Abstract
We quantified the feeding behavior (encounter, attack, capture and ingestion) and demographic parameters (survival and reproduction) of the predatory rotifer Asplanchna sieboldii fed on the prey Plationus patulus previously exposed to microplastics (MPs), Cd or their combination. As compared to controls, capture and ingestion rates of P. patulus by A. sieboldii decreased by 71 and 61%, respectively, with prey previously exposed to mixed MPs and Cd treatment. Life table data showed that the predator died earlier in controls than when fed on prey exposed to both Cd and microplastics. Regardless of the prey treatment, the offspring production by A. sieboldii increased as the available prey numbers increased (from 1 to 4 ind. ml−1). Compared to controls, the fecundity rate of the predator decreased when contaminated prey was offered as food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epicontinental aquatic ecosystems, more than other natural environments, are exposed constantly to pollutants, because freshwater is used for various industrial and urban activities and receives a wide variety of wastes (Demirak et al. 2006). In addition, runoffs during the rainy season of the irrigated lands transport toxicants, which indirectly affect aquatic systems (Mateo-Sagasta et al. 2017). Aquatic ecosystems an withstand toxicants, but excessive discharge of pollutants interferes with biological interactions (Beeby 1993). Many pollutants threaten the health of different groups of aquatic organisms from bacteria to fishes; detergents, fertilizers, insecticides and heavy metals affects aquatic life. Plastic pollution also deteriorates water quality (Abowei and Sikoki 2005).
Global plastic production has been steadily increasing, almost exponentially for the last six decades, leading to 6300 million tons of plastic waste of which only < 10% has been recycled (Greyer et al. 2017; Plastics Europe 2019). Polystyrene is one of the main components of plastics, and because of mismanagement or lack of appropriate technology to recycle plastic wastes, this pollutant reaches aquatic ecosystems as large and small microplastics (Dris et al. 2018; Eerkes-Medrano et al. 2015).
Microplastics are emerging pollutants consisting of a wide variety of small particles and synthetic polymer fibers with diameters varying from 100 nm to about 5 mm (Lusher et al. 2017). Because of their small size, microplastics are ingested by a great diversity of aquatic fauna at different trophic levels (Hall et al. 2015; Alfonso et al. 2020). Birds that feed on fish or other aquatic prey suffer from plasticosis (Charlton-Howard et al. 2023). An additional impact of microplastics on aquatic fauna is associated with their ability to adsorb organic and inorganic chemicals from the ambient waters (Bakir et al. 2014; Llorca et al. 2018).
Cadmium (Cd) is one of the most toxic heavy metals with no known biological role. Despite this, it is common in the aquatic environment after being released from alloys, galvanization, pigments, batteries, and plastics (Wright and Welbourn 2002; Yang et al. 2018). Because species of zooplankton in nature are exposed to both microplastics and cadmium compounds, ecotoxicological evaluations are needed using their combined effects and such studies are rare in literature (Snell and Joaquim-Justo 2007).
The interaction between contaminants can vary in different ways. Antagonistic interactions between selenium and mercury have been reported where the former, an essential metal, reduces the toxicity of the latter (Khan and Wang 2009). In some other cases, the contaminants produce a synergistic effect reported in study on microplastics contaminated with BP-3 (Na et al. 2020). Microplastics with other toxicants combined may have an additive effect. Hence, microplastics affect the aquatic organisms depending on the other contaminants with which they interact (Beiras et al. 2018).
Rotifers are an important component of freshwater zooplankton; they belong to the phylum Rotifera with more than 2100 species (Wallace et al. 2019). Given their ecological importance in the aquatic food chain and ease of handling in the laboratory, they are widely used as bioassay organisms (Wallace et al. 2006). Behavioral traits such as the feeding rates and demographic variables such as lifespan and reproductive rates are sensitivite to toxicant stress. Sublethal toxicity tests mainly consider life history variables (Snell and Joaquim-Justo 2007; Guo et al. 2012).
