Abstract
Reference gas adsorption isotherms are useful for validating equilibrium data obtained from various techniques and for ensuring that experimental systems are operating correctly. In this work, we extend an interlaboratory study on a NIST reference zeolite (Na-Y, RM8850) to two additional temperatures above and below the original 298.15 K, validating the results via independent measurements using two different techniques. Volumetric experiments on a novel Adsorption Differential Volumetric Apparatus (ADVA-270) were carried out at The University of Edinburgh, and gravimetric experiments were performed at Hiden Isochema using a proprietary XEMIS microbalance. Both techniques provided highly accurate results and an excellent match between the two independent measurements using less than 150 mg of sample. Absolute equilibrium data were modelled using a Langmuir-virial isotherm to obtain an accurate concentration dependence of the heat of adsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several industrial processes operate at high pressure, including, for example, CH4 and H2 separation and storage [1, 2], pre-combustion CO2 capture [3], H2 purification [4], CO2 capture combined with H2 purification [5], air separation and biogas upgrading [6, 7]. In order to reliably design any high pressure process, accurate adsorption isotherms are required. Despite the availability of a significant amount of high pressure data in the literature, round-robin campaigns for H2, CO2 and CH4 have often shown poor reproducibility [8,9,10,11,12,13,14,15,16]. Reviews on the reproducibility of H2 equilibrium measurements have identified and addressed typical experimental pitfalls that can easily be extended to the majority of high pressure measurements [2, 8, 17]. Key weak-points include temperature control and uniformity within the apparatus, pressure transducer calibration, description of gas non-ideality, leaks and gas impurities [2]. These can often be addressed with some simple experimental checks such as blank runs and validation of the measurements against reference isotherms and/or other apparatuses, leading to a significant improvement in the quality of the measurements [2, 8, 17]. Nevertheless, providing a suitable material is selected, interlaboratory exercises to generate reference isotherms are valuable tools that have highlighted the challenges, faced by even the most experienced laboratories, in performing accurate high pressure adsorption measurements [18].
A series of interlaboratory round-robin efforts have been led by the US NIST (National Institute of Standards and Technology) FACT Lab, with the objective of generating reference isotherms on standard adsorbents to calibrate or validate adsorption measurements performed using any instrument [19,20,21]. Among these, a 2020 study was used to generate a reference high pressure CH4 isotherm for the reference material RM8850 (Na-Y zeolite) at 298.15 K. The work saw the involvement of 20 different institutions, producing 28 datasets in total, all generated using either gravimetric (11) or volumetric (16) instruments [19]. The datasets measured using a XEMIS microbalance at the Hiden Isochema laboratories and the ADVA-270 volumetric system at The University of Edinburgh were successfully included among the ones used to generate the reference isotherm.
Table 1 provides a summary of the number of instruments and the range of sample masses used for volumetric and gravimetric experiments. Clearly, the majority of tests were carried out with less than 1 g of material and among those only four systems (all gravimetric) used less than 100 mg. Three of these measured CH4 adsorption below 2 MPa and only the Hiden Isochema XEMIS microbalance was used in the whole pressure range, up to 7.5 MPa. This provides a relevant snapshot of the capabilities of high-pressure systems and how they meet the requirements of novel adsorbents. ADVA-270, using ~ 400 mg of Na-Y, was among the four volumetric apparatuses that used the smallest sample masses. For high accuracy in equilibrium measurements, one should maximise the sample mass used, but on the other hand, prototype adsorbents are often synthesised in small batches of ~ 100 mg or less. New metal–organic frameworks (MOFs), for example, are typically synthesized in small vials, limiting sample sizes to 50–100 mg [22]. New or modified zeolites are more commonly synthesised in research laboratories at double the scale. This allows to minimize the use of corrosive chemicals such as hydrofluoric acid and, if expensive templates and solvents are used, multiple conditions can be explored to optimise the adsorbents or catalysts. Therefore, with the continuous development of new materials, it is equally important that instruments are able to test small sample masses while maintaining high accuracy.
With regard to standard samples, long-term stability and data reproducibility make Na-Y zeolite a very attractive reference material. Despite being a relatively simple adsorption system, however, where a non-polar molecule, CH4, adsorbs on a well-defined zeolite, literature data are limited and reported measurements and simulations show qualitative and quantitative differences in the equilibrium isotherms, heat of adsorption and its concentration dependence [23,24,25,26,27,28,29,30,31].
In this work, we extend the results of the 2020 round-robin study on Na-Y by expanding the temperature range of the CH4 isotherms to temperatures 15 K above and below the reference one. Additional isotherms for the reference material can be used to check potential inaccuracies when temperature control is required, while expanding the isotherm measurements above and below room temperature allows extraction of the temperature dependence of the isotherms. We also present a rigorous thermodynamic analysis of the absolute adsorbed amounts and provide consistent heats of adsorption that can be used to reconcile results found in the literature for the CH4/Na-Y system.
