Abstract
Hydroxychloroquine (HCQ) is a very substantial drug active substance that was approved for emergency use by the FDA during the peak of the COVID-19 pandemic due to its potent antiviral properties. In this study, adsorption and desorption of hydroxychloroquine on sulfur (S)-doped graphene powders were investigated. While the adsorption experiments were carried out in the environment of distilled water (pH 5.0-6.0), HEPES buffer (pH 7.6), and Tris.HCl buffer (pH 8.0) the desorption studies were performed in distilled water. The HCQ adsorbed S-doped graphene powders were characterized by UV-Vis, FT-IR, XRD, BET and TEM techniques. According to UV-Vis measurements, the adsorption efficiency in the HEPES buffer medium at pH 7.6 was the highest (68.72% for H3 (HCQ adsorption with SGr3 graphene in HEPES medium)). FT-IR and XRD analyses confirmed the presence of HCQ on the graphene powders’ surface. While morphological changes on the surfaces of graphene powders were imaged by TEM, BET surface area changes proved the HCQ adsorption. The in vitro toxicity of the developed H3 was found to be lower than that of HCQ alone on the L929 cell line. These fundamental findings of the surface interaction between HCQ and graphene are precious for the design and optimization of a targeted drug based on this molecule and material. The adsorption/desorption features of HCQ onto graphene-based carrier systems which in particular doped with sulfur from functional metals have been investigated for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coronavirus disease (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus. Although most people infected with the virus recover without special treatment after mild to moderate respiratory illness, some become seriously ill and require medical attention. Anyone with COVID-19 can become seriously ill or even die at any age. The virus can be spread in the form of small liquid particles when coughing, sneezing, talking, singing, or breathing from the mouth or nose of an infected person (Ucar et al. [1]; WHO [2]).
CQ (chloroquine) has long been applied in the treatment of malaria and amebiasis. However, with reports of overdose of chloroquine causing acute poisoning and even death due to toxicity, HCQ (hydroxychloroquine sulfate), a chloroquine derivative, was first synthesized in 1946 by adding a hydroxyl group to chloroquine and was shown to be much less (~40%) toxic to animals than chloroquine. HCQ is widely used to treat autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis (Liu et al. [3]).
Many international organizations, including FDA (U.S. Food and Drug Administration) and WHO (World Health Organization), have recommended the use of CQ and HCQ (a less toxic derivative of chloroquine) against various viral diseases (malaria, lupus, rheumatoid arthritis and especially in fighting with SARS-CoV-2 pandemic) (FDA [4]; Liu et al. [3]).
However, in addition to the possible side effects and harmful interactions of HCQ, it has been proven by many clinical studies that drug causes serious problems in patients such as heart complications, headache, dizziness, nausea, stomach pain, loss of appetite, feeling irritable, skin rash and hair loss. Then, in June 2020, the World Health Organization withdrew this recommendation (Bendjeffal et al. [5]; Yao et al. [6]). To eliminate these side effects of HCQ, it has been combined with various platforms such as Ag, Au, and Pt nanoparticles (Rezaee et al. [7]), melanin (Schroeder and Gerber [8]) and kaolin (Bendjeffal et al. [5]). Furthermore, Mohammadi et al. have theoretically investigated the absorption and electrical and structural properties of chloroquine attached to graphene nanoribbon (GNR). The structural and electrical properties and absorption of the chloroquine and GNR complex have been calculated using the density functional theory (DFT) method. In the study, it has been shown that GNR nanostructures are suitable for complexes containing chloroquine and that can be very effective in drug delivery of chloroquine and thus aid in the treatment of COVID-19 (Mohammadi et al. [9]).
Graphene has great potential as a drug delivery system due to its amazing physical and chemical properties such as high loading efficiency and surface area, stability, targeting, wide operating temperature application, non-toxic high biocompatibility, high chemical stability, ease of functionalization, relatively low cost, good conductivity (Arvas et al. [10]; Arvas et al. [11]; Arvas et al. [12]).
By incorporating sulfur (S) atoms into the graphene structure, some properties of graphene are improved. Sulfur atoms have larger diameters than carbon atoms, so electrons are easily polarized, allowing graphene to exhibit high chemical activity. Sulfur added to the graphene structure leads to an increase in bond polarization (Arvas et al. [10]; Arvas et al. [11]). Sulfur doping to graphene powders has been reported to increase the wettability of graphene (Ren et al. [13]). It is also known to increase the adsorption capacity of graphene powders containing heteroatoms like sulfur and nitrogen. Additionally, oxygenated functional groups containing sulfur may increase the interaction of the surface with the guest molecule during adsorption due to the effect of intermolecular forces (Almarri et al. [14]; Mahmoud et al. [15]).
Herein, it was aimed to investigate the adsorption and desorption of HCQ onto the surface of S-doped graphene powders in different environments, including distilled water (pH 5.0-6.0), HEPES buffer (pH 7.6) and Tris.HCl buffer (pH 8.0). Thus, the adsorption of HCQ drug to a graphene-based carrier system will be studied for the first time. The effects of different solvent systems and S-doped graphene structures at different rates on drug adsorption will also be investigated.
2 Materials and methods
2.1 Materials
All reagents were of commercial quality and reagent quality solvents were used without further purification. Ultra-pure water was produced from Millipore Milli-Q system (~18.2 MΩ cm). The HCQ drug was obtained from the principal investigator of Bakırdere research group Prof. Sezgin Bakirdere. Tris.HCl (Tris(hydroxymethyl)aminomethane hydrochloride), and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) were supplied from Sigma-Aldrich.
