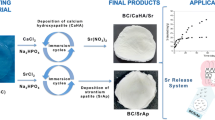
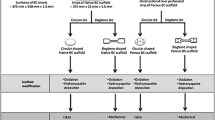
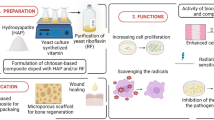
Abstract
The bone regeneration process is complex and challenging and requires the application of biomaterials to promote adequate tissue growth and repair. Biomaterials traditionally used are produced with biocompatible and bioinert metal alloys, not presenting any response in the recipient tissue, whether negative, such as inflammation and infections, or positive, such as rapid and effective healing of the injured tissue. Using biomaterials with an active compound adsorbed in their structure allows a direct interaction between the material and the injured tissue, and consequent modulation of biological responses to promote bone formation. Such biomaterials can facilitate the adhesion of osteoprogenitor cells and other important biological factors for bone tissue regeneration and remodeling. This review explores the importance of considering adsorption during biomaterials production and understanding the bone regeneration process. In addition, focus is given to biomaterials produced from biopolymers based on cellulose and hydroxyapatite, as well as mechanisms of bone regeneration. Challenges remain for optimizing these processes, and the adsorption properties of different materials must be carefully investigated to guarantee adequate interaction with bone tissues and cells. Furthermore, the development of strategies to control the release of adsorbed components is crucial to obtain efficient and targeted bone tissue regeneration.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hao, L., Li, T., Wang, L., Shi, X., Fan, Y., Du, C., Wang, Y.: Mechanistic insights into the adsorption and bioactivity of fibronectin on surfaces with varying chemistries by a combination of experimental strategies and molecular simulations. Bioact. Mater. 6, 3125–3135 (2021). https://doi.org/10.1016/j.bioactmat.2021.02.021
Huang, B., He, M., Zhang, K., Sun, S., Lin, Z., Chen, D., Li, T., Chen, X.: Rotation-induced secondary structure losses and bioactivity changes of bone morphogenetic protein-2 on strontium-substituted hydroxyapatite surfaces. Appl. Surf. Sci. 511, 145623 (2020). https://doi.org/10.1016/j.apsusc.2020.145623
Toledano, M., Carrasco-Carmona, Á., Medina-Castillo, A.L., Toledano-Osorio, M., Osorio, R.: Protein adsorption and bioactivity of functionalized electrospun membranes for bone regeneration. J. Dent. 102, 103473 (2020). https://doi.org/10.1016/j.jdent.2020.103473
Kalfas, I.H.: Principles of bone healing. Neurosurg. Focus 10, 22–25 (2001). https://doi.org/10.3171/foc.2001.10.4.2
An, J., Leeuwenburgh, S., Wolke, J., Jansen, J.: Mineralization processes in hard tissue. Bone (2016). https://doi.org/10.1016/B978-1-78242-338-6.00005-3
Buck, D.W., Dumanian, G.A.: Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 129, 1314–1320 (2012). https://doi.org/10.1097/PRS.0b013e31824eca94
Qiu, Z., Cui, Y., Wang, X.: Natural Bone Tissue and Its Biomimetic. Elsevier Ltd, Amsterdam (2019)
Komori, T.: What is the function of osteocalcin? J. Oral Biosci. 62, 223–227 (2020). https://doi.org/10.1016/j.job.2020.05.004
Hao, J., Shen, M., Wang, C., Wei, J., Wan, Q., Zhu, Y., Ye, T., Luo, M., Qin, W., Li, Y., Jiao, K., Zhao, B., Niu, L.: Regulation of biomineralization by proteoglycans: from mechanisms to application. Carbohydr. Polym. 294, 119773 (2022). https://doi.org/10.1016/j.carbpol.2022.119773
Akshata, C.R., Harichandran, G., Murugan, E.: Effect of pectin on the crystallization of strontium substituted HA for bone reconstruction application. Colloids Surf. B 226, 113312 (2023). https://doi.org/10.1016/j.colsurfb.2023.113312