2 Experimental
2.1 ADVA-270
Figure 1 presents the schematic diagram and some instrument details of the ADVA-270. The system is based on the differential apparatus designed by Zielinski et al. [32] as a symmetric double branch volumetric apparatus, where the two symmetrical branches (namely, sample and reference) are connected via a differential pressure transducer. In differential volumetric systems, gas is dosed from two dosing volumes (VDS and VDR) to the two uptake volumes (VUS and VUR). The pressure difference between the sample and the reference side is then used to calculate the amount adsorbed using a mass balance. Relying on the differential pressure transducer ensures the same accuracy in the entire pressure range. The ADVA system has system volumes of approximately 12.5 cm3 each, much smaller than previous differential systems, with a dosing-to-uptake volume ratio of ~ 1. This ensures high sensitivity even when working with small sample masses.
ADVA-270 is equipped with a ± 62 kPa differential pressure transducer (Rosemount 3051CD) and an absolute pressure transducer up to 27.6 MPa (Rosemount 3051 T), both with a ± 0.04% full scale accuracy. To improve further the accuracy, volume calibrations and the low pressure portion of the isotherms were determined using an absolute pressure transducer with a range up to 0.2 MPa. VCR fittings allow for simple interchange of the absolute pressure transducers when needed.
Thermocouples are inserted in each volume to directly monitor the gas temperature inside the system. The uptake temperature is controlled using a thermostatted circulating bath with a weir that ensures that the two branches are submerged to the same level. The dosing section is enclosed in an insulation box to dampen the effects of fluctuations of the external temperature.
Sample activation is carried out in-situ using an external furnace while the sample is kept under vacuum using a Pfeiffer HiCube 80 Eco pump station (down to 10–8 kPa). The regeneration procedure of the sample consisted of a slow temperature ramp of 1 K/min from room temperature to 383 K, with a holding time of 2 h, before a second ramp to 623 K, followed by an overnight hold at this temperature.
To determine reliably the dry mass of the sample, the methodology developed by Hampson and Rees was used [33]. The sample to be tested is taken from a batch of material kept under a controlled humidity environment (75% RH, NaCl saturated solution [34]) from which the percentage of water loss was determined from a thermal gravimetric analysis. The final dry mass loaded in ADVA-270 was 77 mg.
2.2 XEMIS
The Hiden Isochema XEMIS is a gravimetric gas sorption analyser which allows high pressure isotherms to be measured up to 17 MPa. The main feature of the system is that, differently from many other differential balances, the sample and reference side are perfectly symmetrical. This means that both branches are exposed to the same gas pressure and temperature throughout the experiment for improved accuracy. This gas sorption analyser is completely automated and can span a wide range of pressures.
The microbalance chamber and pressure handling system is constructed entirely of 316L stainless steel and Swagelok VCR tubing and components. Pressure in the chamber is changed and maintained using stepper motor-controlled Swagelok U Series bellows valves with PCTFE stem tips. Temperature is measured using a Pt100 (Class 1/10 DIN) Platinum Resistance Thermometer (PRT). To ensure high accuracy in the entire pressure range the system is equipped with a low and a high pressure transducer (up to 0.1 and 17 MPa, respectively), both of which are Druck (Baker Hughes) UNIK 5000 devices, with a quoted manufacturer’s accuracy of ± 0.04% of full scale. The microbalance has a weight resolution of 0.2 μg with a weighing range of 200 mg and a maximum balance capacity of 5 g. The long term stability is ± 5 μg.
The apparatus allows thermal regeneration in-situ under vacuum (< 10–7 kPa), using an Edwards nEXT85H turbomolecular pump, backed by a Vacuubrand MD1S membrane pump, for oil-free operation, to minimise contamination. Thermal activation of the Na-Y sample was performed by first evacuating the microbalance chamber, with the sample at room temperature, for 15.5 h, before applying a temperature ramp of 1 K/min from room temperature to 393 K and holding for 6 h under vacuum. A second temperature ramp of 1 K/min from 393 to 623 K was then applied, before holding under vacuum for a further 12 h. Sample temperature during activation was controlled using a furnace providing ± 0.1 K setpoint control. The sample mass is monitored throughout the activation process. Upon loading, the sample mass was 180.13 mg; after thermal activation, the dry mass of the sample was 134.86 mg. The sample density used for buoyancy corrections was 2.53 g/cm3. For the reported experiments, the sample was held in a cylindrical stainless steel mesh container. Use of a low volume, high density material for the holder minimised background buoyancy contributions. Sample temperature was controlled throughout the adsorption experiment using a Grant ecocool 150R refrigerated recirculation bath, with ± 0.05 K setpoint control.