2.2 Methods
2.2.1 Synthesis and electrochemical characterisation of the S-doped graphenes
The synthesis of S-doped graphene powders (SGr1-SGr4) was carried out with a classical three-electrode electrochemical system. In the system where Graphite Rod (Ø6X200E, 99.9%, 0.5 cm diameter, MERSEN Istanbul Ind. Pro. Inc) was used as the working electrode, Ag/AgCl (in 3.0 M KCl) was used as the reference electrode and Pt wire (1mm diameter, 99.9%) was used as the counter electrode. 5.0 M H2SO4 solution, which was also used as electrolyte in our previous studies (Arvas et al. [11]; Gürsu et al. [16]), was used as the electrolyte. S-doped graphene powders were produced with cyclic voltammetry at different potential ranges using Gamry Reference 3000 Potentiostat/Galvanostat. The potential ranges and electrolyte compositions used in the production of S-doped graphene powders were shown in Table 1. UV-Vis, FT-IR, BET, XRD and TEM analyzes were used for the characterization of S-doped graphene powders (SGr1-SGr4). UV-Vis analyzes were performed with a Perkin Elmer Lambda 35/PG Instruments T80+ instrument at a wavelength range of 200-800 nm. FT-IR analyses were performed with a PerkinElmer, Spectrum 100 device in the frequency range of 4000-650 cm-1 using the KBr technique (Cebeci et al. [17]; Ucar [18]; Ucar et al. [19]). XPS analyses were performed using Thermo Scientific K-Alpha model XPS (Al Ka = 1468.3 eV) and Casa XPS software. For BET analysis, surface areas and pore sizes were calculated by obtaining DFT and N2 adsorption-desorption isotherms. XRD patterns were obtained with PANalytical brand X'Pert PRO device (Anode:Cu, 40 mA, 40 kV, CuKα1 =1.54059 Angstrom). TEM analysis was performed using the Hitachi HighTech HT7700 device.
2.2.2 Adsorption of the HCQ on the S-doped graphene surfaces
The HCQ adsorption studies were realized using 5 mg/mL HCQ initial concentration in three different solvent systems, including distilled water (pH 5.0-6.0), HEPES buffer (pH 7.6) and Tris.HCl buffer (pH 8.0). HCQ was adsorbed on four different S-doped graphene powders (SGr1-SGr4) surfaces. For this, 5 mg of each S-doped graphene powders (SGr1-SGr4) were dispersed with 5 mL of distilled water or buffer solutions. Sonication was applied at 100% amplitude for 15 minutes in continuous mode with a probe-type sonicator (Bandelin Sonopuls, Germany). 5 mg of the HCQ was dissolved in the solvent in which the graphene types was dispersed and added to the medium where graphene powders made the solution black. All mixtures were stirred at 500 rpm at room temperature overnight. The next day, HCQ adsorbed graphene powders made with the three solvent systems (W1-W4 (adsorption in distilled water with SGr1-SGr4 graphene powders), H1-H4 (adsorption in HEPES with SGr1-SGr4 graphene powders), T1-T4 (adsorption in Tris with SGr1-SGr4 graphene powders)) were precipitated by centrifugation (Nuve, NF 800R) at 6000 rpm for 15 min. After the supernatants were collected, the precipitated HCQ adsorbed graphene powders were washed with distilled water and the above-mentioned centrifugation process was applied again. HCQ adsorbed graphene powders precipitated in the last step were lyophilized and stored at +4 °C. The remaining HCQ was determined from the supernatants using UV-Vis spectrophotometer at a maximum wavelength of 343 nm. The amount of adsorbed HCQ was calculated using the UV-Vis calibration curve.
2.2.3 Desorption of the HCQ from the S-doped graphene surfaces
Desorption of the HCQ from the S-doped graphene surfaces was studied in distilled water (approximately pH 6.5) as an eluent to simulate in-vivo conditions (Al-Bayati and Ahmed [20]). A certain amount of HCQ adsorbed S-doped graphene was taken and dispersed in distilled water by sonication for 15 minutes. The samples were incubated in a water bath (N-Biotek, NB-303, orbital type) at 200 rpm at 37 °C. The samples were centrifuged (Nuve, NF 800R) at 14000 rpm for 5 minutes at regular intervals and the supernatants were analyzed by UV-Vis. The desorption process was continued by adding distilled water to the precipitates for 30 days.
2.2.4 In vitro cell culture assay
In the cell culture experiments, L929 mouse fibroblast cell line was used for cultivation. Fibroblast cells were cultured in 75 cm2 bottle canted necked vented flasks (TPP). DMEM-L as supplement medium was enriched by 10% fetal bovine serum (FBS) and %1 penicillin-1% L-glutamin solution. The cells were incubated at 37 °C, in a humidified atmosphere of 5% CO2 and 99.9% humidity and passaged enzymatically with 0.25% (w/v)trypsin-1 mM EDTA and subculturing was done by briefly rinsed the cell layer with 0.25% (w/v) trypsin-0.53 mM EDTA solution to remove all traces of serum.
MTS assay was used to evaluate cytotoxicity on L929 mouse fibroblast cell line of H3. The MTS ((3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt), CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega) protocol relies on the reduction of the MTS tetrazolium compound by living mammalian cells to produce a colored formazan dye dissolved in a cell culture medium. Briefly, the cells (1 × 105 cells/mL) were implanted on 96-well plates and cultured overnight. Various concentrations ranging from 0.1 to 0.3 mg/mL (0.1; 0.15; 0.20; 0.25; 0.30 mg/mL, n = 4) of the H3 solved in deionized water were added onto cells and incubated the cells for 24 h and 48 h periods of time at 37 °C. Following the incubation periods, 10 μL of MTS reagent in 100 μL of growth medium was added to each well and the plate was incubated for 2 hours at 37 °C. As a final step, colorimetric density in well plates was measured by means of an ELISA reader (Thermo Scientific Multiskan GO Microplate, USA) at 510 nm for each well. DMSO which is well known cytotoxic activity agent was used as a positive control and culture medium was used as negative control. The percentage of the viable cells was calculated using the equation 1:
2.3 Statistical analysis
Statistical analyses of the results were carried out using MS Excel 2016 and OriginPro 9.0 programs. A p-value (a p-value is a technique you can use to quantify the discrepancy between your predicted values and the actual values.) less than 0.05 was considered to be significant. All experiments were repeated at least three times. The obtained data were given as mean ± standard deviation.
3 Results and discussion
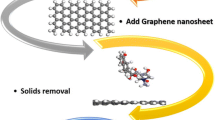
The synthetic route of the graphene synthesis and drug adsorption applied in the study was summarized in Schema 1.
