Abstract
The need to extend the range of potential uses for the Simulated Moving Bed Reactor (SMBR) leads the development of new operational strategies that enable the equipment to perform increasingly difficult reactions and separations. Solketal is the valorization product from the reaction between glycerol and acetone, a thermodynamic limited process that faces miscibility and selectivity issues. In this work, the ModiCon SMBR is proposed and optimized aiming to overcome the difficulties of solketal synthesis in the Conventional SMBR. The optimization is based on the “Reactive-Separation Volumes” methodology, a well-established procedure followed for optimizing numerous other SMBR processes. The results demonstrate that the new SMBR operating mode can produce solketal with a purity of 97%, reaching a productivity of over 9.69 \({\text{kg}}_{{{\text{Solk}}}} {\text{ L}}_{{{\text{Ads}}}}^{{ - {1}}} {\text{ day}}^{{ - {1}}}\), 38% higher than the Multifeed, another non-conventional strategy proposed in the open literature for the synthesis of solketal. The desorbent consumption is 6.76 \({\text{L}}_{{{\text{Desorbent}}}} \,{\text{kg}}_{{{\text{Prod}}}}^{ - 1} ,\) 35% higher than the Multifeed. The most impressive result is the decrease in the reactants consumption of 12%. Such results demonstrate that this strategy intensifies the process even more than other non-conventional operating strategies proposed in the literature, which makes it more appealing to be implemented in the large scale solketal production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biofuels are among the most viable alternatives for replacing fossil fuels in the transportation sector, together with hydrogen, electric vehicles, and low carbon fuels [1,2,3]. The industrial production and distribution of biofuels are well-established, with the proper infrastructure already installed. Besides, applying them in automotive engines requires little modification of equipment [4]. The main drawback of this industry is that it generates large amounts of glycerol as by-product, in the proportion of 10 wt% of the total mass of diesel produced [5].
Owing to its abundance, which leads to a low cost, and to its physicochemical properties, glycerol is a platform chemical that has been deeply studied to find new valorisation routes [5]. A promising subproduct of this chemical is solketal, an auspicious fuel additive due to improving fuel efficiency and stability [6]. Furthermore, reinserting solketal in the biodiesel production chain complies with the Circular Economy model, which is of great economic and environmental interest [5].
For solketal synthesis, equimolar amounts of glycerol and acetone react in an acid environment, forming water as by-product (Fig. 1). The reaction is reversible, therefore one of the main difficulties is the thermodynamic limitation. This can be surpassed by adding one of the reactants in great excess (often acetone) or continuously removing one of the products from the reaction medium [7]. Considering the reactants’ cost and the energetic cost of downstream separation processes, it is much more advantageous to remove one of the products from the reaction medium instead of adding a large excess of acetone. With that in view, multifunctional reactors that combine reaction and separation in a single unit, have been proposed to improve the productivity while diminishing post-treatment costs. Among these new technologies, the most common are fixed-bed reactors, reactive distillation, membrane reactors, and simulated moving bed reactors (SMBR) [5, 7,8,9,10]. These technologies have been a valuable tool for improving the performance of thermodynamic limited reactions [5].
Glycerol reaction with acetone to produce solketal and water as by-product [11]
The SMBR is a sorption-enhanced Process Intensification strategy that improves the efficiency of reactive systems by combining separation by adsorption and chemical reaction using a hybrid stationary phase with catalytic activity and adsorption selectivity between the products. This way, by adsorbing one of the reaction products, the reaction equilibrium shifts towards product formation [12]. Besides, since the SMBR can achieve nearly complete conversion, the outlet streams are simple mixtures of mainly one of the reaction products and the eluent.
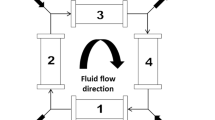
The SMBR is based on the well-established Simulated Moving Bed (SMB) separation technology. The SMB, in turn, is based on the True Moving Bed (TMB), a continuous chromatographic unit that promotes the countercurrent contact between the solid and liquid phases to improve the separation. The conventional TMB is composed of two inlet and two outlet streams positioned at different locations and that divide the unit into four sections. The effectiveness of the separation is related to the flow rate of the solid and liquid phases in each section [13].
The SMB avoids the complications associated with moving the solid phase by periodically shifting the inlet and outlet positions in the direction of the fluid flow in a series of fixed-bed columns (which simulates the solid movement). The duration of the period between each shift is named time switch (t*), and one cycle is complete when the streams reach their initial position [13]. The appropriate separation depends on the sufficient residence time of the species inside the columns, which is directly related to the t* and to the streams flow rate.