2.3 Materials
Experiments were carried out on the NIST reference material RM8850. This is a Na exchanged Y zeolite, with well-defined Si/Al and Na/Al ratios of 2.547 ± 0.037 and 0.997 ± 0.018, respectively. The complete textural and chemical characterisation of the adsorbent are given elsewhere [35]. The purity of CH4 was > 99.995% for both instruments, as per the 2020 NIST paper [19]. At the high pressures used in this work (up to 7.5 MPa), the gas behaviour deviates from ideality, therefore an appropriate Equation of State (EoS) must be used. Gas density, fugacity and the compressibility factor were determined using the Setzmann and Wagner EoS [36], as recommended by NIST [37, 38].
3 Results and discussion
3.1 CH4 excess adsorption isotherms
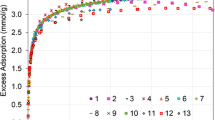
Figure 2 shows the direct comparison of the methane excess adsorption isotherms at 298.15 K measured by ADVA-270 and XEMIS, including also the reference data generated during the NIST interlaboratory study [19]. Isotherms from the two instruments show excellent agreement and, as expected, all equilibrium data fall within the ± 5% acceptability criteria for the NIST reference isotherm [19]. For further validation, CH4 isotherms on both instruments were extended to two additional temperatures (283.15 and 313.15 K). A comparison of the excess isotherms at the three temperatures between the two instruments is presented in Fig. 3.
CH4 excess isotherm at 298.15 K. Empty squares: ADVA-270; Filled circles: XEMIS; Continuous line: NIST Reference Isotherm [19]; Dashed lines: ± 5% acceptability criteria
Clearly, isotherms from both instruments show excellent agreement over the entire pressure and temperature range, demonstrating the high level of accuracy of both techniques. Excess isotherms also show a maximum of the adsorbed amount in the pressure range of 5–6 MPa. Equilibrium data shown in Figs. 2 and 3 are provided in Table S1 and S2 in the Supplementary Information.
3.2 Absolute isotherms and thermodynamic model
High pressure measurements are often reported in the literature as excess isotherms. While this is a convenient way of comparing experimental data between different techniques, any rigorous thermodynamic description of adsorption requires the use of absolute adsorption [39,40,41,42]. This means that experimental equilibrium data need to be converted to absolute. For a microporous adsorbent the conversion is straightforward [39, 42]:
where \(c\) is the gas molar density. The micropore volume, \({V}_{micro}\), used for the conversion to absolute was 0.358 cm3/g, as reported by Nguyen et al. [35].
The isotherms at the three temperatures need to be matched accurately in order to obtain derived quantities. The virial isotherm model provides enough flexibility to describe adsorption data in broad pressure and temperature ranges. In its original form, however, it does not account for the presence of a finite saturation capacity [43]. The presence of the saturation limit was included by Myers in his Langmuir-virial formulation [44], which can be expressed as:
where \(\theta =\frac{n}{{n}_{S}}\) is the surface coverage; \({b}_{P}\) is the affinity and \({n}_{S}\) is the saturation capacity. Written in this form, one can see that the term on the LHS and the first term on the RHS correspond to the Langmuir isotherm, while the additional terms are the virial expansion, with \(B,C,\dots\) the dimensionless virial coefficients describing the nature of interactions among pairs of molecules, triplets of molecules etc.
The saturation capacity, \({n}_{S}\), is temperature independent, given the rigid structure of the Na-Y framework, while the affinity and the virial coefficients have a first order dependency on the temperature reciprocal following [45], e.g. for the second virial coefficient:
The differential enthalpy of adsorption, \(-\Delta H\), can be defined as [42]:
For the Langmuir-virial isotherm model used in this work, it assumes the form:
Equation 5 expresses the relationship between the adsorbed phase concentration and the differential enthalpy of adsorption as a polynomial function.
If the saturation capacity is known, the remaining parameters of the Langmuir-virial isotherm can be determined via direct calculation using the method of polynomials orthogonal to summation [45] developed for the virial isotherm. In the case of the FAU topology, Farmahini et al. [46] have established that the Gurvich rule is accurate to approximately 2%. Therefore, using the density of liquid methane at the normal boiling point, a value of \({n}_{S}\) = 9.425 mol/kg was determined.
The mathematical nature of the virial isotherm, including the Langmuir-virial, may easily lead to overfitting. To avoid this potential pitfall, a statistical measure is needed to determine where to stop in an un-biased way. Here the Bayesian Information Criterion (BIC) analysis [47] was used as it allows the determination of the degree of the polynomial from the difference of the BICs of the successive approximations.
Expressed in terms of residual sum of squares (RSS), the BIC can be defined as:
where \({N}_{d}\) is the number of data points and \(k\) the number of parameters estimated by the model.
The absolute adsorption isotherms, including both datasets from ADVA-270 and XEMIS, and the Langmuir-virial results are shown in Fig. 4. The Langmuir-virial parameters obtained are reported in Table 2, up to the 4th virial coefficient which was the highest term for which the BIC decreased.