3.1 Electrochemical characterization of the S-doped graphenes (SGr1-SGr4 graphene powders)
S-doped graphene powders (SGr1-SGr4) were synthesized by cyclic voltammetry at a scan rate of 50 mV.s-1 under the conditions specified in section 2.2.1. The synthesis conditions and the properties of the obtained S-doped graphene powders were investigated in our previous studies and characterized by XPS analysis (Arvas et al. [10]; Arvas et al. [11]; Gürsu et al. [16]). The potential range (-1.0 V)-(3.0 V) was used in the synthesis of SGr1 coded graphene powders. As examined in our previous studies, the oxidation peaks formed in the synthesis voltammograms during the scan of the anodic potential were related to the hydroxyl, epoxy, carboxyl and sulfate groups. However, these groups formed during the cathodic scan were reduced and the graphene powders formed were doped with -S.
The potential ranges indicated in Table 1 were determined by considering the potentials where the reduction and oxidation peaks occurred in the synthesis voltammograms. In this way, the selectivity of sulfur-containing oxygenated functional groups formed on the graphene surface was ensured. SGr2 coded graphene powders were synthesized by cyclic voltammetry in the potential range of -0.4 V to 3.0 V. Since the potential in the cathodic direction was scanned less than the SGr1 coded graphene powder, the sulfur-containing oxygenated functional groups formed on the surface of SGr2 coded graphene were reduced less than SGr1 coded graphene. In the synthesis of SGr3 coded graphene powders, a potential range of 0.8 V to 3.0 V was used with the same approach. Finally, the synthesis of SGr4 coded graphene powders was synthesized in the potential range of 1.4 V to 3.0 V. Since the potential range used in the synthesis of SGr4 coded graphene powder was the narrowest, the oxygen-containing functional groups remained unreduced on the graphene surface. XPS analysis was performed for S-doped graphene powders coded SGr1, SGr2, SGr3, SGr4 and shown in Figs. 1, 2, 3, and 4, respectively. In Figure 1a, the peaks obtained at approximately 284.2 eV, 286.36 eV, 288.46 eV and 291.3 eV in the C1s showed. In Figure 1b, the peaks obtained at approximately 531.2 eV, 532.11 and 533.79 eV in the O1s XPS spectrum of SGr1 showed the formation of –C=O, –C-O, O=C-O, respectively. In Figure 1c, the peaks obtained at approximately 169.28 eV and 170.44 eV in the S2P XPS spectrum of SGr1 indicated the formation of –C-SOX-C-. The peaks in the XPS spectra of SGr2, SGr3 and SGr4 graphene powders were related to the same functional groups obtained in SGr1. However, there were certain differences, especially in the intensity of the peaks observed in the SGr2P spectra. The peak intensities of oxygen and sulfur increased due to the presence of oxygen-containing functional groups, especially in SGr3 and SGr4. In XPS analyzes in our previous studies in the literature, the peak intensity attributed to −C−SOx−C bonds was higher, especially in the S2p spectrum of SGr4 coded graphene powder. In addition, the peak density of −C=O bonds was higher in the O1s spectrum of the graphene powders synthesized in the narrower potential range. The difference between the S-doped graphene powders (SGr1-SGr4) produced using Yucel’s method in terms of the presence of functional groups enabled the S-doped graphene powders to show different chemical, physical and electronic properties from each other (Arvas et al. [10]; Arvas et al. [11]; Ersozoglu et al. [21]; Gursu et al. [22]). In addition, graphene powders, which were synthesized in a single step and environmentally friendly, were more advantageous than other known methods since they were produced with a simple production method. Ensuring the selectivity of the functional groups by controlling the potential range provided interesting results in the physical adsorption-desorption of HCQ on S-doped graphene powders, comparable to each other during the application. The cyclic voltammograms obtained during the synthesis of S-doped graphene powders were demonstrated in our previous studies. In previous studies of our team, the effect of the potential range applied in Yucel's Method on the functional groups formed was shown. Due to the high reduction potential of SGr1 coded graphene, oxygenated functional groups formed by oxidation were reduced. In the XPS spectrum of SGr1 coded graphene, the peak intensities of oxygenated functional groups (C−SOx−C, −COOH, −OH, −C2H3O) were lower than those of SGr2, SGr3 and SGr4 coded graphene, respectively, where reduction occurred less (Arvas et al. [10]; Arvas et al. [23]; Arvas et al. [11]). The graphene powders containing the highest amount of oxygenated functional groups were SGr3 and SGr4. Since the reduction potential was kept low during the synthesis of these graphene powders, the functional groups formed by oxidation remained on the graphene surface without being reduced. The presence of negative-terminated functional groups increased the hydrophilic character of graphene and also contributed to the improvement of adsorption kinetics by increasing the intermolecular interaction thanks to these functional groups with high electronegativity.
3.2 Adsorption of the HCQ on the S doped graphene surfaces
3.2.1 UV-Vis analysis
For the quantitative determination of HCQ adsorbed on the graphene surface, a calibration curve of HCQ was created with UV-Vis. HCQ was characterised by bands at 237 nm, 257 nm and double-headed band at 329 nm and 343 nm in UV-Vis scanning, in agreement with the literature (Arguelho et al. [24]; Ferraz et al. [25]; Shaffer et al. [26]; Shrivastava [27]). The obtained absorption spectra were presented in Figure 5a. These values were evaluated as electronic transitions of π→π* (343 nm), n→π* (329 nm) and n→σ* (257 nm) in relation to the presence of chromophore groups in the molecule. For the quantitative analysis of the samples, the last and highest absorption band of quinoline group observed at 343 nm was chosen as the wavelength. Absorbance measurements at this wavelength had a linear relationship with increasing concentrations in water in the range of 0.5-0.005 mg/mL. The calibration curve obtained using 343 nm absorbance was presented in Figure 5b. For the quantitative determination of HCQ, LOD and LOQ were 21.51 and 51.69 ppm, respectively.