Though most rotifers are primary consumers, there are also a few secondary consumers in the phylum Rotifera. For example, predatory species of the genus Asplanchna are common in zooplankton communities (Gilbert 2014). Asplanchna is not a visual predator, but has mechano-receptors to sense its prey. Hence, its feeding behavior is essentially a function of encounter probability. Hence, the relationships among the number of attacks, captures and ingestions that follow random prey encounters, as well as the selectivity based on the chemoreceptors on the ciliary corona often determine the survival of Asplanchna in nature (Gilbert 1980a; Iyer and Rao 1996).
Contaminants also interfere in the feeding process of non-visual predators. For example, inert substances such as soil particles, and detritus consisting of fragments of diatoms are known to decrease the feeding efficiency of rotifers (Gilbert 2022). Different studies also show that microplastics have an indirect effect on food ingestion, assimilation and population growth of planktonic rotifers (Drago and Weithoff 2021; Morgalev et al. 2022). Species of Asplanchna are also affected by the presence of large (< 30 µm) inorganic particles and microplastics that offer no energy to the predators, but occupy space in gut filling. From an ecological perspective, quantitative changes in the feeding behavior of Asplanchna indicate the consequences of its demographic responses when the predators encounter prey contaminated with heavy metals such as cadmium and microplastics. Therefore, Asplanchna exposed to combined effects of microplastics, and cadmium would present quantifiable changes in the demography over time. The life table approach allows quantifying the numerical characteristics of mortality and reproduction in an age-specific way, and this helps to detect non-random sources of death of a cohort population (Krebs 1985).
Therefore, the objective of this study was to evaluate the effect of prey (Plationus patulus (Müller, 1786)) previously exposed to cadmium and microplastics on the feeding behavior and demography of Asplanchna sieboldii (Leydig, 1854) using the life table method.
Materials and methods
Isolation and culture of test species
The herbivorous prey rotifer P. patulus has been cultured in the laboratory for many years. The original strain was isolated from Lake Xochimilco (Mexico City, Mexico). Monoclonal culture of P. patulus was established from a single parthenogenetic female and fed the single-celled alga, Chlorella vulgaris as food. The density of alga chosen for maintaining stock cultures of P. patulus and for the experiments was 1 × 106 cells mL−1 and moderately hard water as medium (EPA medium). The EPA medium was prepared by dissolving 1.9 g of NaHCO3, 1.2 g of CaSO4, 1.2 of MgSO4 and 0.04 g of KCl in 20 L of distilled water (Weber 1993). Chlorella vulgaris was cultured in transparent 2 L culture jars using Bold basal medium (Borowitzka and Borowitzka 1988) supplemented with 0.5 g L−1 of NaHCO3 after every 3rd day. When the alga was in the logarithmic growth phase, it was harvested, centrifuged at 3000 rpm for 5 min. and then resuspended in a small volume distilled water. The concentrated alga was stored at 4 °C until use. To maintain favorable conditions for P. patulus growth, the culture medium was 100% replaced after every 2 days. The pH of the culture medium was maintained at 7.0–7.3. Temperature 25 ± 1 °C and continuous and diffuse light were set for rotifer cultures and for experiments.
The predatory rotifer Asplanchna sieboldii was also isolated from Lake Xochimilco and a monoclonal culture was established starting with a parthenogenetic female providing mixed diets of two brachionid rotifers (Brachionus havanaensis Rousselet, 1911 and Brachionus calyciflorus Pallas, 1766) as food. To prevent sexual reproduction of A. sieboldii in the stock cultures, the predator densities were kept low and the culture medium was changed daily. The other test conditions were similar to those of P. patulus.