Figure 5 shows the calculated differential enthalpy of adsorption in comparison with literature values from both experiments and molecular simulations. From the zero-loading enthalpies of adsorption, it is clear there is a relatively wide range of values varying from 14 to 22 kJ/mol, which is somewhat surprising for a simple adsorption system such as CH4 in a zeolite.
Differential heat of adsorption comparison with literature. Continuous line: Langmuir-virial isotherm from this work. Empty symbols from molecular simulations. Filled symbols from experimental measurements. In the figure: Pellenq et al. [24], Calero et al. [25], Maurin et al. [26], Zhang et al. [27], Neddenriep [23], Guo et al. [28], Mauer et al. [30], Yang et al. [31]
Experimental calorimetry data at different adsorbed phase concentrations were measured by Maurin et al. [26] and Mauer et al. [30]. Maurin et al. [26] used a volumetric system coupled with a Tian-Calvet microcalorimeter and the results were shown to be in good agreement with Grand Canonical Monte Carlo (GCMC) simulations. Both experimental and simulated results show a mild increase in the differential heat of adsorption from ~ 14.5 to 16 kJ/mol. This is attributed to the contribution of the adsorbate–adsorbate interaction of CH4 molecules, which is found to be proportional to the occupancy. Adsorbate-adsorbent interactions remain almost constant to \(-{\Delta H}_{0}\). This indicates an energy homogeneity of Na-Y towards CH4 adsorption, as expected for a non-polar molecule. Similar conclusions had previously been reported by Woods and Rowlison [48] and Pellenq et al. [24], but these authors report a higher value of the zero-loading enthalpy of adsorption. The present results show the expected increasing trend of the enthalpy of adsorption with concentration starting from \(-{\Delta H}_{0}=\) 13.7 kJ/mol. This is in excellent agreement with recent simulations and calorimetric experimental results [25,26,27, 30].
3.2.1 Heat of adsorption from excess data
Literature data also include the use of absolute isotherm models (Langmuir, Tóth, virial, etc.) to regress excess data. This is often common practice and is applied to data that either show no maximum in the adsorbed amount or are limited to the pressure range below the maximum. To assess the effects of such an approach, we compared available excess data in the literature with the Langmuir-virial isotherm fitted to our excess isotherms in different pressure ranges. In each case, the BIC difference was used to determine the order of the concentration dependence that was statistically meaningful. Figure 6 shows the result of this comparison.
The parameters of all the fits are included in the Supplementary Information. It is important to note that even for the lowest pressure fit there is a systematic positive deviation in the derived “enthalpy of adsorption”, varying from approximately 0.5 to 2 kJ/mol. The lower value reflects the effect of the temperature dependence of the gas phase molar density, while the higher value is due to the inevitable increase in deviation from the absolute isotherm at higher pressures. As the data used in the fit approach the maximum of the excess isotherm, the instability in the derived differential enthalpy is more pronounced as the system approaches a singularity.
Figure 7 includes the comparison to the virial isotherm of Talu et al. [49], based on measurements up to 6.3 MPa. In their study, no maximum in the excess isotherm was reported, indicating an inconsistency in the results. The heats reported by Feng et al. [29], are based on a limited set of measurements up to 1 MPa. They used the Clausius–Clapeyron equation to determine the heats, but data at three temperatures were only available up to 1 mol kg–1, indicating that part of the results reported are extrapolated. This is represented in the figure using a continuous line for the range where data were available and a dashed line for the extrapolated results.
4 Conclusions
Methane adsorption isotherms at 283.15, 298.15 and 313.15 K on NIST reference zeolite RM8850 were obtained by two independent laboratories using a gravimetric and a volumetric technique. Both instruments were able to generate highly accurate isotherms up to 7.5 MPa with an excellent match in the entire pressure and temperature range using less than 150 mg of sample. The good agreement between the measurements demonstrates the resolution of both instruments even when working with very low sample masses. This has been identified as one of the challenges for high pressure measurements in both volumetric and gravimetric systems and one essential step to reduce the time required for multicomponent isotherms on these systems. Developing techniques which ensure high accuracy while using small sample size is key to allow accessibility to advanced characterisation techniques to new materials synthesised only in small quantities [18, 50]. The results provide the means to extend the use of the system methane/NIST reference zeolite RM8850 to test equilibrium measuring devices at different temperatures near ambient conditions, beyond the original results of the interlaboratory round robin coordinated by the NIST FACT Lab.
To derive the differential enthalpy of adsorption for this reference system, which is directly comparable to the isosteric heat of adsorption, a Bayesian Information Criterion was used to obtain unbiased isotherm parameters from the regression of the equilibrium data using the Langmuir-Virial isotherm of Myers [44]. The methodology allowed identification of the best regression of the data while avoiding overfitting. The resulting differential enthalpy of adsorption shows a monotonic increase with the adsorbed concentration. This is in very good agreement with recent molecular simulations and experimental calorimetry data from the literature.