The HCQ-graphene powders obtained after adsorption of HCQ in three different solvent environments on the surface of S-doped graphene powders were redispersed in their own solvents and scanned with UV-Vis spectroscopy to determine the adsorption efficiencies. The adsorption efficiency of HCQ was evaluated in terms of the types of solutions used, the pH of the medium, and the types of graphene powders. Adsorption of HCQ on graphene powders, albeit at different rates, was observed in all syntheses accomplished in distilled water (pH 5.0-6.0), HEPES (pH 7.6), and Tris (pH 8.0) medium (Figure 6). The absorption spectra of all the HCQ-graphene powders had the characteristic bands of HCQ at 267nm, 329 and 343 nm. Moreover, the adsorption efficiencies of W1-W4 complexes obtained after adsorption in distilled water medium ranged from 10-20% (Figure 6a, 6b, 6c, 6d). The low ion content of distilled water and the fact that distilled water does not contain some ions that can contribute to adsorption in buffer solutions caused the low efficiency of adsorption performed in distilled water (data not shown). The adsorption process of a drug from the liquid system to the solid surface is immensely related to the solution pH, which affects the surface charge of the adsorbent, as well as the degree of ionisation and speciation of the adsorbate types (Al-Bayati and Ahmed [20]). If the adsorption efficiencies were evaluated in terms of the pH of the medium, the efficiencies were higher in the basic medium than in the acidic medium. When evaluated in terms of adsorbent, the adsorption affinity of HCQ to graphene surface at low pH decreases because of electron transfer between the electron-withdrawing atoms in the structure of HCQ and the hydronium ions. A similar hypothesis was reported by a prior study in which trimethoprim adsorption onto cellulose acetate polymer and attapulgite clay was reduced because of competition exerted by the hydronium ions at low pH (Al-Bayati and Ahmed [20]). The adsorption efficiencies obtained from adsorption performed in HEPES and Tris buffer media, which were two common buffer systems, were given in Table 2. Also a graphical representation of the predicted interactions between HCQ and S-doped graphene powders was demonstrated in Schema 2. Strong hydrogen bridge bonds as well as weak π-π interactions were foreseen to contribute to the adsorption. Compared to distilled water, the adsorption efficiencies were remarkably increased. The HEPES (zwitterionic sulphonic acid buffering agent) and Tris (organic compound extensively used in biochemistry and molecular biology as a component of buffer solutions) that formed the buffers were thought to contribute to the adsorption of HCQ. If the adsorption values in HEPES and Tris buffers were compared, the highest efficiency achieved in both media belongs to SGr3 graphene powders and was calculated as 68.72% and 60.05%, respectively. A difference in up to 25% in the adsorption efficiency of HCQ was detected on the surfaces of graphene powders containing different amounts of sulphur. The presence of various ratios of -S and -SOx functional groups on the surface of each graphene layer in graphene powders ranging from SGr1 to SGr4 explained this change in adsorption efficiency. It can be claimed that the higher adsorption occurs not directly from the buffer components of HEPES and Tris, but with the pH change. Because there was no band change or new band formation between pure water, HEPES, and Tris in the UV-Vis spectra in Figure 6 measured in their buffer systems of each S-doped graphene powder type. The H3 complex produced in the HEPES buffer had the highest adsorption efficiency. This change observed in the UV-Vis region supports that chemical speciation can affect electron transfer. In a study examining the ionophore effect of the HCQ molecule, in the physiological pH range encountered in vivo, molar fractions of two dominant species of HCQ [containing the ionic quinoline group (pKa 7.28) and both ionic quinoline and ionic ternary nitrogen group (pKa 9.76) two species] are shown (Kavanagh et al. [28]). In our study, the minimum adsorption was calculated in the adsorption studies performed in water (pH 5.0-6.0). It is already known that HCQ has maximum ionic ternary nitrogen and ionic quinoline species in this pH range. For S-doped graphene, this pH range corresponds to the decreasing intrinsic state (electron density decrease, the electron loss of SO2-) around C=C. This is explained by the minimum adsorbtion of HCQ on the adsorbing surface. The HEPES buffer (pH 7.6) at provided better adsorption compared to the water medium. In the presence of approximately 50% of both species (ionic ternary nitrogen and ionic quinoline, ionic ternary nitrogen and non-ionic quinoline) in HEPES buffer (pH 7.6), the maximum adsorption rate was reached with 68.72 % (H3). This change may be associated with increased adsorption as because of decreased ionisation and increased intrinsic density in quinoline group (Kang et al. [29]). At this point it may be claimed that HCQ provides better interaction with S-doped graphene powders by electronegativity change because of losing a hydrogen atom at pH 7.6. Due to the presence of both acidic H+ and electrons on S-doped graphene surface at this pH value, the electronic interaction of both dominant species on S-doped adsorbing surface can be predicted. This result is consistent with that of Bendjeffal et al., which achieved maximum HCQ adsorption at pH 7.6 (Bendjeffal et al. [5]). In the adsorption study performed in Tris buffer (pH 8.0), a hyperchromic effect is observed in the adsorption intensity compared to water, with the decrease in the ionic ternary nitrogen species and the increase in the non-ionic quinoline species. In this pH range, the intrinsic density is expected to increase on the S-doped graphene surface. However, since the same situation is valid for HCQ, maximum adsorption was still unobserved. In an existing study in the literature; in which different biological buffers were used for in vivo and in vitro studies, provided that they have the same pH, the effect of different ion types on HCQ species due to buffer differences is disregarded (Kavanagh et al. [28]). In Figure 6a-k, the spectra of S-doped graphene and complexes scanned in water, Tris and HEPES buffers were presented. There was no change in the UV-Vis spectra due to the effect of different ions or an interaction with the molecules of the buffer solutions. In addition, the absorption band at 260 nm for S-doped graphene in all spectra was assigned to the π→π* transition of in C=C of the sp2 C domain, which manifests strongly after S doping, and identifies delocalised electrons in the carbon frame (Kang et al. [29]). In addition, in Table 2, in the spectrums of the complexes of the four species (SGr1-SGr4), it was observed that the π→π* electronic transition shoulder band at approximately 265 nm caused by HCQ intensifies at approximately 267 nm. This change was due to the π* contribution of HCQ because of the interaction of quinoline chromophore group and S-doped graphene.