Polystyrene microspheres
Polystyrene (PS) microparticles with a diameter of 30 μm were acquired from Sigma-Aldrich (Batch BCCC0789). Using the appropriate dilution using distilled water, the quantity of PS was determined using a Sedgewick–Rafter chamber following the safety instructions of the manufacturer. A stock solution of 40 mg L−1 was prepared. The stock solution was subject to ultrasonication at 20 kHz 10 watts for 3 min for homogenization. This procedure was carried out every time the solution was used for the experiments.
Cadmium stock solution
A stock solution of 1 g L−1 of CdCl2 2.5 H2O (Fermont, Batch: 80,702, purity 99.7%) was prepared in a 100 mL volumetric flask with distilled water so that each mL of the solution contained 1 mg of Cd. Previously, all the glassware that was to be used for the tests was cleaned thoroughly (Moody and Lindstrom 1977).
Feeding behavior of A. sieboldii
The feeding behavior of Asplanchna sieboldii was evaluated using twenty-one individuals of P. patulus as prey (7 ind. mL−1) and one predator in petri dishes containing 3 mL of EPA medium. Low density (0.01 × 106 cells mL−1 ) of C. vulgaris added to the medium to keep the prey individuals active. Pre-starved (3 h) adult A. sieboldii, campanulate morphotype, individuals were used as predators. The prey individuals were exposed for 1 h to one of the two sublethal concentrations of Cd (10 and 20% of LC50, 0.009 and 0.018 mg L−1, respectively) and one concentration of microplastics (5 mg L−1). For each treatment, there were 5 replicates. Ten-minute feeding observations were made for quantifying the following parameters: Encounter (E), when there is detection by the predator of the prey; Attack (A), when the predator tries to capture the prey; Capture (C), when the predator holds the prey; Ingestion (I), when the predator consumes the prey (Gilbert 1980a).
The number of E, A, C and I events was statistically evaluated with a one-way ANOVA and Tukey post-hoc tests.
Life table study
Life table demography experiments were conducted in 25-mL capacity glass jars containing 20 mL of the medium. In all, there were 90 test jars (3 prey densities × 6 treatments × 5 replicates). Into each jar we introduced the prey P. patulus of chosen density (1, 2 or 4 ind. mL−1) that was previously exposed to the selected Cd levels (10 or 20% of LC50) for 24 h and a neonate of A. sieboldii (< 3 h) that was never exposed to either microplastics or Cd. The test jars also received alga at 1 × 104 cells mL−1 to keep the prey active. These experiments were conducted at 25 ± 1 °C with continuous, diffuse illumination. Following initiation of life table study, after every 12 h, neonates born if any, from each replicate were counted and discarded. After every 24 h, the surviving predators were transferred to fresh jars containing appropriate test conditions. The experiment was discontinued when the predator in each replicate died. Demographic variables were calculated according to Krebs (1985). For lx and mx data, we derived mean ± SE values using 5 replicates. However, for other variables (average lifespan, life expectancy at birth (E0), gross reproductive rate (GRR), net reproductive rate (NRR), generation time (GT) and the rate of population increase (r per day), we used jackknife method (Meyer et al. 1986) to derive mean ± SE. This was necessary because Asplanchna sieboldii is cannibalistic and feeds on congeners, so life table experiments were initiated with single individuals (1980a), after which the jackknife method was used to pool and analyze the data. Replicates where no reproduction occurred were eliminated from the analyses.
The life table variables were statistically tested using one-way ANOVA for each prey density. For multiple comparisons within a prey density treatment, we used post-hoc Tukey tests. Statistical evaluations were carried out following Kaptein and van den Heuvel (2022) and SigmaPlot version 11.
Results
Feeding rates
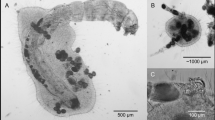
The presence of microplastic spheres in the body of P. patulus was observed. However, only a few microplastic spheres were seen in the rotifer gut. Data on the frequency of feeding events by A. sieboldii (mean ± SE), Encounter, Attack, Capture and Ingestion are presented in Fig. 1. The number of encounters and attacks by the predator on its prey P. patulus exposed to one or the combination of contaminants (microplastics or Cd) was not significantly different. However, the capture decreased significantly (p < 0.05, ANOVA; Table 1) with prey previously exposed to sublethal concentrations of cadmium and microplastics. With the prey exposed to both pollutants, the lowest values of capture and ingestion were obtained, which were lower by 71 and 61%, respectively, as compared to the control.