The analysis was extended also to the direct fitting of excess adsorption equilibrium data to enable results to be compared to additional datasets from the literature. While reasonable agreement was found at lower pressures, it is possible to conclude that it is always better to convert the excess to absolute data for a rigorous thermodynamic approach and that major deviations are observed if excess data above 1 MPa are included. The “excess” heats derived from low pressure data tend to have a difference of the order of 0.5–2 kJ/mol, which is consistent with the deviation between calorimetry and excess isotherm derived results reported by Myers and co-workers [51].
Data availability
Data is provided within the manuscript and in the supplementary information file
Abbreviations
- \({b}_{P}\) :
-
Affinity, Pa–1
- \(B\) :
-
Second virial coefficient, –
- \(c\) :
-
Gas molar density, mol m–3
- \(C\) :
-
Third virial coefficient, –
- \(D\) :
-
Fourth virial coefficient, –
- \(\Delta H\) :
-
Differential enthalpy of adsorption, J mol–1
- \(\Delta {H}^{0}\) :
-
Differential enthalpy of adsorption at zero coverage, J mol–1
- \(k\) :
-
Number of parameters in model, –
- \(n\) :
-
Absolute adsorbed amount, mol kg–1
- \({n}^{ex}\) :
-
Excess adsorbed amount, mol kg–1
- \({n}_{s}\) :
-
Saturation capacity, mol kg–1
- \({N}_{d}\) :
-
Number of experimental data points, –
- \(f\) :
-
Fugacity, Pa
- \(P\) :
-
Pressure, Pa
- \(R\) :
-
Ideal Gas constant, J mol–1 K–1
- \(T\) :
-
Temperature, K
- \({V}_{micro}\) :
-
Micropore specific volume, m3 kg–1
- \(Z\) :
-
Compressibility factor, –
- \(\theta\) :
-
Surface coverage, –
References
Bhatia, S.K., Myers, A.L.: Optimum conditions for adsorptive storage. Langmuir 22, 1688–1700 (2006). https://doi.org/10.1021/la0523816
Broom, D.P., Hirscher, M.: Improving reproducibility in hydrogen storage material research. ChemPhysChem 22, 2141–2157 (2021). https://doi.org/10.1002/CPHC.202100508
Gür, T.M.: Carbon dioxide emissions, capture, storage and utilization: review of materials, processes and technologies. Prog. Energy Combust. Sci. 89, 100965 (2022). https://doi.org/10.1016/J.PECS.2021.100965
Luberti, M., Ahn, H.: Review of polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 47, 10911–10933 (2022). https://doi.org/10.1016/J.IJHYDENE.2022.01.147
Sharma, I., Friedrich, D., Golden, T., Brandani, S.: Monolithic adsorbent-based rapid-cycle vacuum pressure swing adsorption process for carbon capture from small-scale steam methane reforming. Ind. Eng. Chem. Res. 59, 7109–7120 (2020). https://doi.org/10.1021/acs.iecr.9b05337
Sircar, S.: Applications of Gas Separation by Adsorption for the Future. Adsorpt. Sci. Technol. 19, 347–366 (2001). https://doi.org/10.1260/0263617011494222
Oreggioni, G.D., Luberti, M., Reilly, M., Kirby, M.E., Toop, T., Theodorou, M., Tassou, S.A.: Techno-economic analysis of bio-methane production from agriculture and food industry waste. Energy Procedia. 123, 81–88 (2017). https://doi.org/10.1016/j.egypro.2017.07.252
Broom, D.P., Hirscher, M.: Irreproducibility in hydrogen storage material research. Energy Environ. Sci. 9, 3368–3380 (2016). https://doi.org/10.1039/C6EE01435F
Zlotea, C., Moretto, P., Steriotis, T.: A Round Robin characterisation of the hydrogen sorption properties of a carbon based material. Int. J. Hydrogen Energy 34, 3044–3057 (2009). https://doi.org/10.1016/j.ijhydene.2009.01.079
Gensterblum, Y., Van Hemert, P., Billemont, P., Busch, A., Charriere, D., Li, D., Krooss, B.M., De Weireld, G., Prinz, D., Wolf, K.-H.: European inter-laboratory comparison of high pressure CO2 sorption isotherms. I: Activated carbon. Carbon 47, 2958–2969 (2009). https://doi.org/10.1016/j.carbon.2009.06.046
Gensterblum, Y., Van Hemert, P., Billemont, P., Battistutta, E., Busch, A., Krooss, B.M., De Weireld, G., Wolf, K.-H.: European inter-laboratory comparison of high pressure CO2 sorption isotherms II: Natural coals. Int. J. Coal Geol. 84, 115–124 (2010). https://doi.org/10.1016/j.coal.2010.08.013