3.2.2 FT-IR analysis
FT-IR spectrum of the pure HCQ was presented in Figure 7. 3357 cm−1 refers to the N−H stretching vibration of HCQ. A small peak observed at 3102 cm−1 corresponds to the stretching vibration of the O−H group of HCQ. 2941 cm−1 belongs to CH2 bands. The other bands appeared at 1614 cm−1 for C=C stretching, 1554 cm−1 for N−H stretching, 1458 cm−1 for CH2 bending, and 1114 cm−1 for C=N bending. Similarly, the C−Cl stretching peak is located at 850 cm−1. These data of HCQ were compatible with the literature (Armutcu and Piskin [30]; Wang et al. [31]).
The FT-IR spectra of the H1-H4 samples adsorbed in the HEPES buffer were shown in Figure 8 in comparison with SGr1-SGr4 graphene powders. For the SGr1-SGr4, the narrow and sharp peaks in the range of 1450–1100 cm−1 includes the contributions from the C−S, S=O, and S−O stretchings. While some peaks of free graphene powders did not change after HCQ adsorption, some new peaks were observed in HCQ adsorbed samples, especially in the fingerprint region. In addition, wide bands seen around 3430 cm−1 in all four samples and not in graphene powders were associated with the similar characteristic N−H peak in the same region of the HCQ. Also, it can be comprehended that the O−H band of HCQ at around the 3100 cm-1 was damped due to the hydrogen bridge interaction between sulfinyl (−HS=O) groups of graphene powders and O−H. Figure 9 represented the FT-IR spectra of the T1-T4 samples adsorbed in the Tris buffer in comparison with SGr1-SGr4 graphene powders. Interestingly, no significant change was observed in the 3400 cm−1 bands in the Tris medium before and after the adsorption, unlike the HEPES medium. In adsorption with HEPES buffer, ionic tertiary nitrogen, and ionic quinoline increase by approximately 50% with decreasing ionization in the quinoline group, that is, the N−H form is more. In this way, adsorption can occur with a loss of electronegativity over the H atom. At the same pH, there are both acidic and basic H atoms in graphene powders. Therefore, HCQ may have been adsorbed in both types without any problems. In Tris medium, ionic tertiary nitrogen decreases, and nonionic quinoline increases. However, intrinsic density is high on the graphene powder surface and HCQ. Therefore, there has been a decrease in adsorption efficiency and the N−H band may not be clearly visible in FT-IR due to this electron density.
In T1-T4 samples, HCQ adsorption in the Tris medium was evident from the intensity change and/or shifting around the 1630-1640 cm−1 bands. In addition, it was determined that the 1560-1570 cm−1 bands, which were not in the spectrum of graphene but in the samples, corresponded to the 1550 cm−1 band in the spectrum of HCQ.
3.2.3 XRD analysis
XRD analyses of S-doped graphene powders (SGr1-SGr4) were performed before and after the HCQ adsorption in HEPES and Tris buffer media (Figures 10 and 11). The effect of defects due to S-doping on lattice strain was investigated by XRD (Wang et al. [31]). The Bragg equation (nλ=2d sinθ) was used to calculate the d-values and to determine the distances between the graphene layers (Lee et al. [32]). The diffraction peaks of SGr1, SGr2, SGr3 and SGr4 graphene powders have 2θ values of 26.3° (d=3.387Å), 26.3° (d=3.388Å), 26.23° (d=3.397Å), and 26.40° (d=3.376Å) points were attributed to the (002) planes, respectively (Figure 6). It was observed that as the number of sulfur-containing oxygenated functional groups increased, the d-spaces also enhanced (Lee et al. [32]; Quan et al. [33]). It was also reported that the widening at the diffraction points around 2θ values of 42°observed in all S-doped graphene powders was related to the edge doping of graphite and graphene (Lee et al. [32]; Wang et al. [34]). Diffraction peaks of H1, H2, H3, and H4 samples with 2θ values of 26.25° (d=3.394Å), 26.32° (d=3.385Å), 26.34° (d=3.382 Å), and 26.24° (d=3.395Å) attributed to the (002) planes (Figure 10). Especially in H3 and H4 samples containing more oxygenated functional groups, more changes occurred in the (002) planes compared to SGr3 and SGr4. Especially, this change in the H3 sample was associated with the high HCQ adsorption efficiency with the interaction. and the diffraction peaks with 2θ values of 26.22° (d=3.398 Å) were attributed to the (002) planes. Diffraction peaks of T1, T2, T3 and T4 samples with 2θ values of 26.19° (d=3.402 Å), 26.15° (d=3.407 Å), 26.24° (d=3.395 Å) and 26.22° (d=3.398 Å) respectively attributed to (002) planes (Figure 11).
3.2.4 BET and DFT analysis
BET and DFT analyzes were performed to calculate the surface areas and the pore volumes of SGr1-SGr4 graphene powders and HCQ adsorbed graphene powders (H1-H4 and T1-T4). N2 adsorption and desorption isotherms and DFT analysis of SGr1-SGr4 graphene powders and HCQ adsorbed graphene powders (H1-H4 and T1-T4) were shown in Figures 12, 13 and 14, respectively (Ren et al. [35]; Yang et al. [36]). Calculated surface areas (m2/g) and pore volumes (cc/g) were shown in Table 3 (Ramezani and Dehghani [37]). In SGr1-SGr4 graphene powders, the highest surface area (97.87 m2/g) and pore volume (0.187cc/g) were obtained in SGr2 coded S-doped graphene powders. In HCQ adsorbed graphene powders (H1-H4) in HEPES buffer, the highest surface area and pore volume were obtained in the H3 coded graphene sample and their values were 434,031 m2/g and 0.647 cc/g, respectively (Table 3). These results were directly related to the adsorption efficiency of H3. Although the highest surface area was obtained at SGr2, the physical adsorption efficiency with HCQ increased due to the excess of sulfur-containing oxygenated functional groups (−SO2-, −SO42-, −HS=O, −CS, −SO) in SGr3 coded graphene powder as depicted in Schema 2. The highest surface area and pore volume in (T1-T4) samples were calculated in T3 coded sample and their values were 77.931 m2/g and 0.160 cc/g, respectively. As a result, the positive effect of sulfur-containing oxygenated functional groups on HCQ adsorption was understood. The higher surface areas and pore volumes in both HEPES and Tris buffers were due to the effect of sulfur-containing oxygen groups in these graphene powders. The point to be noted here was that the number of functional groups containing higher amounts of oxygen formed on the surface of SGr4 coded graphene was higher than that of SGr3, since it was synthesized in a narrower range. As seen in Schema 2, it was observed that oxygen-containing sulfur groups contributed to the formation of a large number of hydrogen bonds with the secondary amine groups of HCQ. However, the concentration of oxygen-containing functional groupson the graphene surface in the narrow potential range resulted in the formation of graphene powders with higher layer numbers during synthesis process. Hence, HCQ interacted with less graphene layer during physical adsorption. Therefore, the adsorption efficiency of HCQ was realized on the surfaces of SGr3 coded graphene powders in both HEPES and Tris buffers. Since the −SO3H functional group in the HEPES structure has a highly electronegative character, it increased the surface area of multilayer SGrs by further weakening the weakened Wan der Walls forces in the interlayer plenary planes and preventing the agglomeration of the layers. The reason why the highest surface area alteration was observed in H3, one of the SGr containing the highest amount of oxygenated functional groups, may be due to the high electronegativity of these oxygenated functional groups. We believe that physical intermolecular interactions play an active role in this part and are not directly related to the pH of HEPES (pH 7.6) and Tris (pH 8.0) buffers.