Akshata, C.R., Murugan, E., Harichandran, G.: Alginate templated synthesis, characterization and in vitro osteogenic evaluation of strontium-substituted hydroxyapatite. Int J Biol Macromol. 252, 126478 (2023). https://doi.org/10.1016/j.ijbiomac.2023.126478
Luz, E.P.C.G., das Chagas, B.S., de Almeida, N.T., de Fátima Borges, M., Andrade, F.K., Muniz, C.R., Castro-Silva, I.I., Teixeira, E.H., Popat, K., de Freitas Rosa, M., Vieira, R.S.: Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater. Sci. Eng. C. 116, 111175 (2020). https://doi.org/10.1016/j.msec.2020.111175
Ataie, M., Nourmohammadi, J., Seyedjafari, E.: Carboxymethyl carrageenan immobilized on 3D-printed polycaprolactone scaffold for the adsorption of calcium phosphate/strontium phosphate adapted to bone regeneration. Int. J. Biol. Macromol. 206, 861–874 (2022). https://doi.org/10.1016/j.ijbiomac.2022.03.096
Ayawei, N., Ebelegi, A.N., Wankasi, D.: Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 1–11 (2017). https://doi.org/10.1155/2017/3039817
Da̧browski, A: Adsorption—from theory to practice. Adv. Colloid Interface Sci. 93, 135–224 (2001). https://doi.org/10.1016/S0001-8686(00)00082-8
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley-Interscience, New York (1984)
Kalam, S., Abu-Khamsin, S.A., Kamal, M.S., Patil, S.: Surfactant adsorption isotherms: a review. ACS Omega 6, 32342–32348 (2021). https://doi.org/10.1021/acsomega.1c04661
Ponec, V., Knor, Z., Cerny, S.: Adsorption on Solids. (2018)
Pourhakkak, P., Taghizadeh, A., Taghizadeh, M., Ghaedi, M., Haghdoust, S.: Fundamentals of adsorption technology. Presented at the (2021)
Teng, Y., Wang, R., Wu, J.: Study of the fundamentals of adsorption systems. Appl. Therm. Eng. 17, 327–338 (1997). https://doi.org/10.1016/S1359-4311(96)00039-7
Yang, X., Wan, Y., Zheng, Y., He, F., Yu, Z., Huang, J., Wang, H., Ok, Y.S., Jiang, Y., Gao, B.: Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem. Eng. J. 366, 608–621 (2019). https://doi.org/10.1016/j.cej.2019.02.119
Hadi, P., Xu, M., Ning, C., Sze Ki Lin, C., McKay, G.: A critical review on preparation, characterization and utilization of sludge-derived activated carbons for wastewater treatment. Chem. Eng. J. 260, 895–906 (2015). https://doi.org/10.1016/j.cej.2014.08.088
Fiyadh, S.S., AlSaadi, M.A., Jaafar, W.Z., AlOmar, M.K., Fayaed, S.S., Mohd, N.S., Hin, L.S., El-Shafie, A.: Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 230, 783–793 (2019). https://doi.org/10.1016/j.jclepro.2019.05.154
Wu, K., Li, Y., Liu, T., Huang, Q., Yang, S., Wang, W., Jin, P.: The simultaneous adsorption of nitrate and phosphate by an organic-modified aluminum-manganese bimetal oxide: adsorption properties and mechanisms. Appl. Surf. Sci. 478, 539–551 (2019). https://doi.org/10.1016/j.apsusc.2019.01.194
Gao, X., Guo, C., Hao, J., Zhao, Z., Long, H., Li, M.: Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 164, 4423–4434 (2020). https://doi.org/10.1016/j.ijbiomac.2020.09.046
Patel, H.: Fixed-bed column adsorption study: a comprehensive review. Appl. Water Sci. 9, 45 (2019). https://doi.org/10.1007/s13201-019-0927-7
Vidali, G., Ihm, G., Kim, H.-Y., Cole, M.W.: Potentials of physical adsorption. Surf. Sci. Rep. 12, 135–181 (1991). https://doi.org/10.1016/0167-5729(91)90012-M
Agboola, O.D., Benson, N.U.: Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: a review. Front. Environ. Sci. (2021). https://doi.org/10.3389/fenvs.2021.678574