Gasparik, M., Rexer, T.F.T., Aplin, A.C., Billemont, P., De Weireld, G., Gensterblum, Y., Henry, M., Krooss, B.M., Liu, S., Ma, X., Sakurovs, R., Song, Z., Staib, G., Thomas, K.M., Wang, S., Zhang, T.: First international inter-laboratory comparison of high-pressure CH4, CO2 and C2H6 sorption isotherms on carbonaceous shales. Int. J. Coal Geol. 132, 131–146 (2014). https://doi.org/10.1016/J.COAL.2014.07.010
Goodman, A.L., Busch, A., Duffy, G.J., Fitzgerald, J.E., Gasem, K.A.M., Gensterblum, Y., Krooss, B.M., Levy, J., Ozdemir, E., Pan, Z., Robinson, R.L., Schroeder, K., Sudibandriyo, M., White, C.M.: An Inter-laboratory Comparison of CO2 Isotherms Measured on Argonne Premium Coal Samples. Energy Fuels 18, 1175–1182 (2004). https://doi.org/10.1021/ef034104h
Hurst, K.E., Parilla, P.A., O’Neill, K.J., Gennett, T.: An international multi-laboratory investigation of carbon-based hydrogen sorbent materials. Appl. Phys. A 122, 1–9 (2016). https://doi.org/10.1007/s00339-015-9537-x
Moretto, P., Zlotea, C., Dolci, F., Amieiro, A., Bobet, J.-L., Borgschulte, A., Chandra, D., Enoki, H., De Rango, P., Fruchart, D., Jepsen, J., Latroche, M., Llamas Jansa, I., Moser, D., Sartori, S., Wang, S., Zan, J.: A round robin test exercise on hydrogen absorption/desorption properties of a magnesium hydride based material. Int. J. Hydrogen Energy. 38, 6704–6717 (2013). https://doi.org/10.1016/j.ijhydene.2013.03.118
Hurst, K.E., Gennett, T., Adams, J., Allendorf, M.D., Balderas-Xicohténcatl, R., Bielewski, M., Edwards, B., Espinal, L., Fultz, B., Hirscher, M., Hudson, M.S.L., Hulvey, Z., Latroche, M., Liu, D.-J., Kapelewski, M., Napolitano, E., Perry, Z.T., Purewal, J., Stavila, V., Veenstra, M., White, J.L., Yuan, Y., Zhou, H.-C., Zlotea, C., Parilla, P.: An international laboratory comparison study of volumetric and gravimetric hydrogen adsorption measurements. ChemPhysChem 20, 1997–2009 (2019). https://doi.org/10.1002/cphc.201900166
Broom, D.P., Thomas, K.M.: Gas adsorption by nanoporous materials: Future applications and experimental challenges. MRS Bull. 38, 412–421 (2013). https://doi.org/10.1557/mrs.2013.105
Broom, D.P.: Challenges in characterizing adsorbents for gas storage and separation. Adsorption (2023). https://doi.org/10.1007/s10450-023-00424-9
Nguyen, H.G.T., Sims, C.M., Toman, B., Horn, J., van Zee, R.D., Thommes, M., Ahmad, R., Denayer, J.F.M., Baron, G.V., Napolitano, E., Bielewski, M., Mangano, E., Brandani, S., Broom, D.P., Benham, M.J., Dailly, A., Dreisbach, F., Edubilli, S., Gumma, S., Möllmer, J., Lange, M., Tian, M., Mays, T.J., Shigeoka, T., Yamakita, S., Hakuman, M., Nakada, Y., Nakai, K., Hwang, J., Pini, R., Jiang, H., Ebner, A.D., Nicholson, M.A., Ritter, J.A., Farrando-Pérez, J., Cuadrado-Collados, C., Silvestre-Albero, J., Tampaxis, C., Steriotis, T., Řimnáčová, D., Švábová, M., Vorokhta, M., Wang, H., Bovens, E., Heymans, N., De Weireld, G.: A reference high-pressure CH4 adsorption isotherm for zeolite Y: results of an interlaboratory study. Adsorption 26, 1253–1266 (2020). https://doi.org/10.1007/s10450-020-00253-0
Nguyen, H.G.T., Espinal, L., van Zee, R.D., Thommes, M., Toman, B., Hudson, M.S.L., Mangano, E., Brandani, S., Broom, D.P., Benham, M.J., Cychosz, K., Bertier, P., Yang, F., Krooss, B.M., Siegelman, R.L., Hakuman, M., Nakai, K., Ebner, A.D., Erden, L., Ritter, J.A., Moran, A., Talu, O., Huang, Y., Walton, K.S., Billemont, P., De Weireld, G.: A reference high-pressure CO2 adsorption isotherm for ammonium ZSM-5 zeolite: results of an interlaboratory study. Adsorption 24, 531–539 (2018). https://doi.org/10.1007/s10450-018-9958-x
Nguyen, H.G.T., Toman, B., van Zee, R.D., Prinz, C., Thommes, M., Ahmad, R., Kiska, D., Salinger, J., Walton, I.M., Walton, K.S., Broom, D.P., Benham, M.J., Ansari, H., Pini, R., Petit, C., Adolphs, J., Schreiber, A., Shigeoka, T., Konishi, Y., Nakai, K., Henninger, M., Petrzik, T., Kececi, C., Martis, V., Paschke, T., Mangano, E., Brandani, S.: Reference isotherms for water vapor sorption on nanoporous carbon: results of an interlaboratory study. Adsorption 29, 113–124 (2023). https://doi.org/10.1007/s10450-023-00383-1