Life table data
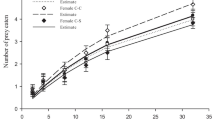
Survivorship curves of A. sieboldii showed that when fed on the prey previously exposed to 10%Cd, its survival increased compared to the higher concentration of Cd or controls. In treatments where prey were exposed previously to both Cd and microplastics, the predator population died earlier than in other treatments (Fig. 2). The offspring production in A. sieboldii started after 24 h. In general, in controls and treatments with Cd and microplastics, the offspring production increased as the available prey numbers increased. Compared to controls, the fecundity rate of the predator decreased when the contaminated prey was offered as food (Fig. 3).
The average lifespan of A. sieboldii ranged from 3.3 to 5.2 days and life expectancy at birth ranged from 2.8 to 4.5 days. Under all the three offered prey densities, both average lifespan and life expectancy at birth of the predator were significantly affected (p < 0.05, Tukey test) when fed prey previously exposed to 20%Cd + MPs or 20%Cd only. However, at the prey densities of 1 and 2 ind. mL−1 and at lower levels of Cd (10%) level, there was a significant increase in both these variables. The reproductive period decreased with increasing concentration of cadmium (from 10 to 20%). Both gross (GRR) and net reproductive (NRR) rates of predators ranged from 1 to 8 offspring female−1 lifespan−1. Higher GRR and NRR were recorded in controls when fed prey at the density of 4 ind. mL−1. Generation time (GT) varied from 2–3 days, depending on treatment. Single and combined contaminants caused significant decrease (< 0.05, Tukey test) in the reproductive rates (GRR and NRR) of the predator even at the highest offered prey density (4 ind. mL−1). The population growth rate of A. sieboldii in controls ranged from 0.12 to 0.55 day−1 while in treatments with contaminant-exposed prey, it ranged from 0.15 to 0.99 d−1 (Table 2).
Discussion
The effects of interactions between contaminants such as metals (Sarma et al. 2006), pesticides (Faust et al. 1994) and microplastics with other contaminants (Rehse et al. 2018) are commonly analyzed by rapid acute toxicity tests (LC50) and at a single food chain level, usually herbivores (Wallace et al. 2006). The LC50 24 h of Cd obtained in this work was 0.9 mg L−1, which was close to that reported in literature (Snell and Joaquim-Justo 2007). With only MPs, P. patulus had a LC50 of 90 mg L−1. Therefore, we used for cadmium 10 and 20% of LC50 and for MPs 10% of the LC50. Despite the useful information generated from chronic trials (Liang et al. 2021), there are few studies that have evaluated the ecotoxicological effects on diet exposed to pollutants (Sarma et al. 1998). Although some studies on the food chain effects of toxicants are available in literature (Van Kirk and Hill 2007), less information is available on the effects of two pollutants at higher trophic levels such as the results of the present study.
The prey rotifer P. patulus had only a few microspheres probably because the size of the spheres used here, although large-bodied rotifers of Brachionidae are capable of feeding on food particles up to 50 µm (Gilbert 2022). We did not quantify the MPs in the gut of either prey or the predator.
