganic phosphorus from sediment to water, facilitating the development of Actinobacteria. This possibility can be supported by RDA analysis, which highlights the orthophosphate content as a significant environmental factor influencing the developement of Actinobacteria. However, despite year-to-year bacterial composition changes, microbial activity and abundance remained relatively stable. However, microbial activity and abundance were higher in summer than in autumn for both years, with high correlation between microbial abundance and activity, likely due to factors like temperature, light, and nutrient availability favouring microbial growth and metabolic activity (Crump and Hobbie 2005; Sun et al. 2017; Wang et al. 2016).
In our study, the spatial variation has been taken into account to differentiate between sampling sites (seven sites), between before (JSO with respect to Seine-Valenton WWTP) and after (CLR, VSS with respect to Seine-Valenton, and BOU with regard to Seine-Aval) WWTP discharge, between upstream (JSO, CLR and VSS) and downstream (BOU and TSS) sites, between rivers (Seine, Marne and Oise). Comparing sites before and after WWTP discharge, we observed significantly higher microbial abundance and activity downstream than upstream of the WWTP. This suggests that the WWTP effluent dischage can contribute to an increase in microbial activity and abundance in the river. Additionally, microbial abundance was higher upstream of Paris compared to downstream, although no changes in activity were noted. This constrasts with our observations of the physico-chemical parameters, where we noticed a nutrient accumumation from upstream to downstream, consistent with findings from previous studies (Gücker et al. 2006; Marti et al. 2004; Spänhoff et al. 2007; Waiser et al. 2011). Several factors may contribute to these variations. The low-charge activated sludges used in the Seine Valenton WWTP, an upstream site, may be linked to the higher microorganisms abdundance upstream of Paris. Changes in microbial activity can lag behind changes in abundance, as microorganisms need time to adapt to new conditions or to recover from environmental disturbances (Sveen et al. 2024). Thus, no overall activity difference may be observed despite higher upstream abundance. Variability between sampling sites is less pronounced, indicating the dominant factors shaping the microbial communities in the Seine River are primarily related to seasonal and annual changes rather than spatial differences. According to available data, the effluents were well regulated for all physico-chemical parameters before being discharged into the Seine River. Thus, the ecosystem of the Seine River, in the Paris conurbation, may have relatively uniform environmental conditions across the various sampling sites, leading to reduced variability in microbial communities among sites. Regarding downstream and upstream samples, downstream samples displayed lower bacterial diversity compared to upstream samples, as indicated by Shannon and Chao1 indices, suggesting potential effects of accumulation phenomena. Downstream water showed a higher abundance of Bacteroidota and Patescibacteria phyla compared to upstream. Bacteroidota, commontly found in human intestines (Dowd et al. 2008; Shanks et al. 2011), may indicate the influence of excessive faecal matter and contaminant accumulation from high population density areas in the Paris conurbation. Patescibacteria groups are known for encoding active enzymes that degrade complex organic matter into simple molecules, a process observed in previous studies (Dong et al. 2021; Gu et al. 2023). Specifically, the dominance of Patescibacteria was associated with the presence of complex organic compounds like PolyPh and PolyAr. While the latter are significantly or completely removed during the water treatment process, other compounds promoting the development of this phylum may be introduced by the effluents from the Seine Centre, Seine Aval and Seine Grésillons WWTPs. At the phylum level, Proteobacteria showed similar relative abundance between upstream and downstream samples, yet significant variations were noted at the class level. Notably, the Gammaproteobacteria class dominated all surface water samples of the Seine River, consistent with findings by (Ge et al. 2021). Their study revealed that Gammaproteobacteria is predominant in the urbanised area, followed by Alphaproteobacteria and Betaproteobacteria. This is because many members of the Gammaproteobacteria originate from anthropogenic or zoonotic sources and are widely considered transient passengers (Newton et al. 2011; Sun et al. 2017) that may have been transported by the WWTP effluent. It is interesting also to note that no class was detected for Betaproteobacteria, despite its significance and abundance in other studies, particularly in surface water in the Dongjiang River in China (Liu et al. 2012; Sun et al. 2017). This absence can be explained by varying selection pressures. Betaproteobacteria are fast-growing, nutrient-dependent, making them vulnerable to predation, whereas, Alpha- and Gamma-proteobacteria are better adapted to low nutrient environments and can degrade complex organic compounds such as humic substances (Sun et al. 2017). Given the nutrient-rich nature of the Seine River, which mainly contains recalcitrant organic compounds from WWTP effluents, and their ability to degrade them, Alpha- and Gamma-proteobacteria are likely to thrive. The downstream sites exhibited greater abundance of Gammaproteobacteria, while the upstream sites showed higher abundance of Alphaproteobacteria. This finding is in good agreement with similar results presented by (Marti and Balcázar 2014), who found higher Gammaproteobacteria abundance downstream in the Ter River; constrasting with (Yuan et al. 2023), who observed the opposite trend in the North Canal River, China. These authors explained those variations between upstream and downstream sites for this class as being related to WWTP, due to the presence of residual contaminants in the effluent. To better understand these variations, the measurement of contaminant levels in term of emerging organic contaminants in water and the correlation between microbial diversity and contaminant need to be further investigated.