Furukawa, H.: Synthesis and characterization of metal-organic frameworks. In: Glover, T.G., Mu, B. (eds.) Gas Adsorption in Metal-Organic Frameworks: Fundamentals and Applications, pp. 17–81. CRC Press, Boca Raton (2018). https://doi.org/10.1201/9780429469770
Neddenriep, R.J.: Sodium cation adsorption sites in zeolite types X and Y. J. Colloid Interface Sci. 28, 293–304 (1968). https://doi.org/10.1016/0021-9797(68)90133-1
Pellenq, R.J.-M., Tavitian, B., Espinat, D., Fuchs, A.H.: Grand canonical monte carlo simulations of adsorption of polar and nonpolar molecules in NaY Zeolite. Langmuir 12, 4768–4783 (1996). https://doi.org/10.1021/LA9600356
Calero, S., Dubbeldam, D., Krishna, R., Smit, B., Vlugt, T.J.H., Denayer, J.F.M., Martens, J.A., Maesen, T.L.M.: Understanding the role of sodium during adsorption: a force field for alkanes in sodium-exchanged faujasites. J. Am. Chem. Soc. 126, 11377–11386 (2004). https://doi.org/10.1021/ja0476056
Maurin, G., Bourrelly, S., Llewellyn, P.L., Bell, R.G.: Simulation of the adsorption properties of CH4 in faujasites up to high pressure: Comparison with microcalorimetry. Microporous Mesoporous Mater. 89, 96–102 (2006). https://doi.org/10.1016/j.micromeso.2005.09.024
Zhang, J., Burke, N., Zhang, S., Liu, K., Pervukhina, M.: Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem. Eng. Sci. 113, 54–61 (2014). https://doi.org/10.1016/j.ces.2014.04.001
Guo, X., Zhang, P., Navrotsky, A.: The thermodynamics of gas absorption and guest-induced flexibility in zeolite Y. Microporous Mesoporous Mater. 294, 109893 (2020). https://doi.org/10.1016/j.micromeso.2019.109893
Feng, L., Shen, Y., Wu, T., Liu, B., Zhang, D., Tang, Z.: Adsorption equilibrium isotherms and thermodynamic analysis of CH4, CO2, CO, N2 and H2 on NaY zeolite. Adsorption 26, 1101–1111 (2020). https://doi.org/10.1007/s10450-020-00205-8
Mauer, V., Bläker, C., Pasel, C., Bathen, D.: Energetic characterization of faujasite zeolites using a sensor gas calorimeter. Catalysts 11, 98 (2021). https://doi.org/10.3390/catal11010098
Yang, X., SadeghiPouya, E., Xiao, G., Richter, M., May, E.F.: High-pressure gravimetric measurements for binary gas adsorption equilibria and comparisons with Ideal Adsorbed Solution Theory (IAST). Langmuir 39, 13521–13533 (2023). https://doi.org/10.1021/acs.langmuir.3c01569
Zielinski, J.M., Coe, C.G., Nickel, R.J., Romeo, A.M., Cooper, A.C., Pez, G.P.: High pressure sorption isotherms via differential pressure measurements. Adsorption 13, 1–7 (2007). https://doi.org/10.1007/s10450-007-9005-9
Hampson, J.A., Rees, L.V.C.: Adsorption of ethane and propane in silicalite-1 and zeolite NaY: determination of single components, mixture and partial adsorption data using an isosteric system. J. Chem. Soc. Faraday Trans. 1(89), 3169–3176 (1993). https://doi.org/10.1039/FT9938903169
Stokes, R.H., Robinson, R.A.: Standard solutions for humidity control at 25° C. Ind. Eng. Chem. 41, 2013 (1949)
Nguyen, H.G.T., Tao, R., Van Zee, R.D.: Porosity, powder x-ray diffraction patterns, skeletal density, and thermal stability of NIST zeolitic reference materials RM 8850, RM 8851, and RM 8852. J. Res. Natl. Inst. Stand. Technol. 126, 126407 (2021). https://doi.org/10.6028/jres.126.047
Setzmann, U., Wagner, W.: A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 20, 1061–1155 (1991). https://doi.org/10.1063/1.555898
Lemmon, E.W., Bell, I.H., Huber, M.L., McLinden, M.O.: NIST standard reference database 23: reference fluid thermodynamic and transport properties-REFPROP, version 10.0. National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg (2018). https://doi.org/10.18434/T4/1502528