Kumar, E., Bhatnagar, A., Hogland, W., Marques, M., Sillanpää, M.: Interaction of inorganic anions with iron-mineral adsorbents in aqueous media—a review. Adv. Colloid Interface Sci. 203, 11–21 (2014). https://doi.org/10.1016/j.cis.2013.10.026
Gayathiri, M., Pulingam, T., Lee, K.T., Sudesh, K.: Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism. Chemosphere 294, 133764 (2022). https://doi.org/10.1016/j.chemosphere.2022.133764
Ali, I., Gupta, V.K.: Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2006). https://doi.org/10.1038/nprot.2006.370
Foroutan, R., Peighambardoust, S.J., Hosseini, S.S., Akbari, A., Ramavandi, B.: Hydroxyapatite biomaterial production from chicken (femur and beak) and fishbone waste through a chemical less method for Cd2+ removal from shipbuilding wastewater. J. Hazard. Mater. 413, 125428 (2021). https://doi.org/10.1016/j.jhazmat.2021.125428
Sirajudheen, P., Poovathumkuzhi, N.C., Vigneshwaran, S., Chelaveettil, B.M., Meenakshi, S.: Applications of chitin and chitosan based biomaterials for the adsorptive removal of textile dyes from water—a comprehensive review. Carbohydr. Polym. 273, 118604 (2021). https://doi.org/10.1016/j.carbpol.2021.118604
Giorgio, I., Dell’Isola, F., Andreaus, U., Alzahrani, F., Hayat, T., Lekszycki, T.: On mechanically driven biological stimulus for bone remodeling as a diffusive phenomenon. Biomech. Model. Mechanobiol. 18, 1639–1663 (2019). https://doi.org/10.1007/s10237-019-01166-w
Mikai, A., Ono, M., Tosa, I., Nguyen, H.T.T., Hara, E.S., Nosho, S., Kimura-Ono, A., Nawachi, K., Takarada, T., Kuboki, T., Oohashi, T.: BMP-2/β-TCP Local Delivery for Bone Regeneration in MRONJ-Like Mouse Model. Int. J. Mol. Sci. 21, 7028 (2020). https://doi.org/10.3390/ijms21197028
Wei, F., Flowerdew, K., Kinzel, M., Perotti, L.E., Asiatico, J., Omer, M., Hovell, C., Reumers, V., Coathup, M.J.: Changes in interstitial fluid flow, mass transport and the bone cell response in microgravity and normogravity. Bone Res. 10, 65 (2022). https://doi.org/10.1038/s41413-022-00234-9
Marongiu, G., Dolci, A., Verona, M., Capone, A.: The biology and treatment of acute long-bones diaphyseal fractures: overview of the current options for bone healing enhancement. Bone Rep. 12, 100249 (2020). https://doi.org/10.1016/j.bonr.2020.100249
Maruyama, M., Rhee, C., Utsunomiya, T., Zhang, N., Ueno, M., Yao, Z., Goodman, S.B.: Modulation of the inflammatory response and bone healing. Front. Endocrinol. (2020). https://doi.org/10.3389/fendo.2020.00386
Al-Bari, A.A., Al Mamun, A.: Current advances in regulation of bone homeostasis. FASEB BioAdv. 2, 668–679 (2020). https://doi.org/10.1096/fba.2020-00058
Duan, L., Liang, Y., Xu, X., Wang, J., Li, X., Sun, D., Deng, Z., Li, W., Wang, D.: Noncoding RNAs in subchondral bone osteoclast function and their therapeutic potential for osteoarthritis. Arthritis Res. Ther. 22, 279 (2020). https://doi.org/10.1186/s13075-020-02374-x
Xie, Y., Hu, C., Feng, Y., Li, D., Ai, T., Huang, Y., Chen, X., Huang, L., Tan, J.: Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 7, 233–245 (2020). https://doi.org/10.1093/rb/rbaa006
Civitelli, R., Ziambaras, K.: Calcium and phosphate homeostasis: concerted interplay of new regulators., (2011)
Balasooriya, I.L., Chen, J., Korale Gedara, S.M., Han, Y., Wickramaratne, M.N.: Applications of nano hydroxyapatite as adsorbents: a review. Nanomaterials 12, 2324 (2022). https://doi.org/10.3390/nano12142324
Yang, Y., Xiao, Y.: Biomaterials regulating bone hematoma for osteogenesis. Adv. Healthc. Mater. 9, 2000726 (2020). https://doi.org/10.1002/adhm.202000726
Zheng, K., Kapp, M., Boccaccini, A.R.: Protein interactions with bioactive glass surfaces: a review. Appl. Mater. Today 15, 350–371 (2019). https://doi.org/10.1016/j.apmt.2019.02.003