Regarding the interactions of A. sieboldii and its contaminated prey, there are five important variables which determine the feeding behavior of an aquatic predator: (1) the swimming speed of prey, (2) the size of the prey, (3) the swimming speed of the predator, (4) density of the food and (5) the visual field of the predator (Salt 1987). There were no significant differences in the encounter rates between the predator and its prey that was exposed to the two contaminants at the concentrations used in this study. This implies that there was possibly no significant change in the swimming pattern of P. patulus. As for the swimming speed of the predator, A. sieboldii, all individuals were of bell-shaped morphotype and were not directly exposed to contaminants. So, the changes in the number of attacks, captures and ingestion could depend on the visual field of the predator. However, Asplanchna is a non-visual predator, and its receptive field depends on the sensory organs that detect the vibrations in the medium produced by the prey (Dumont 1977). The chemo- and mechano-receptors are located around the ciliary corona of the predator have a role in these feeding responses (Gilbert 1980b). Decreased capture and ingestion of the prey treated with cadmium and microplastics was most evident when the predator was offered P. patulus with combined contaminants, as reported in previous studies (Joanidopoulos and Marwan 1998; Liess et al. 2020). It has been shown that A. sieboldii did not capture its clones and congeners despite constantly encountering them (Gilbert 1977, 1980a). However, our study is one of the few studies where the possible action of these chemo- and mechano-receptors failed to capture and ingest prey when contaminated with toxicants. This might be due to the lower levels of contaminants used here.
In the life table variables, it was recorded that survival increased with 10% Cd and was affected by the presence of MPs and the combination of both contaminants. Although fecundity of the predator increased when fed on the prey exposed to the combination of pollutants, this had generated a negative effect on further survival, i.e., a higher energy cost of reproduction leading to reduced life expectancy. This response has been reported for several herbivorous rotifer species, from both freshwater and marine ecosystems (Xiao-Ping et al. 2020; Zhang et al. 2021). Population growth rates showed that A. sieboldii fed on prey exposed to the combination of pollutants possibly accelerated their metabolism, increased their offspring but reduced life expectancy. The higher offspring production under lower stressful levels of toxicants, including heavy metals, is the hormetic effect possibly to overcompensate the stress effects (Tang et al. 2019). Hormetic effects have some important consequences in the community structure of zooplankton, which are often under-reported (Calabrese and Mattson 2017). These variations make populations more susceptible, especially across generations (González-Pérez et al. 2021).
The statistical analysis showed that the toxicants interactions (Cd and/or MPs) with prey density affected the survival and reproduction of predator A. sieboldii. This suggests that possibly P. patulus transfers Cd, MPs or their combination to its predators (Drago and Weithoff 2021). Information on the accumulation and/or the transfer of pollutants within a single zooplankton group such as rotifers suggests the ecological relevance of Asplanchna. This work highlights that microplastics and heavy metals together affect the ingestion and demography of predatory A. sieboldii through the prey P. patulus. Gama-Flores et al. (2007) have found a similar response, as reported here, where the demography of the predatory A. brightwellii was affected through ingesting prey exposed to copper and cadmium. Sarma et al. (1998) have also reported that Asplanchna brightwellii was negatively affected when fed on prey previously exposed to a pesticide.
Conclusions
For Mexican freshwater ecosystems, the Official Mexican Standard NOM-001-ECOL-1996 allows a maximum discharge of 200 μg L−1 of Cd, and for microplastics, there is still no maximum permissible limit. The Cd concentrations used in this work have been recorded in freshwater ecosystems with values < 20 μg L−1 of Cd (Bervoets et al. 2009), and 10 mg L−1 of microplastics (Dong et al. 2020). For the experiments conducted in this work, the prey P. patulus was exposed to concentrations that are within the permissible limits of Cd and natural levels of microplastics. It is evident that there are adverse effects of microplastics and cadmium on the feeding behavior and demography of the predator A. sieboldii. This study further suggests that Cd and MPs affect the prey capturability by Asplanchna. Predator’s survival, reproduction and population growth rates were affected due to the presence of Cd, microplastics or their mixture. Further studies are needed to quantify the biotransfer/bioaccumulation of the chosen contaminants in the predator–prey interactions within the Rotifera. For such studies, life table approach may not provide enough material for chemical analysis. Other population level studies such as population dynamics of predatory rotifers will yield sufficient biomass for bioaccumulation/biotransfer studies.