3.2.5 TEM analysis
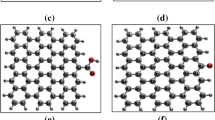
TEM could display the differences in the surface morphology and textures of graphene powders before and after the physical drug adsorption onto the surface. TEM images of S-doped graphene powders before and after the HCQ adsorption were exhibited in Figure 11. From the TEM image of non-adsorbed graphene powders (Figure 15a), it could be obviously noticed that the graphene powders were formed of individual layered structures. It could be seen from the images in Figures 15b and c that the drug molecule (HCQ) in cubic shape enter between these layers in the final graphene products obtained in HEPES and Tris medium. Moreover, no significant agglomeration on the surface of both T3 and H3 samples which means that HCQ was adsorbed well and persistently on the graphene powders. Compared to the T3 sample, the higher ratio of cubic structures in the H3 sample supported the adsorption efficiency values obtained from UV-Vis analyses, and other spectroscopic measurement results.
3.3 Desorption of the HCQ from the S doped graphene surfaces
The desorption profile of the HCQ drug from S-doped graphene surface was evaluated in distilled water at 37 °C. Figure 16a and b demonstrated the desorption pattern of HCQ from graphene powders at various time intervals for a period of up to 720 h. The desorption pattern of HCQ was similar in both T3 and H3 samples, with a maximum desorption rate of approximately 100 µg in the period of first six hours. This may be sufficient to provide a requested regional concentration amount of drug within the first few hours. Following this rapid release, HCQ exhibited a slower and gradual release and after two weeks the HCQ release reached an equilibrium state. These findings were substantial in that S-doped graphene powders offer a good sustainable desorption profile and show that S doped graphene powders can be used as a drug delivery system (Croitoru et al. [38]). It is known that thanks to the controlled release and extended long half-life of this produced material, its excretion from the targeted biological tissue will decrease and the active substance will be absorbed at a higher level by the tissues. Considering the renal clearance and cellular uptake of HCQ observed in daily dose intakes, it is of vital importance that this drug can be administered with a controlled release, which ensures the delivery of high doses locally, especially in individuals with diseases such as renal failure (FDA [39]).
3.4 In Vitro Cytotoxicity Assay
It was previously emphasized that HCQ is of great importance in the treatment of autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis disease characterized by chronic, widespread inflammation, and multisystem organ damage (Lam et al. [40]). During the fight against COVID-19, it has come up again with both its administration of the high doses rate as therapeutic and cytotoxic effect.
According to the known precise biodistribution mechanisms of the hydroxychloroquine molecule; therapeutically, in humans, it can travel into cell organelles, where it accumulates and causes the pH to rise. Increased pH value prevents the dimerization of alpha and beta chains of major histocompatibility complex (MHC) class II in the cell, inhibits the cell's antigen presentation, and thus reduces the inflammatory response. Increased pH in endosomes prevents virus particles (such as SARS-CoV and SARS-CoV-2) from benefiting from their fusion and intracellular activity. For example; HCQ inhibits the terminal glycosylation of the ACE-2 (angiotensin-converting enzyme type 2), the receptor targeted by the COVID-19 virus for cell entry. Thus, a weaker molecular interaction occurs between the COVID-19 spike protein and the enzyme (Fox [41]; Vincent et al. [42]; Wang et al. [43]). Hydroxychloroquine has ultra-rapid oral absorption, high bioavailability, a long elimination half-life, and an extensive volume of distribution during the elimination phase. Therefore, in cases where high dose drug delivery administration is required, such as in COVID19 therapy, serious toxicity occurs rapidly within 1-3 hours after ingestion (Yusuf et al. [44])
In this study, MTS assay was performed to analyze the cytotoxic potential of HCQ as the cargo of a delivery vehicle in L929 mouse fibroblast cell line. The viability of L929 mouse fibroblast cell treated with H3 prepared at various concentrations (0.1; 0.15; 0.20; 0.25; 0.30 mg/mL, n = 4) decreased for H3 with increasing concentration (101.7-50.2% (24h) and 96.8-56.7% (48h)) (Figure 17). Also, molecule indicated toxicity with IC50 (half-maximal inhibitory concentration) values of 0.33 mg/mL (24h) and 0.35 mg/mL (48h) (Figure 17). While the IC50 value of the HCQ molecule in the cytotoxicity study conducted with murine microglial cell line, BV-2 was determined as 12.76 µg/mL (24h) (Račková and Csekes [45]), in this paper, this value was determined as 330 µg/mL (24h) for HCQ adsorbed to a carrier system, H3 with the L929 mouse fibroblast cell line. According to a different toxicity study conducted with human lung fibroblast cells, the cytotoxicity value determined was 18.77 µg/mL (48h) (Yang et al. [46]), while in this paper, this value was determined as 350 µg/mL (48h) with the L929 mouse fibroblast cell line.