Caballé-Serrano, J., Abdeslam-Mohamed, Y., Munar-Frau, A., Fujioka-Kobayashi, M., Hernández-Alfaro, F., Miron, R.: Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: a systematic review. Arch. Oral Biol. 100, 57–68 (2019). https://doi.org/10.1016/j.archoralbio.2019.02.006
Ivanets, A.I., Kitikova, N.V., Shashkova, I.L., Roshchina, M.Y., Srivastava, V., Sillanpää, M.: Adsorption performance of hydroxyapatite with different crystalline and porous structure towards metal ions in multicomponent solution. J. Water Process Eng. 32, 100963 (2019). https://doi.org/10.1016/j.jwpe.2019.100963
Izquierdo-Barba, I., Santos-Ruiz, L., Becerra, J., Feito, M.J., Fernández-Villa, D., Serrano, M.C., Díaz-Güemes, I., Fernández-Tomé, B., Enciso, S., Sánchez-Margallo, F.M., Monopoli, D., Afonso, H., Portolés, M.T., Arcos, D., Vallet-Regí, M.: Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 83, 456–466 (2019). https://doi.org/10.1016/j.actbio.2018.11.017
Liu, Z., Yamada, S., Otsuka, Y., Peñaflor Galindo, T.G., Tagaya, M.: Surface modification of hydroxyapatite nanoparticles for bone regeneration by controlling their surface hydration and protein adsorption states. Dalt. Trans. 51, 9572–9583 (2022). https://doi.org/10.1039/D2DT00969B
Ma, K., Cui, H., Zhou, A., Wu, H., Dong, X., Zu, F., Yi, J., Wang, R., Xu, Q.: Mesoporous hydroxyapatite: synthesis in molecular self-assembly and adsorption properties. Microporous Mesoporous Mater. 323, 111164 (2021). https://doi.org/10.1016/j.micromeso.2021.111164
Seims, K.B., Hunt, N.K., Chow, L.W.: Strategies to control or mimic growth factor activity for bone, cartilage, and osteochondral tissue engineering. Bioconjug. Chem. 32, 861–878 (2021). https://doi.org/10.1021/acs.bioconjchem.1c00090
You, Y., Keqi, Q., Huang, Z., Ma, R., Shi, C., Li, X., Liu, D., Dong, M., Guo, Z.: Sodium alginate templated hydroxyapatite/calcium silicate composite adsorbents for efficient dye removal from polluted water. Int. J. Biol. Macromol. 141, 1035–1043 (2019). https://doi.org/10.1016/j.ijbiomac.2019.09.082
Bal, Z., Kaito, T., Korkusuz, F., Yoshikawa, H.: Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 3, 521–544 (2020). https://doi.org/10.1007/s42247-019-00063-3
Gavinho, S.R., Bozdag, M., Kalkandelen, C., Regadas, J.S., Jakka, S.K., Gunduz, O., Oktar, F.N., Graça, M.P.F.: An eco-friendly process to extract hydroxyapatite from sheep bones for regenerative medicine: structural, morphologic and electrical studies. J. Funct. Biomater. 14, 279 (2023). https://doi.org/10.3390/jfb14050279
Hou, X., Zhang, L., Zhou, Z., Luo, X., Wang, T., Zhao, X., Lu, B., Chen, F., Zheng, L.: Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 13, 187 (2022). https://doi.org/10.3390/jfb13040187
Ielo, I., Calabrese, G., De Luca, G., Conoci, S.: Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 23, 9721 (2022). https://doi.org/10.3390/ijms23179721
Gorgieva, T.: Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials 9, 1352 (2019). https://doi.org/10.3390/nano9101352
Lin, D., Liu, Z., Shen, R., Chen, S., Yang, X.: Bacterial cellulose in food industry: current research and future prospects. Int. J. Biol. Macromol. 158, 1007–1019 (2020). https://doi.org/10.1016/j.ijbiomac.2020.04.230
Swingler, S., Gupta, A., Gibson, H., Kowalczuk, M., Heaselgrave, W., Radecka, I.: Recent advances and applications of bacterial cellulose in biomedicine. Polymers (Basel) 13, 412 (2021). https://doi.org/10.3390/polym13030412