Conclusion
Changes in the bacterial communities were evaluated using high-throughput sequencing, and the relationships between microbial parameters and physico-chemincal properties were investigated. The dominant phyla identified in the Seine River were Proteobacteria, Actinobacteriota, Bacteroidota, and Cyanobacteria. The presence of the Alphaproteobacteria and Gammaproteobacteria suggests that the Seine River water contains nutrients in the form of recalcitrant organic compounds. The results show a strong temporal variability and a lower spatial variability in the bacterial community structure of surface water in the Seine River. It is interesting to note that the bacterial composition of the Seine River in 2020 was clearly different from that in 2021, probably due to the lower water flow rate, which allowed Cyanobacteria to develop, leading to changes in the composition of the bacterial communities. Higher microbial activity and abundance were found in summer compared to autumn, likely due to temperature and orthophosphate content. Moreover, spatial variation in bacterial composition was observed between upstream and downstream sites, and between before and after of the Seine Valenton-WWTP, suggesting a contribution due to either the wastewater treatment processess or an accumulation phenomena. For example, Gammaproteobacteria were higher in the upstream sites than downstream, potentially linked with the presence of residual contaminants in the effluent. Additional measures of emerging contaminants and other pollutants are needed to better understand these variability. Our results provide a basic understanding of the microbial community in the Seine River, serving as a reference for assessing the impact of the recent introduction of wastewater disinfection by performic acid.
References
Abia ALK, Alisoltani A, Keshri J, Ubomba-Jaswa E (2018) Metagenomic analysis of the bacterial communities and their functional profiles in water and sediments of the Apies River, South Africa, as a function of land use. Sci Total Env 616–617:326–334. https://doi.org/10.1016/j.scitotenv.2017.10.322
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951. https://doi.org/10.1016/S0038-0717(00)00244-3
Albaggar AKA (2014) Investigation of bacterial community composition and abundance in a lowland arable catchment. University of East Anglia
Bagagnan S, Guérin-Rechdaoui S, Rocher V, Alphonse V, Moilleron R, Jusselme MD (2024) Spatial and temporal characteristics of microbial communities in the Seine river in the greater Paris area under anthropogenic perturbation. Heliyon 10(9):e30614. https://doi.org/10.1016/j.heliyon.2024.e30614
Behera SK, Kim HW, Oh J-E, Park H-S (2011) Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci Total Environ 409:4351–4360. https://doi.org/10.1016/j.scitotenv.2011.07.015
Bryant JA, Aylward FO, Eppley JM, Karl DM, Church MJ, DeLong EF (2016) Wind and sunlight shape microbial diversity in surface waters of the North Pacific Subtropical Gyre. ISME J 10:1308–1322. https://doi.org/10.1038/ismej.2015.221
Castro-Hermida JA, García-Presedo I, Almeida A, González-Warleta M, Correia Da Costa JM, Mezo M (2008) Contribution of treated wastewater to the contamination of recreational river areas with Cryptosporidium spp. and Giardia duodenalis. Water Res 42:3528–3538. https://doi.org/10.1016/j.watres.2008.05.001
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Crump BC, Hobbie JE (2005) Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50:1718–1729. https://doi.org/10.4319/lo.2005.50.6.1718
Dong X, Zhang C, Li W, Weng S, Song W, Li J, Wang Y (2021) Functional diversity of microbial communities in inactive seafloor sulfide deposits. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiab108
Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, Edrington TS (2008) Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol 8:125. https://doi.org/10.1186/1471-2180-8-125
Dray S, Dufour A-B (2007) The ade4 Package: implementing the duality diagram for ecologists. J Stat Softw 22(1):20. https://doi.org/10.18637/jss.v022.i04