Cytotoxicity results prove that H3, the HCQ carrier system, significantly reduces the cellular cytotoxicity rate of the active ingredient, the HCQ molecule. In addition to the high therapeutic effect of the HCQ molecule, its high cytotoxic effect poses a great limitation in the application of the therapeutic dose of the drug, especially in advanced disease. It is clear that the cytotoxicity limitation has been largely overcome with the carrier system produced by adsorbing the HCQ molecule. However, the therapeutic effect of H3 can be definitively demonstrated, especially if it is supported by comprehensive in vitro and in vivo studies involving different healthy cell lines and the COVID-19 virus.
4 Conclusion
In this study, the adsorption of hydroxychloroquine (HCQ) on S-doped graphene powders was realized utilizing three different solvent systems, distilled water, Tris, and HEPES. The effect of pH of the medium and the S doping of graphene on adsorption was also investigated. The adsorption efficiencies were determined by UV-Vis analyses. The outcomes of the study presented that the HEPES buffer system with a pH of 7.6 and SGr3 graphene powders displayed the best HCQ adsorption conditions. Additionally, functional group changes, surface properties, and morphological images of HCQ adsorbed graphene powders were studied by FT-IR, XRD, BET, and TEM. A tremendous increase of 377% in the surface area of H3 relative to SGr3 due to HCQ adsorption was determined in the HEPES buffer medium. According to the in vitro toxicity assay performed on the L929 cell line, H3 was found to be more biocompatible than HCQ alone with the IC50 value of 330 µg/mL and 12.76 µg/mL for H3 and HCQ, respectively. This study is pioneering research that will pave the way for using S doped graphene powders produced by the electrochemical method in drug release systems and a lot of different biomedical applications.
Data Availability
No datasets were generated or analysed during the current study.
References
Ucar, B., Acar, T., Arayici, P.P., Derman, S.: A nanotechnological approach in the current therapy of COVID-19: model drug oseltamivir-phosphate loaded PLGA nanoparticles targeted with spike protein binder peptide of SARS-CoV-2. Nanotechnology 32, 485601 (2021)
WHO, The Corona Virus Disease 2019 (COVID-19) (2020) https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Liu, J., et al.: Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6, 1–4 (2020)
FDA (2020) FDA Drug Safety Communication: FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/media/137250/download. Accessed 20 May 2021
Bendjeffal. H., Ziati, M., Aloui, A., Mamine, H., Metidji, T., Djebli, A., Bouhedja, Y., : Adsorption and removal of hydroxychloroquine from aqueous media using Algerian kaolin: Full factorial optimisation, kinetic, thermodynamic, and equilibrium studies. Intl J Environ Anal Chem 103, 9, 1–22 (2021)
Yao, X., et al.: In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical infectious diseases 71, 732–739 (2020)
Rezaee P, Akbari M, Morad R, Koochaki A, Maaz M, Jamshidi Z. First principle simulation of coated hydroxychloroquine on Ag, Au and Pt nanoparticle as a potential candidate for treatment of SARS-CoV-2 (COVID-19) 11, 1, 2131, (2021)
Schroeder, R., Gerber, J.: Chloroquine and hydroxychloroquine binding to melanin: some possible consequences for pathologies. Toxicol Rep 1, 963–968 (2014)
Mohammadi S, Esmailpour M, Mohammadi M Chloroquine drug and Graphene complex for treatment of COVID-19, (2020) e-print
Arvas, M.B., Gençten, M., Sahin, Y.: A two-dimensional material for high capacity supercapacitors: S-doped graphene. Intl J Energ Res 44, 1624–1635 (2020)
Arvas, M.B., Karatepe, N., Gencten, M., Sahin, Y.: Fabrication of high-performance symmetrical coin cell supercapacitors by using one step and green synthesis sulfur doped graphene powders. New J Chem 45, 6928–6939 (2021)
Arvas, M.B., Karatepe, N., Gencten, M., Sahin, Y.: One-step synthesis of nitrogen-doped graphene powders and application of them as high-performance symmetrical coin cell supercapacitors in different aqueous electrolyte. Intl J Energ Res 46, 7348–7373 (2022)
Ren, X., et al.: Improved interfacial floatability of superhydrophobic and compressive S, N co-doped graphene aerogel by electrostatic spraying for highly efficient organic pollutants recovery from water. Appl Surf Sci 457, 780–788 (2018)
Almarri, M., Ma, X., Song, C.: Role of surface oxygen-containing functional groups in liquid-phase adsorption of nitrogen compounds on carbon-based adsorbents. Energy & Fuels 23, 3940–3947 (2009)
Mahmoud, A.M., El-Wekil, M.M., Mahnashi, M.H., Ali, M.F., Alkahtani, S.A.: Modification of N, S co-doped graphene quantum dots with p-aminothiophenol-functionalized gold nanoparticles for molecular imprint-based voltammetric determination of the antiviral drug sofosbuvir. Microchimica Acta 186, 1–8 (2019)
Gürsu, H., Güner, Y., Dermenci, K.B., Gençten, M., Savaci, U., Turan, S., Şahin, Y.: A novel green and one-step electrochemical method for production of sulfur-doped graphene powders and their performance as an anode in Li-ion battery. Ionics 26, 4909–4919 (2020)
Cebeci, C., Ucar, B., Acar, T., Erden, I.: Colorimetric detection of hydrogen peroxide with gadolinium complex of phenylboronic acid functionalized 4 5-diazafluorene. Inorganica Chimica Acta 522, 120386 (2021)
Ucar, B.: Synthesis and characterization of natural lanthanum labelled DOTA-Peptides for simulating radioactive Ac-225 labeling. Appl Radiat Isot 153, 108816 (2019)
Ucar, B., Acar, T., Pelit-Arayici, P., Demirkol, M.O., Mustafaeva, Z.: A new radio-theranostic agent candidate: Synthesis and analysis of (ADH-1) c-EDTA conjugate. Fresenius Environ Bull 27, 4751–4758 (2018)