Ahn, S.-J., Shin, Y.M., Kim, S.E., Jeong, S.I., Jeong, J.-O., Park, J.-S., Gwon, H.-J., Seo, D.E., Nho, Y.-C., Kang, S.S., Kim, C.-Y., Huh, J.-B., Lim, Y.-M.: Characterization of hydroxyapatite-coated bacterial cellulose scaffold for bone tissue engineering. Biotechnol. Bioprocess. Eng. 20, 948–955 (2015). https://doi.org/10.1007/s12257-015-0176-z
Torgbo, S., Sukyai, P.: Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 237, 121868 (2019). https://doi.org/10.1016/j.matchemphys.2019.121868
Núñez, D., Cáceres, R., Ide, W., Varaprasad, K., Oyarzún, P.: An ecofriendly nanocomposite of bacterial cellulose and hydroxyapatite efficiently removes lead from water. Int. J. Biol. Macromol. 165, 2711–2720 (2020). https://doi.org/10.1016/j.ijbiomac.2020.10.055
Wen, Y., Liu, J., Jiang, L., Zhu, Z., He, S., He, S., Shao, W.: Development of intelligent/active food packaging film based on TEMPO-oxidized bacterial cellulose containing thymol and anthocyanin-rich purple potato extract for shelf life extension of shrimp. Food Packag. Shelf Life 29, 100709 (2021). https://doi.org/10.1016/j.fpsl.2021.100709
Valls, C., Cusola, O., Roncero, M.B.: Evaluating the potential of ozone in creating functional groups on cellulose. Cellulose 29, 6595–6610 (2022). https://doi.org/10.1007/s10570-022-04694-4
Solomevich, S.O., Dmitruk, E.I., Bychkovsky, P.M., Nebytov, A.E., Yurkshtovich, T.L., Golub, N.V.: Fabrication of oxidized bacterial cellulose by nitrogen dioxide in chloroform/cyclohexane as a highly loaded drug carrier for sustained release of cisplatin. Carbohydr. Polym. 248, 116745 (2020). https://doi.org/10.1016/j.carbpol.2020.116745
Luz, E.P.C.G., Chaves, P.H.S., de Vieira, L.A.P., Ribeiro, S.F., de Borges, M.F., Andrade, F.K., Muniz, C.R., Infantes-Molina, A., Rodríguez-Castellón, E., de Rosa, M.F., Vieira, R.S.: In vitro degradability and bioactivity of oxidized bacterial cellulose- hydroxyapatite composites. Carbohydr. Polym. 237, 116174 (2020). https://doi.org/10.1016/j.carbpol.2020.116174
Vasconcelos, N.F., Andrade, F.K., de Vieira, L.A.P., Vieira, R.S., Vaz, J.M., Chevallier, P., Mantovani, D., de Borges, M.F., de Rosa, M.F.: Oxidized bacterial cellulose membrane as support for enzyme immobilization: properties and morphological features. Cellulose 27, 3055–3083 (2020). https://doi.org/10.1007/s10570-020-02966-5
Luo, H., Xiong, G., Hu, D., Ren, K., Yao, F., Zhu, Y., Gao, C., Wan, Y.: Characterization of TEMPO-oxidized bacterial cellulose scaffolds for tissue engineering applications. Mater. Chem. Phys. 143, 373–379 (2013). https://doi.org/10.1016/j.matchemphys.2013.09.012
Yang, M., Zhen, W., Chen, H., Shan, Z.: Biomimetic design of oxidized bacterial cellulose-gelatin-hydroxyapatite nanocomposites. J. Bionic Eng. 13, 631–640 (2016). https://doi.org/10.1016/S1672-6529(16)60334-7
Abou-Zeid, R.E., Awwad, N.S., Nabil, S., Salama, A., Youssef, M.A.: Oxidized alginate/gelatin decorated silver nanoparticles as new nanocomposite for dye adsorption. Int. J. Biol. Macromol. 141, 1280–1286 (2019). https://doi.org/10.1016/j.ijbiomac.2019.09.076
Georgouvelas, D., Abdelhamid, H.N., Li, J., Edlund, U., Mathew, A.P.: All-cellulose functional membranes for water treatment: adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 264, 118044 (2021). https://doi.org/10.1016/j.carbpol.2021.118044
Rahmatika, A.M., Goi, Y., Kitamura, T., Widiyastuti, W., Ogi, T.: TEMPO-oxidized cellulose nanofiber (TOCN) decorated macroporous silica particles: synthesis, characterization, and their application in protein adsorption. Mater. Sci. Eng. C 105, 110033 (2019). https://doi.org/10.1016/j.msec.2019.110033
Cao, S., Li, Q., Zhang, S., Liu, K., Yang, Y., Chen, J.: Oxidized bacterial cellulose reinforced nanocomposite scaffolds for bone repair. Colloids Surf. B 211, 112316 (2022). https://doi.org/10.1016/j.colsurfb.2021.112316