Similarly to the SMB the SMBR is conventionally composed of four sections organized in a closed loop, each operating at specific flow rates, with two inlet and two outlet streams, synchronous t*, constant flow rates and constant feed concentration [13]. The t* takes even more relevance on the SMBR performance, once it determines the residence time and, consequently, to what extent the reaction will occur [14].
Solketal synthesis was studied and optimized in the Conventional SMBR using Amberlyst-35, a hybrid solid with adsorptive and catalytic activity. The results were not encouraging, since the high characteristic diffusion times of the species, the low conversion, the low adsorption selectivity between acetone and solketal and the highly different selectivity of glycerol and acetone dramatically limited the process performance, making its practical implementation unfeasible [10]. Furthermore, unlike other SMBR processes in which one of the reactants may be used as eluent, due to the miscibility limitation between acetone and glycerol, an auxiliary solvent had to be used to guarantee that the system is single phased at all times and in all points of the unit. This also impairs the performance to some extent [10].
Following the same trend of the SMB, the SMBR operation has been scrutinized to develop new processes, modes of operation and stationary phases able to produce new chemicals or to enhance the productivity of already consolidated processes. Indeed, one of the major advantages of the SMBR technology is its versatility, since the number of degrees of freedom can be manipulated to extend the range of potential applications and reach even better performance parameters. These alternative operating strategies are generally called non-conventional operation modes. The number of sections, the number of inlet and outlet ports, the feed composition and other dynamic configurations can be modified with relatively simple adaptations on the physical equipment [15,16,17,18].
One of the most studied SMBR non-conventional operations is the VariCol, which consists of asynchronously shifting the port position within the time switch, to vary the number of columns in each section [19]. Since each section of the SMBR is responsible for performing a task (adsorption, desorption, and chemical reaction), it may be useful to increase/decrease the area where each process takes place depending on how limited they are. Manipulating the size of the reactive area and increasing the reactants’ interaction is especially useful for thermodynamic limited reactions, such as solketal synthesis.
The Multifeed SMBR was studied and optimized specifically for this reaction. This strategy proposes to feed the reactants in different positions, acetone near the extract port and glycerol near the raffinate, to take advantage of the different affinities of the species with the stationary phase and promote their countercurrent contact. This way, since the contact time of the reactants was enhanced and the raffinate contamination was avoided due to the larger distance between the acetone feed port and solketal withdrawn port, higher flow rates could be employed. Ultimately, the goal of increasing the unit productivity was achieved [10].
The periodically Modulated Feed Concentration strategy (ModiCon) is one of the most thoroughly studied SMB operating strategies, but not for reactive processes. This is a dynamic operating strategy of modulating the feed stream concentration throughout the switching time, this way the unit benefits from having at least two more degrees of freedom: the length of the subintervals of the time switch, and the feed concentration in each of these subintervals. Agrawal et al. produced one of the few works on the ModiCon SMBR, where the esterification of propylene glycol methyl ether acetate (PMA) from 1-methoxy-2-propanol (PM) and acetic acid was studied using a hybrid stationary phase. In this process, PM is used as reactant and desorbent, therefore in the first fraction of the time switch, this compound is fed alone and in the second a reactive mixture with acetic acid is fed [20].
Nonetheless, the ModiCon SMBR may be even more advantageous when none of the reactants can be used as desorbent (as solketal synthesis) due to enhancing their contact. For producing solketal using this operating strategy, glycerol (the most retained reactant) is fed in the first fraction of the time switch, followed then by acetone. This way, similarly to the effect in the Multifeed SMBR, the countercurrent contact of the reactants improves their interaction and, consequently, solketal output. Furthermore, as the acetone concentration front is further apart from the raffinate port because it is inserted later, it is possible to reduce the raffinate stream contamination.
The main goal of this work is developing and optimizing solketal synthesis in the ModiCon SMBR. This process is proposed for the first time in the open literature. To evaluate the improvement of implementing such strategy, the process is compared with the Multifeed SMBR proposed by Faria et al. [10]. In the present work, both non-conventional operations are modelled and optimized considering the same unit, an SMBR pilot plant available at our laboratories. The optimization methodology proposed by Faria et al. is applied to define the main SMBR design variables of the Multifeed SMBR, namely sectional flow rates, configuration and time switch [10].