Data availability
The data presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
Abowei JFN, Sikoki FD (2005) Management and control of water pollution double trust. Publications Company, Port Harcourt, Nigeria, p 236
Alfonso MB, Scordo F, Seitz C, Manstretta GMM, Ronda AC, Arias AH, Tomba JP, Silva LI, Perillo GME, Piccolo MC (2020) First evidence of microplastics in nine lakes across Patagonia (South America). Sci Total Environ 733:139385. https://doi.org/10.1016/j.scitotenv.2020.139385
Bakir A, Rowland SJ, Thompson RC (2014) Transport of persistent organic pollutants by microplastics under estuarine conditions. Estuary Coast Shelf Sci 140:14–21
Beeby A (1993) Applying ecology. Chapman and Hall, London/New York, p 441
Bervoets L, Van Campenhout K, Reynders H, Knapen D, Covaci A, Blust R (2009) Bioaccumulation of microcontaminants and biomarker responses in caged carp (Cyprinus Carpio). Ecotoxicol Environ Saf 72(3):720–728
Borowitzka MA, Borowitzka LJ (1988) Micro-algal Biotechnology. Cambridge University Press, Cambridge, UK, p 477
Calabrese EJ, Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis 3:13. https://doi.org/10.1038/s41514-017-0013-z
Charlton-Howard HS, Bond AL, Lavers R-AJ (2023) ‘Plasticosis’: characterising macro- and microplastic-associated fibrosis in seabird tissues. J Hazard Mater 450:131090
Demirak A, Yilmaz F, Tuna AL, Ozdemir N (2006) Heavy metals in water, sediments and Leuciscus cephalus of a stream in southwestern Turkey. Chemosphere 63(9):1451–1458
Dong M, Luo Z, Jiang Q, Xing X, Zhang Q, Sun Y (2020) The rapid increase of microplastics in the lake’s urban sediments. Sci Rep 10:848
Drago C, Weithoff G (2021) Variable fitness response of two rotifer species exposed to microplastics particles: the role of food quantity and quality. Toxics 9(11):305
Dris R, Imhof HK, Löder MGJ, Gasperi J, Laforsch C, Tassin B (2018) Microplastic pollution in freshwater systems: methodological challenges, occurrence and sources. In: Zeng EY (ed) Contamination by microplastics in aquatic environments, an emerging matter of environmental urgency. Elsevier, Amsterdam, pp 51–93
Dumont HJ (1977) Biotic factors in the population dynamics of rotifers. Arch Hydrobiol Beih 8:98–122
Eerkes-Medrano D, Thompson RC, Aldridge DC (2015) Microplastics in freshwater systems—a review of emerging threats, identification of knowledge gaps, and prioritization of research needs. Water Res 75:63–82
Plastics Europe (2019) Plastics—The Facts 2019. https://www.plasticseurope.org/it/ resources/publications/1804-plastics-facts-2019, 42 pp. https://www.plasticseurope.org/it/resources/publications/1804-plastics-facts-2019
Faust M, Altenburger R, Boedeker W, Grimme LH (1994) Algae toxicity of binary combinations of pesticides. Bull Environ Pollut Toxicol 53(1):134–141
Gama-Flores JL, Castellanos-Páez ME, Sarma SSS, Nandini S (2007) Effect of pulsed exposure to heavy metals (copper and cadmium) on some population variables of Brachionus calyciflorus Pallas (Rotifera: Brachionidae: Monogononta). Hydrobiologia 593(1):201–208
Geyer R, Jambeck JR, Law KL (2017) Production, use and destination of all plastics ever manufactured. Adv Sci 3(7):e1700782
Gilbert JJ (1977) Control of feeding behavior and selective cannibalism in the rotifer Asplanchna. Freshwat Biol 7:337–341
Gilbert JJ (1980a) Feeding in the rotifer Asplanchna: behaviour, cannibalism, selectivity, prey defenses and impact on rotifer communities. In: Kerfoot WC (ed) Evolution and Ecology of Zooplankton communities. The University Press of New England, Hanover, pp 158–172