Al-Bayati, R.A., Ahmed, A.S.: Adsorption-desorption of trimethoprim antibiotic drug from aqueous solution by two different natural occurring adsorbents International. J Chem 3, 21 (2011)
Ersozoglu, M.G., Gursu, H., Gumrukcu, S., Sarac, A.S., Sahin, Y.: Single Step Electrochemical Semi-Exfoliated S-Doped Graphene-Like Structures from Commercial Carbon Fiber as Efficient Metal-Free Catalyst for Hydrogen Evolution Reaction. ChemElectroChem 9, e202101455 (2022)
Gursu, H., Gencten, M., Sahin, Y.: Preparation of sulphur-doped graphene-based electrodes by cyclic voltammetry: a potential application for vanadium redox flow battery. Int J Electrochem Sci 13, 875–885 (2018)
Arvas, M.B., Gürsu, H., Gencten, M., Sahin, Y.: New Approach Synthesis of S. N Co-Doped Graphenes for High-Performance Supercapacitors Chem Select 7, e202200360 (2022)
Arguelho, M.L.P., Andrade, J.F., Stradiotto, N.R.: Electrochemical study of hydroxychloroquine and its determination in plaquenil by differential pulse voltammetry. J Pharm Biomed Anal 32, 269–275 (2003)
Ferraz, L.R., et al.: Quality by design in the development and validation of analytical method by ultraviolet-visible spectrophotometry for quantification of hydroxychloroquine sulfate. Intl J Pharm Sci Res 5, 4666 (2014)
Shaffer, B., Cahn, M.M., Levy, E.J.: Absorption of antimalarial drugs in human skin: spectroscopic and chemical analysis in epidermis and corium. J Investig Dermatol 30, 341–345 (1958)
Shrivastava, A.: Analytical methods for the determination of hydroxychloroquine in various matrices. Int J Appl Pharm 4, 55–61 (2020)
Kavanagh, O.N., et al.: Hydroxychloroquine does not function as a direct zinc ionophore. Pharmaceutics 14, 899 (2022)
Kang, S., Jeong, Y.K., Jung, K.H., Son, Y., Kim, W.R., Ryu, J.H., Kim, K.M.: One-step synthesis of sulfur-incorporated graphene quantum dots using pulsed laser ablation for enhancing optical properties. Optics Express 28, 21659–21667 (2020)
Armutcu, C., Piskin, S.: Evaluation of controlled hydroxychloroquine releasing performance from calcium-alginate beads. Hittite J Sci Eng 8, 255–263 (2021)
Wang, H., Lu, W., Ke, L., Wang, Y., Zhou, J., Rao, P.: Effect of hydroxychloroquine sulfate on the gelation behavior, water mobility and structure of gelatin. Colloids Surf A: Physicochem Eng Aspects 633, 127849 (2022)
Lee, J., Noh, S., Pham, N.D., Shim, J.H.: Top-down synthesis of S-doped graphene nanosheets by electrochemical exfoliation of graphite: metal-free bifunctional catalysts for oxygen reduction and evolution reactions. Electrochimica Acta 313, 1–9 (2019)
Quan, B., et al.: Solvothermal-derived S-doped graphene as an anode material for sodium-ion batteries Advanced. Science 5, 1700880 (2018)
Wang, X., Li, G., Seo, M.H., Hassan, F.M., Hoque, M.A., Chen, Z.: Sulfur Atoms Bridging Few-Layered MoS2 with S-Doped Graphene Enable Highly Robust Anode for Lithium-Ion Batteries. Adv Energy Mater 5, 1501106 (2015)
Ren, X., Ma, H., Zhang, T., Zhang, Y., Yan, T., Du, B., Wei, Q.: Sulfur-doped graphene-based immunological biosensing platform for multianalysis of cancer biomarkers. ACS Appl Mater Inter 9, 37637–37644 (2017)
Yang, S., Zhi, L., Tang, K., Feng, X., Maier, J., Müllen, K.: Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv Funct Mater 22, 3634–3640 (2012)
Ramezani, Z., Dehghani, H.: Effect of nitrogen and sulfur co-doping on the performance of electrochemical hydrogen storage of graphene. Intl J Hydr Energ 44, 13613–13622 (2019)
Croitoru, A.-M., et al.: Novel Graphene Oxide/Quercetin and Graphene Oxide/Juglone. Nanostruct Platforms Effect Drug Deliv Syst Biomed Appl Nanomater 12, 1943 (2022)
FDA (2017) PLAQUENIL®Hydroxychloroquine sulfate tablets, USP. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf. Accessed 17 Apr 2021
Lam, LC., Vinita ,D., Yadav, Victor J., Mihal.: An uncommon correlation of rheumatoid arthritis and Lupus Nephritis: A case report on the unusual progression of Lupus Nephritis 14, 8 (2022)
Fox, R.I.: Mechanism of action of hydroxychloroquine as an antirheumatic drug. In Sem Arthr Rheum 23, 2, 82–91, (1993)
Vincent, M.J., et al.: Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2, 1–10 (2005)
Wang, M., et al.: Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30, 269–271 (2020)
Yusuf, I.H., Charbel Issa, P., Ahn, S.J.: Hydroxychloroquine-induced retinal toxicity. Front Pharmacol 14, 1196783 (2023)
Račková, L., Csekes, E.: Redox aspects of cytotoxicity and anti-neuroinflammatory profile of chloroquine and hydroxychloroquine in serum-starved BV-2 microglia. Toxicol Appl Pharmacol 447, 116084 (2022)
Yang, J., et al.: Cytotoxicity evaluation of chloroquine and hydroxychloroquine in multiple cell lines and tissues by dynamic imaging system and physiologically based pharmacokinetic model. Front Pharmacol 11, 574720 (2020)
Acknowledgement
The authors would like to thank Prof. Sezgin Bakirdere for his valuable comments and contributions to the research. The authors also gratefully acknowledge Gamze Nur Kara for her support in cell culture studies.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
T.A., M.B.A., B.A and B.U. carried out the experiments, M.B.A., T.A., and Y.S. contributed to conception, design and wrote the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acar, T., Arvas, M.B., Arvas, B. et al. Adsorption and desorption of hydroxychloroquine onto sulphur doped graphene powders as a potential drug for COVID-19: physicochemical investigation, surface chemistry and in vitro cytotoxicity evaluation. Adsorption (2024). https://doi.org/10.1007/s10450-024-00506-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00506-2