Drury B, Rosi-Marshall E, Kelly JJ (2013) Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl Environ Microbiol 79:1897–1905. https://doi.org/10.1128/AEM.03527-12
Findlay S (2010) Stream microbial ecology. J North Am Benthol Soc 29:170–181. https://doi.org/10.1899/09-023.1
Flipo N, Lestel L, Labadie P, Meybeck M, Garnier J (2021) Trajectories of the Seine River Basin. In: Flipo N, Labadie P, Lestel L (eds) The Seine River basin, The handbook of environmental chemistry. Springer International Publishing, Cham. https://doi.org/10.1007/698_2019_437
Ge Y, Lou Y, Xu M, Wu C, Meng J, Shi L, Xia F, Xu Y (2021) Spatial distribution and influencing factors on the variation of bacterial communities in an urban river sediment. Environ Pollut 272:115984. https://doi.org/10.1016/j.envpol.2020.115984
George I, Anzil A, Lanckman R, Meyer N, Berthe T, Petit F, Fechner L, Servais P (2012) Communautés microbiennes dans les rivières du bassin de la Seine : diversité et lien avec la présence de polluants chimiques. (PIREN-Seine—phase 6)
Goetghebuer L (2019) Les communautés bactériennes de rivières: étude de la dynamique des espèces au sein d’une communauté modèle. Université Libre de Belgique
Gu Z, Bao M, He C, Chen W (2023) Transformation of dissolved organic matter in landfill leachate during a membrane bioreactor treatment. Sci Total Environ 856:159066. https://doi.org/10.1016/j.scitotenv.2022.159066
Gücker B, Brauns M, Pusch MT (2006) Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J N Am Benthol Soc 25(2):313–329
Guo D, Lintern A, Webb JA, Ryu D, Liu S, Bende-Michl U, Leahy P, Wilson P, Western AW (2019) Key factors affecting temporal variability in stream water quality. Water Resour Res 55:112–129. https://doi.org/10.1029/2018WR023370
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. https://doi.org/10.1890/04-0922
Ibekwe AM, Leddy MB, Bold RM, Graves AK (2012) Bacterial community composition in low-flowing river water with different sources of pollutants. FEMS Microbiol Ecol 79:155–166. https://doi.org/10.1111/j.1574-6941.2011.01205.x
Jin D, Kong X, Cui B, Jin S, Xie Y, Wang X, Deng Y (2018) Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci Rep 8:13368. https://doi.org/10.1038/s41598-018-31706-w
Korajkic A, Parfrey LW, McMinn BR, Baeza YV, VanTeuren W, Knight R, Shanks OC (2015) Changes in bacterial and eukaryotic communities during sewage decomposition in Mississippi river water. Water Res 69:30–39. https://doi.org/10.1016/j.watres.2014.11.003
Kruczyńska A, Kuźniar A, Banach A, Jurczyk S, Podlewski J, Słomczewski A, Marzec-Grządziel A, Sochaczewska A, Gałązka A, Wolińska A (2023) Changes in the mycobiome structure in response to reduced nitrogen fertilization in two cropping systems of maize. Sci Total Environ 904:166343. https://doi.org/10.1016/j.scitotenv.2023.166343
Lestel L, Carré C, Mouchel J-M, Meybeck M, Briand C, Servais P, Haghe J-P, Euzen A (2021) Chapitre 2—Paris et la Seine : l’impossible équipement d’une agglomération. Les rivières urbaines et leur pollution Indisciplines. Éditions Quæ, Versailles, pp 23–78
Li J, Sun Y, Zhang X, Pan C, Zhang S, Zheng B (2022) Water quality and microbial community in the context of ecological restoration: a case study of the Yongding River, Beijing, China. Int J Environ Res Public Health 19:13056. https://doi.org/10.3390/ijerph192013056
Liu Z, Huang S, Sun G, Xu Z, Xu M (2012) Phylogenetic diversity, composition and distribution of bacterioplankton community in the Dongjiang River, China. FEMS Microbiol Ecol 80:30–44. https://doi.org/10.1111/j.1574-6941.2011.01268.x
Liu L, Yang J, Yu X, Chen G, Yu Z (2013) Patterns in the composition of microbial communities from a subtropical river: effects of environmental Spatial and Temporal Factors. PLoS ONE 8:e81232. https://doi.org/10.1371/journal.pone.0081232
Liu T, Zhang AN, Wang J, Liu S, Jiang X, Dang C, Ma T, Liu S, Chen Q, Xie S, Zhang T, Ni J (2018) Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome. https://doi.org/10.1186/s40168-017-0388-x
Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473–474:619–641. https://doi.org/10.1016/j.scitotenv.2013.12.065