Since the Multifeed and the ModiCon SMBR improvement is based on the same principles of maximizing the contact of the reactants and keeping acetone concentration front further apart from the raffinate collection port, the Multifeed optimization was seen as an exploration step of the ModiCon optimization. The ModiCon optimization, in turn, was seen as the exploitation step, and followed the approach of defining the Reactive Separation Region. In practice, the optimal points of the Multifeed SMBR were used as inputs for the ModiCon optimization. Based on previous studies, the best combination of stationary phase and desorbent is Amberlyst-35 and ethanol, respectively, at 303 K to assure the miscibility and the reaction compounds and achieve the best conversion [11, 21].
2 Simulated moving bed reactor
The SMBR is a chromatographic reactor that operates continuously and promotes the countercurrent contact between the liquid and stationary phase to improve the separation [13]. The unit considered is a pilot-scale SMBR installed at our laboratories. It is composed of 8 columns divided into four sections, with two inlet and two outlet streams and synchronous shift of these streams. The fixed-bed columns are equally packed with the dimensions and properties described in Table 1.
The stationary phase selected was Amberlyst-35, a macroreticular ion exchange resins composed by a styrene divinylbenzene polymeric matrix functionalized with sulfonic groups. It is a hybrid solid that acts simultaneously as catalyst and adsorbent. The most relevant physical–chemical properties of Amberlyst-35 can be found elsewhere [10].
3 Mathematical model
3.1 Fundamental adsorption and reaction data
The independent studies of the adsorption and of the reaction are essential to predict the behaviour of the process and allow better design, modelling, and optimization. In a previous work, a solvent and catalyst/adsorbent screening was performed, and ethanol and Amberlyst-35 were selected as the mobile and stationary phase of the sorption enhanced reaction between acetone and glycerol [21].
The chemical equilibrium data was determined by experiments performed in a batch reactor between 303 and 323 K. The estimated standard enthalpy and a free Gibbs were − 20.1 ± 1.1 kJ mol−1 and 1.4 ± 0.3 kJ mol−1, respectively [7]. As for the reaction kinetics, the experimental data could be described by the Langmuir–Hinshelwood-Hougen-Watson reaction rate law (Eq. 1) expressed in terms of the activities \(a\) (calculated using the UNIFAC model) [5, 7].
The reaction activation energy, \({E}_{a}\), was 69.0 ± 6.6 kJ mol−1, the pre-exponential factor, \({k}_{c,0}\), was 492 ± 93 \({\text{mol kg}}_{{{\text{Cat}}}}^{ - 1} \,{\text{s}}^{ - 1} ,\) \({T}_{ref}\) was 313.15 K and the water adsorption constant, \({K}_{S,W}\), was 14.4 ± 3.1. The experiments were performed considering the presence of internal mass transfer resistance and under experimental conditions that guarantee the absence of external mass transfer resistance.
The adsorption equilibrium isotherm of each compound with ethanol over Amberlyst-35 were determined in a previous work through the frontal analysis methodology at three temperatures, 303 K, 313 K and 323 K. The methodology consists of sequentially feeding binary mixtures composed of ethanol and one of the reactants/products to a fixed-bed reactor until it gets saturated with the feed mixture. The competitive multicomponent Langmuir adsorption model (Eq. 2) could accurately describe the results [11].
where \({\overline{q} }_{i}\) represents the adsorbed concentration for component \(i\), \({Q}_{i}\) is the monolayer capacity, \({K}_{i}\) is the equilibrium constant, and \({\overline{C} }_{p,i}\) the average concentration in the particle pores.
3.2 Mathematical model and performance parameters
Relying on mathematical models to estimate the design variables and predict the dynamic behavior of the SMBR is of great value for comparing the performance of different operating strategies before implementing the process. The SMBR model considers the following assumptions: (1) isothermal operation; (2) plug-flow model with axial dispersion and negligible radial dispersion; (3) constant packing porosity and bed length; (4) velocity variations due to changes in the bulk composition; (5) internal and external mass transfer resistances lumped into a global mass transfer coefficient; (6) multicomponent adsorption equilibrium described by the competitive Langmuir adsorption isotherm model. The non-idealities of the system were estimated and considered by determining the activity coefficients through the UNIFAC method.