Wsoo, M.A., Shahir, S., Mohd Bohari, S.P., Nayan, N.H.M., Razak, S.I.A.: A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: a new perspective. Carbohydr. Res. 491, 107978 (2020). https://doi.org/10.1016/j.carres.2020.107978
Yadav, N., Hakkarainen, M.: Degradable or not? Cellulose acetate as a model for complicated interplay between structure, environment and degradation. Chemosphere 265, 128731 (2021). https://doi.org/10.1016/j.chemosphere.2020.128731
Heinze, T., Liebert, T.: 4.2 Chemical characteristics of cellulose acetate. Macromol. Symp. 208, 167–238 (2004). https://doi.org/10.1002/masy.200450408
Maia, M.T., Luz, É.P.C.G., Andrade, F.K., de Rosa, M.F., de Borges, M.F., Arcanjo, M.R.A., Vieira, R.S.: Advances in bacterial cellulose/strontium apatite composites for bone applications. Polym. Rev. 61, 736–764 (2021). https://doi.org/10.1080/15583724.2021.1896543
Eslah, S., Nouri, M.: Fabrication of electrically conductive cellulose acetate/polyaniline/WO3 nanocomposite nanofibers with potential applications in electrochemical devices. Polym. Sci. Ser. A. 61, 345–356 (2019). https://doi.org/10.1134/S0965545X19030040
El-Naggar, M.E., Abdel-Karim, A., Radwan, E.K., Sharmoukh, W., Wassel, A.R., Bayoumy, A.M., Ibrahim, M.: Experimental and theoretical investigations on fouling resistant cellulose acetate/SiO2 NPs/PEDOT ultrafiltration nanocomposite membranes. J. Clean. Prod. 324, 129288 (2021). https://doi.org/10.1016/j.jclepro.2021.129288
Al-Harbi, N., Hussein, M.A., Al-Hadeethi, Y., Umar, A.: Cellulose acetate-hydroxyapatite-bioglass-zirconia nanocomposite particles as potential biomaterial: synthesis, characterization, and biological properties for bone application. Eng. Sci. (2021). https://doi.org/10.30919/es8d528
Zhong, Z., Qin, J., Ma, J.: Cellulose acetate/hydroxyapatite/chitosan coatings for improved corrosion resistance and bioactivity. Mater. Sci. Eng., C 49, 251–255 (2015). https://doi.org/10.1016/j.msec.2015.01.020
Kołodziejska, B., Stępień, N., Kolmas, J.: The influence of strontium on bone tissue metabolism and its application in osteoporosis treatment. Int. J. Mol. Sci. 22, 6564 (2021). https://doi.org/10.3390/ijms22126564
Luz, E.P.C.G., de Borges, M.F., Andrade, F.K., de Rosa, M.F., Infantes-Molina, A., Rodríguez-Castellón, E., Vieira, R.S.: Strontium delivery systems based on bacterial cellulose and hydroxyapatite for guided bone regeneration. Cellulose. 25, 6661–6679 (2018). https://doi.org/10.1007/s10570-018-2008-8
de Soares, A.L.B., Maia, M.T., Gomes, S.D.L., da Silva, T.F., Vieira, R.S.: Polysaccharide-based bioactive adsorbents for blood-contacting implant devices. Braz. J. Chem. Eng. (2022). https://doi.org/10.1007/s43153-022-00253-3
Acknowledgements
This research was financially supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq—442734/2020-4).
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq-442734/2020-4).
Author information
Authors and Affiliations
Contributions
ALdBS: investigation, methodology, formal analysis, writing—original draft, visualization. EPCGL: formal analysis, writing—review and editing, visualization, and RSV: formal analysis, resources, validation, review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Brito Soares, A.L., Luz, E.P.C.G. & Vieira, R.S. Adsorption processes for forming biomaterials of cellulose and hydroxyapatite for applications in bone tissue regeneration. Adsorption (2024). https://doi.org/10.1007/s10450-024-00441-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00441-2