Luo X, Xiang X, Huang G, Song X, Wang P, Fu K (2019) Bacterial abundance and physicochemical characteristics of water and sediment associated with hydroelectric dam on the Lancang River China. Int J Environ Res Public Health 16:2031. https://doi.org/10.3390/ijerph16112031
Lv X, Ma B, Yu J, Chang SX, Xu J, Li Y, Wang G, Han G, Bo G, Chu X (2016) Bacterial community structure and function shift along a successional series of tidal flats in the Yellow River Delta. Sci Rep 6:36550. https://doi.org/10.1038/srep36550
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinforma Oxf Engl 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Magurran AE (2004) Measuring biological diversity. John Wiley & Sons, Hoboken
Mahé F, Rognes T, Quince C, de Vargas C, Dunthorn M (2014) Swarm: robust and fast clustering method for amplicon-based studies. PeerJ 2:e593. https://doi.org/10.7717/peerj.593
Mai Y, Lai Z, Li X, Peng S, Wang C (2018) Structural and functional shifts of bacterioplanktonic communities associated with spatiotemporal gradients in river outlets of the subtropical Pearl River Estuary, South China. Mar Pollut Bull 136:309–321. https://doi.org/10.1016/j.marpolbul.2018.09.013
Marti E, Balcázar JL (2014) Use of pyrosequencing to explore the benthic bacterial community structure in a river impacted by wastewater treatment plant discharges. Res Microbiol 165:468–471. https://doi.org/10.1016/j.resmic.2014.04.002
Marti E, Aumatell J, Godé L, Poch M, Sabater F (2004) Nutrient retention efficiency in streams receiving inputs from wastewater treatment plants. J Environ Qual 33:285–293. https://doi.org/10.2134/jeq2004.2850
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet. J 17(1):10–12. https://doi.org/10.14806/ej.17.1.200
Meziti A, Tsementzi D, Kormas K, Karayanni H, Konstantinidis KT (2016) Anthropogenic effects on bacterial diversity and function along a river-to-estuary gradient in Northwest Greece revealed by metagenomics. Environ Microbiol 18(12):4640–4652. https://doi.org/10.1111/1462-2920.13303
Mikhailov IS, Zakharova YR, Bukin YS, Galachyants YP, Petrova DP, Sakirko MV, Likhoshway YV (2019) Co-occurrence networks among bacteria and microbial eukaryotes of Lake Baikal during a spring phytoplankton bloom. Microb Ecol 77:96–109. https://doi.org/10.1007/s00248-018-1212-2
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A Guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49. https://doi.org/10.1128/MMBR.00028-10
Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, Caceres MD, Durand S, Evangelista HBA, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, McGlinn D, Ouellette M-H, Cunha ER, Smith T, Stier A, Braak CJFT, Weedon J (2022) Vegan: community ecology package. Community Ecology Package 2(9):1–295
Ouyang L, Chen H, Liu X, Wong MH, Xu F, Yang X, Xu W, Zeng Q, Wang W, Li S (2020) Characteristics of spatial and seasonal bacterial community structures in a river under anthropogenic disturbances. Environ Pollut 264:114818. https://doi.org/10.1016/j.envpol.2020.114818
Paijens C, Bressy A, Frère B, Tedoldi D, Mailler R, Rocher V, Neveu P, Moilleron R (2021) Urban pathways of biocides towards surface waters during dry and wet weathers: assessment at the Paris conurbation scale. J Hazard Mater 402:123765. https://doi.org/10.1016/j.jhazmat.2020.123765
Paijens C, Tedoldi D, Frère B, Mailler R, Rocher V, Moilleron R, Bressy A (2022) Biocidal substances in the Seine River: contribution from urban sources in the Paris megacity. Environ Sci Water Res Technol 8:2358–2372. https://doi.org/10.1039/D2EW00253A
Pandey PK, Kass PH, Soupir ML, Biswas S, Singh VP (2014) Contamination of water resources by pathogenic bacteria. AMB Express 4:51. https://doi.org/10.1186/s13568-014-0051-x
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590-596. https://doi.org/10.1093/nar/gks1219
R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-Proj.org
Raimonet M, Cazier T, Rocher V, Laverman AM (2017) Nitrifying kinetics and the persistence of nitrite in the Seine River. Fr J Environ Qual 46:585–595. https://doi.org/10.2134/jeq2016.06.0242