Gilbert JJ (1980b) Developmental polymorphism in the rotifer Asplanchna sieboldi: three distinct female morphotypes, controlled by the level of vitamin E in the diet, or tocopherol, allow this small aquatic organism to adapt quickly to environmental changes. Am Sci 68(6):636–646
Gilbert JJ (2014) Morphological and behavioral responses of a rotifer to the predator Asplanchna. J Plankton Res 36:1576–1584
Gilbert JJ (2022) Food niches of planktonic rotifers: Diversification and implications. Limnol Oceanogr 67:2218–2251
González-Pérez BK, Sarma SSS, Castellanos-Páez ME, Nandini S (2021) Effects of the endocrine disruptor 4-nonylphenol on the demography of rotifers Plationus patulus and Brachionus havanaensis: a multigenerational study. J Environ Sci Health, Part A 56(13):1357–1366. https://doi.org/10.1080/10934529.2021.1997281
Guo R, Ren X, Ren H (2012) Evaluation of the toxic effects of dimethoate to rotifer using swimming behavior. Bull Env Cont Toxicol 89:568–571
Hall NM, Berry KLE, Rintoul L, Hoogenboom MO (2015) Ingestion of microplastics by scleractine corals. Mar Biol 162:725–732
Iyer N, Rao TR (1996) Predatory rotifer responses Asplanchna intermedia to prey species that differ in vulnerability: laboratory and field studies. Freshw Biol 36(3):521–533
Joanidopoulos KD, Marwan W (1998) Specific behavioural responses triggered by identified mechanosensory receptor cells in the apical field of the giant rotifer Asplanchna sieboldi. J Exp Biol 201:169–177
Kaptein M, van den Heuvel E (2022) Statistics for data scientists undergraduate topics in computer science. Springer, New York, p 321
Khan MAK, Wang F (2009) Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 28(8):1567–1577
Krebs CJ (1985) Ecology; experimental analysis of distribution and abundance, 3rd edn. Harper & Row, New York, p 592
Liang Y, Yang X, Wang Y, Liu R, Gu H, Mao L (2021) Influence of polystyrene microplastics on rotifer (Brachionus calyciflorus) growth, reproduction, and antioxidant responses. Aquat Ecol 55:1097–1111
Liess M, Henz S, Shahid N (2020) Modeling the synergistic effects of toxicant mixtures. Environ Sci Eur 32:119. https://doi.org/10.1186/s12302-020-00394-7
Llorca M, Schirinzi G, Martínez M, Barceló D, Farré M (2018) Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ Pollut 235:680–691
Lusher AL, Hollman PCH, Mendoza-Hill JJ (2017) Microplastics in fisheries and aquaculture. Status of knowledge on their occurrence and implications for aquatic organisms and food safety. FAO Technical Paper on Fisheries and Aquaculture No. 615. Rome, p 147
Mateo-Sagasta J, Zadeh SM, Turral H, Burke J (2017) Water pollution from agriculture: a global review. Executive summary. FAO, Rome, p 29
Meyer JS, Ingersoll CG, McDonald LL, Boyce MS (1986) Estimation of uncertainty in population growth rates: jackknife vs. bootstrap techniques. Ecology 67:1156–1166
Moody JR, Lindstrom RM (1977) Selection and cleaning of plastic containers for the storage of trace element samples. Anal Chem 49:2264–2267
Morgalev Y, Dyomin V, Morgalev S, Davydova A, Morgaleva T, Kondratova O, Polovtsev I, Kirillov N, Olshukov A (2022) Environmental contamination with micro- and nanoplastics changes the phototaxis of euryhaline zooplankton to paired photostimulation. Water 14(23):3918