Huber, M.L., Lemmon, E.W., Bell, I.H., McLinden, M.O.: The NIST REFPROP database for highly accurate properties of industrially important fluids. Ind. Eng. Chem. Res. 61, 15449–15472 (2022). https://doi.org/10.1021/acs.iecr.2c01427
Brandani, S., Mangano, E., Sarkisov, L.: Net, excess and absolute adsorption and adsorption of helium. Adsorption 22, 261–276 (2016). https://doi.org/10.1007/s10450-016-9766-0
Brandani, S., Mangano, E., Luberti, M.: Net, excess and absolute adsorption in mixed gas adsorption. Adsorption 23, 569–576 (2017). https://doi.org/10.1007/s10450-017-9875-4
Salem, M.M.K., Braeuer, P., Szombathely, M.V., Heuchel, M., Harting, P., Quitzsch, K., Jaroniec, M.: Thermodynamics of high-pressure adsorption of argon, nitrogen, and methane on microporous adsorbents. Langmuir 14, 3376–3389 (1998). https://doi.org/10.1021/la970119u
Myers, A.L., Monson, P.A.: Physical adsorption of gases: the case for absolute adsorption as the basis for thermodynamic analysis. Adsorption 20, 591–622 (2014). https://doi.org/10.1007/S10450-014-9604-1
Czepirski, L., Jagiełło, J.: Virial-type thermal equation of gas—solid adsorption. Chem. Eng. Sci. 44, 797–801 (1989). https://doi.org/10.1016/0009-2509(89)85253-4
Myers, A.L.: Equation of state for adsorption of gases and their mixtures in porous materials. Adsorption 9, 9–16 (2003). https://doi.org/10.1023/A:1023807128914
Taqvi, S.M., LeVan, M.D.: A simple way to describe nonisothermal adsorption equilibrium data using polynomials orthogonal to summation. Ind. Eng. Chem. Res. 36, 419–423 (1997). https://doi.org/10.1021/ie960366d
Farmahini, A.H., Limbada, K., Sarkisov, L.: Comment on the applicability of the Gurvich rule for estimation of pore volume in microporous zeolites. Adsorption 28, 219–230 (2022). https://doi.org/10.1007/s10450-022-00364-w
Raftery, A.E.: Bayesian model selection in social research. Sociol. Methodol. 25, 111–163 (1995). https://doi.org/10.2307/271063
Woods, G.B., Rowlinson, J.S.: Computer simulations of fluids in zeolites X and Y. J. Chem. Soc. Faraday Trans. 85, 765–781 (1989). https://doi.org/10.1039/F29898500765
Talu, O., Zhang, S.Y., Hayhurst, D.T.: Effect of cations on methane adsorption by NaY, MgY, CaY, SrY, and BaY zeolites. J. Phys. Chem. 97, 12894–12898 (1993). https://doi.org/10.1021/j100151a043
Gibson, J.A.A., Mangano, E., Shiko, E., Greenaway, A.G., Gromov, A.V., Lozinska, M.M., Friedrich, D., Campbell, E.E.B., Wright, P.A., Brandani, S.: Adsorption materials and processes for carbon capture from gas-fired power plants: AMPGas. Ind. Eng. Chem. Res. 55, 3840–3851 (2016). https://doi.org/10.1021/ACS.IECR.5B05015
Dunne, J.A., Mariwala, R., Rao, M., Sircar, S., Gorte, R.J., Myers, A.L.: Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6, and SF6 on silicalite. Langmuir 12, 5888–5895 (1996). https://doi.org/10.1021/la960495z
Acknowledgements
We thank Prof. Paul A. Wright from the University of St Andrews for his comments on the sample masses typically obtained in laboratory scale synthesis of novel MOFs and zeolites.
Funding
The authors declare that no funds, grants, or other support were received for the preparation of this work.
Author information
Authors and Affiliations
Contributions
E.M. and S.B.: Supervision, Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing. R.R.: Investigation, Formal analysis, Data Curation, Visualization, Writing - Original Draft, Writing - Review & Editing. A.T.: Software, Formal analysis, Data Curation, Writing - Review & Editing . D.B.: Conceptualization, Methodology, Writing - Review & Editing. M.D.G and M.J.B.: Investigation, Formal analysis, Data Curation, Writing - Review & Editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rea, R., Tavitian, A., Broom, D.P. et al. Validated methane adsorption isotherms up to 7.5 MPa on a reference Na-Y zeolite at near ambient temperatures. Adsorption 30, 1529–1538 (2024). https://doi.org/10.1007/s10450-024-00518-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-024-00518-y