Rath KM, Fierer N, Murphy DV, Rousk J (2019) Linking bacterial community composition to soil salinity along environmental gradients. ISME J 13:836–846. https://doi.org/10.1038/s41396-018-0313-8
Rhymes J, Jones L, Lapworth DJ, White D, Fenner N, McDonald JE, Perkins TL (2015) Using chemical, microbial and fluorescence techniques to understand contaminant sources and pathways to wetlands in a conservation site. Sci Total Environ 511:703–710. https://doi.org/10.1016/j.scitotenv.2014.12.085
Rocher V, Laverman AM, Gasperi J, Azimi S, Guérin S, Mottelet S, Villières T, Pauss A (2015) Nitrite accumulation during denitrification depends on the carbon quality and quantity in wastewater treatment with biofilters. Environ Sci Pollut Res 22:10179–10188. https://doi.org/10.1007/s11356-015-4196-1
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Savio D, Sinclair L, Ijaz UZ, Parajka J, Reischer GH, Stadler P, Blaschke AP, Blöschl G, Mach RL, Kirschner AKT, Farnleitner AH, Eiler A (2015) Bacterial diversity along a 2600 km river continuum: river bacterioplankton diversity. Env Microbiol 17:4994–5007. https://doi.org/10.1111/1462-2920.12886
Schmidt TM (2006) The maturing of microbial ecology. Int Microbiol off J Span Soc Microbiol 9:217–223
Servais P, Billen G, Garcia-Armisen T, George I, Goncalves A, Thibert S, (2009) La contamination microbienne dans le bassin de la Seine, Programme PIREN-Seine. Agence de l’eau Seine-Normandie
Shang J, Zhang W, Gao Y, Li Y, Wu H (2023) Dam-induced flow alternations drive the regime shift towards a cyanobacteria-dominated microbiota state in the Yangtze River. Water Res 244:120527. https://doi.org/10.1016/j.watres.2023.120527
Shanks OC, Kelty CA, Archibeque S, Jenkins M, Newton RJ, McLellan SL, Huse SM, Sogin ML (2011) Community structures of fecal bacteria in cattle from different animal feeding operations. Appl Environ Microbiol 77:2992–3001. https://doi.org/10.1128/AEM.02988-10
Shao K, Yao X, Xie G, Wu Y, Hu Y, Tang X, Gao G (2019) Detectable levels of bacterial pathogens in the rivers of the lake Chaohu Basin, China. Int J Environ Res Public Health 16:4857. https://doi.org/10.3390/ijerph16234857
Soto Ramos IM, Crooke B, Seegers B, Cetinić I, Cambazoglu MK, Armstrong B (2023) Spatial and temporal characterization of cyanobacteria blooms in the Mississippi Sound and their relationship to the Bonnet Carré Spillway openings. Harmful Algae 127:102472. https://doi.org/10.1016/j.hal.2023.102472
Spänhoff B, Bischof R, Böhme A, Lorenz S, Neumeister K, Nöthlich A, Küsel K (2007) Assessing the impact of effluents from a modern wastewater treatment plant on breakdown of coarse particulate organic matter and benthic macroinvertebrates in a lowland river. Water Air Soil Pollut 180:119–129. https://doi.org/10.1007/s11270-006-9255-2
Staley C, Unno T, Gould TJ, Jarvis B, Phillips J, Cotner JB, Sadowsky MJ (2013) Application of Illumina next-generation sequencing to characterize the bacterial community of the Upper Mississippi River. J Appl Microbiol 115:1147–1158. https://doi.org/10.1111/jam.12323
Sun W, Xia C, Xu M, Guo J, Sun G (2017) Seasonality affects the diversity and composition of bacterioplankton communities in Dongjiang River, a drinking water source of Hong Kong. Front Microbiol 8:1644
Sveen TR, Hannula SE, Bahram M (2024) Microbial regulation of feedbacks to ecosystem change. Trends Microbiol 32:68–78. https://doi.org/10.1016/j.tim.2023.06.006
Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol Biochem 34:387–401. https://doi.org/10.1016/S0038-0717(01)00199-7
Tu C, Jin Z, Che F, Cao X, Song X, Lu C, Huang W (2022) Characterization of phosphorus sorption and microbial community in lake sediments during overwinter and recruitment periods of cyanobacteria. Chemosphere 307:135777. https://doi.org/10.1016/j.chemosphere.2022.135777
Turner S, Pryer KM, Miao VPW, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA Sequence analysis. J Eukaryot Microbiol 46:327–338. https://doi.org/10.1111/j.1550-7408.1999.tb04612.x