Rehse S, Kloas W, Zarfl C (2018) Microplastics reduce the short-term effects of environmental pollutants. Part I: the effects of bisphenol a on freshwater zooplankton are lower in the presence of polyamide particles. Int J Environ Res Public Health 15(2):280
Salt GW (1987) The components of feeding behavior in rotifers. Hydrobiologia 147:271–281
Sarma SSS, Nandini S, Araiza MAF (1998) Effect of methyl parathion-treated prey (Brachionus calyciflorus) on the population growth of the predator Asplanchna sieboldi (Rotifera). Bull Environ Contam Toxicol 61(1):135–142
Sarma SSS, Martínez-Jerónimo R-PT, Nandini S (2006) Effect of cadmium and chromium toxicity on the demography and population growth of Brachionus calyciflorus and Brachionus patulus (Rotifera). J Environ Sci Health Part A 41(4):543–558
Snell TW, Joaquim-Justo C (2007) Workshop on rotifers in ecotoxicology. Hydrobiologia 593:227–232
Tang S, Liang J, Xiang C, Xiao Y, Wang X, Wu J, Li G, Cheke RA (2019) A general model of hormesis in biological systems and its application to pest management. J R Soc Interface 16(157):20190468
Van Kirk RW, Hill SL (2007) Demographic model predicts trout population response to selenium based on individual-level toxicity. Ecol Model 206:407–420
Wallace RL, Snell WT, Nogrady T (2006) Rotifera biology, ecology and systematics. Kenobi Productions, Ghent & Backhuys, Kerkwerve, Netherlands, p 299
Wallace RL, Snell TW, Walsh EJ, Sarma SSS, Segers H (2019) Chapter 8. Phylum Rotifera. Keys to Palaearctic Fauna. In: Thorp and Covich’s Freshwater Invertebrates. Volume IV. Fourth Edition. Academic Press/Elsevier. 219–267 https://doi.org/10.1016/B978-0-12-385024-9.00008-3
Weber CI (1993) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 4th ed. United States Environmental Protection Agency, Cincinnati, Ohio, EPA/600/4–90/027F, xv + 293 pp
Wright DA, Welbourn P (2002) Metals and other inorganic chemicals. In: Wright DA, Welbourn P (eds) Environmental toxicology. Cambridge University Press, Cambridge, pp 249–348
Xiao-Ping X, Yi-Long X, Xing-Ming W (2020) Effects of DDT and dicofol on the demographics of the life table of freshwater rotifer Brachionus calyciflorus Pallas. Pol J Environ Stud 29(2):1945–1951
Yang J, Xie Y, Jeppe K, Long S, Pettigrove V, Zhang X (2018) Sensitive community responses of microbiota to copper in sediment toxicity test. Toxicol Chem 37(2):599–608
Zhang X, Tang X, Yang Y, Sun Z, Ma W, Tong X, Wang C, Zhang X (2021) Responses of reproduction, population growth and metabolome of the marine rotifer Brachionus plicatilis to tributylphosphate (TnBP). Environ Pollut 273:116462
Acknowledgements
Two anonymous reviewers have improved our presentation. JAH-L received a scholarship from CONAHCyT (Mexico). This work was supported by UNAM-PAPIIT- IG200820. The APC of this work has been covered by the UNAM-Springer Nature Agreement.
Funding
This work was supported by the Mexican funding agencies: CONACyT (CVU-1046849, SNII-18723, SNII- 20520) and UNAM-PAPIIT- IG200820.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest or competing interests.
Additional information
Handling editor: Télesphore Sime-Ngando, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hernández-Lucero, J.A., Sarma, S.S.S. & Nandini, S. Behavioral and demographic responses of the predatory rotifer Asplanchna sieboldii (Leydig, 1854) fed prey (Plationus patulus (Müller, 1786)) previously exposed to cadmium and microplastics. Aquat Ecol 58, 239–248 (2024). https://doi.org/10.1007/s10452-023-10061-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-023-10061-7