Waiser MJ, Tumber V, Holm J (2011) Effluent-dominated streams. Part 1: Presence and effects of excess nitrogen and phosphorus in Wascana Creek, Saskatchewan. Can Environ Toxicol Chem 30:496–507. https://doi.org/10.1002/etc.399
Wang Y, Sheng H-F, He Y, Wu J-Y, Jiang Y-X, Tam NF-Y, Zhou H-W (2012) Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl Environ Microbiol 78:8264–8271. https://doi.org/10.1128/AEM.01821-12
Wang Y, Liu L, Chen H, Yang J (2015) Spatiotemporal dynamics and determinants of planktonic bacterial and microeukaryotic communities in a Chinese subtropical river. Appl Microbiol Biotechnol 99:9255–9266. https://doi.org/10.1007/s00253-015-6773-0
Wang P, Chen B, Yuan R, Li C, Li Y (2016) Characteristics of aquatic bacterial community and the influencing factors in an urban river. Sci Total Environ 569–570:382–389. https://doi.org/10.1016/j.scitotenv.2016.06.130
Wang L, Zhang J, Li H, Yang H, Peng C, Peng Z, Lu L (2018) Shift in the microbial community composition of surface water and sediment along an urban river. Sci Total Environ 627:600–612. https://doi.org/10.1016/j.scitotenv.2018.01.203
Wang H, Liu X, Wang Y, Zhang S, Zhang G, Han Y, Li M, Liu L (2023) Spatial and temporal dynamics of microbial community composition and factors influencing the surface water and sediments of urban rivers. J Environ Sci 124:187–197. https://doi.org/10.1016/j.jes.2021.10.016
Weiskopf SR, Rubenstein MA, Crozier LG, Gaichas S, Griffis R, Halofsky JE, Hyde KJW, Morelli TL, Morisette JT, Muñoz RC, Pershing AJ, Peterson DL, Poudel R, Staudinger MD, Sutton-Grier AE, Thompson L, Vose J, Weltzin JF, Whyte KP (2020) Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci Total Environ 733:137782. https://doi.org/10.1016/j.scitotenv.2020.137782
Xie Y, Fan J, Zhu W, Amombo E, Lou Y, Chen L, Fu J (2016) Effect of heavy metals pollution on soil microbial diversity and bermudagrass genetic variation. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00755
Yuan S, Zhang W, Li W, Li Z, Wu M, Shan B (2023) Shifts in the bacterial community caused by combined pollutant loads in the North Canal River. China J Environ Sci 127:541–551. https://doi.org/10.1016/j.jes.2022.05.026
Zar JH (2014) Biostatistical analysis. Pearson Education Limited, London
Zhao Z, Zhao R, Qiu X, Wan Y, Lee L (2022) Structural diversity of bacterial communities and its relation to environmental factors in the surface sediments from main stream of Qingshui River. Water 14:3356. https://doi.org/10.3390/w14213356
Acknowledgements
This research was supported by the MeSeine Innovation programme. We would like to express our gratitude to the SIAAP technical team for their help in sampling and carrying out physicochemical measurements.We thank to the MIGALE bioinformatics center at INRAE (MIGALE, INRAE, 2020. Migale bioinformatics Facility, https://doi.org/10.15454/1.5572390655343293E12) for providing help and/or computing and/or storage resources.We thank to the PRAMMICs platform (OSU EFLUVE—UPEC) for making of the Safac dispositif.
Funding
Open access funding provided by Université Paris-Est Créteil.
Author information
Authors and Affiliations
Contributions
Sadia Bagagnan: conceptualization, methodology, data analysis, drafting of the original version; My Dung Jusselme: supervision, conceptualization, methodology, data analysis, drafting, revision & editing; Régis Moilleron: conceptualization, supervision, drafting, revision & editing, funding acquisition; Anthony Marconi: supervision and investigation; Sabrina Guérin-Rechdaoui: supervision and investigation; Vincent Rocher: supervision and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that might appear to influence the work reported in this paper.
Additional information
Handling Editor:
Man Xiao
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bagagnan, S., Guerin-Rechdaoui, S., Marconi, A. et al. Overview of microbial communities in the surface water of the Seine River to understand their response to climate change and human activities. Aquat Ecol (2024). https://doi.org/10.1007/s10452-024-10124-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10452-024-10124-3