In previous studies, the mathematical model developed was simplified by developing an analogous TMBR model [10, 11]. This strategy reduces the computational effort by directly using the steady-state equations to solve the model, rather than solving the system dynamically until reaching the cyclic steady state. This simplification is also used in the present work for the simulations of the Multifeed operating strategy. As for the ModiCon, since it is a dynamic non-conventional operational strategy, it is not possible to numerically solve the model by using steady-state equations. The equations for the dynamic model are described below:
Mass Balance—Bulk:
Throughout this work, the indexes \(i\) and \(j\) will always refer to the species and the SMBR section, respectively. \({C}_{i,j}\) represents the bulk concentration, \({u}_{j}\) is the interstitial fluid velocity, \({\varepsilon }_{b}\) the bed porosity, \({r}_{p}\) the particle radius, \({k}_{L,i,j}\) the global mass transfer coefficient, \({D}_{ax,j}\) the axial dispersion coefficient, \(t\) is time, and \(z\) is the axial coordinate.
Interstitial fluid velocity variation (obtained from the global mass balance to the system):
\(V_{M,i}\) is the molar volume of component \(i\).
Mass Balance—Particle:
where \({\varepsilon }_{p}\) is the particle porosity, and \({\rho }_{b}\) is the bulk density. The variables \({\nu }_{i}\) and \(\mathfrak{R}\) are related to the chemical reaction and represent component’s \(i\) stoichiometric coefficient and the reaction rate based on the local intraparticle composition, respectively. Equation (5) represents the mass balance in the particle for the SMBR model. For the Multifeed modelling using the TMBR, it is necessary to account for the solid velocity, therefore the mass balance in the particle is represented by Eq. 6:
Initial and Danckwerts boundary conditions:
where \({x}_{i}\) is the molar fraction of component \(i\) and \(L\) is the column length.
Global mass transfer coefficient:
where \({k}_{ext}\) and \({k}_{int}\) are the external and internal mass transfer coefficients, respectively. The internal mass transfer coefficient was estimated by Eq. 14 [22], while the external mass transfer coefficient was estimated by the Wilson and Geankopolis correlation [23] (Eq. 15).
where \({D}_{i, m}\) is the molecular diffusivity coefficient of a compound in a mixture, \({\tau }_{p}\) is the particle tortuosity, \({Sh}_{p}\) and \({Re}_{p}\) are the Sherwood and Reynolds numbers relative to the particle, respectively, described by Eqs. 16 and 17, and \(Sc\) is the Schmidt number (Eq. 18).
where \(\rho\) is the fluid phase density and \({\eta }_{m}\) is the mixture viscosity.
To compute the molecular diffusivity, \({D}_{i,m}\), the infinite dilution diffusivities must be first estimated through the Scheibel correlation [24]:
where \({D}_{i,j}^{0}\) is the diffusion coefficient for a dilute solute \(i\) in a solvent \(j\), \(T\) is the temperature, \({\eta }_{j}\) is the viscosity of solvent \(j\). Then, for multicomponent systems, the Perkins and Geankoplis correlation [25] can be used to predict \({D}_{i,m}\):
Finally, the effective diffusion coefficient (\({D}_{eff,i}\)) is estimated as follows.
The inlet and outlet stream concentrations are estimated by the mass balance to the nodes, represented by the following equations:
where \(Q\) represents the fluid flow rate and the indexes \(I\), \(II\), \(III\) and \(IV\) the sections of the SMBR. The indexes \(Rec\), \(D\), \(X\), \(R\) and \(F\) refer to the recycle, desorbent, extract, raffinate and feed streams. The variables \({n}_{D}\), \({n}_{X}\), \({n}_{F}\) and \({n}_{R}\) mean the number of the column immediately after the desorbent, extract, feed and raffinate nodes, which are incremented by the number of positions each stream moves in the direction of the fluid flow.
In the ModiCon operating strategy, the time switch (\({t}^{*}\)) may be divided in as many subintervals as desired, with the feed concentration (\({C}_{F, i}\)) pattern admitting as many configurations as possible [26]. Equations 26 and 27 represent these variables:
To completely describe the system, the performance of the SMBR is quantitatively assessed through the Raffinate and Extract Purity (\(PuR\) and \(PuX\)), limiting reactant conversion (\({X}_{lim}\)), raffinate productivity (\(PR\)), and desorbent consumption (\(DC\)). All the performance parameters are estimated considering the average concentration over a complete cycle.
3.3 Numerical solution
The model equations were numerically solved using the commercial software General PROcess Modelling System (gPROMS), version 7.0.7. A method of orthogonal collocation in finite elements (OCFEM) was used, discretizing the axial domain of each SMBR column in 30 finite elements, with two interior collocation points in each element. The resulting system of ordinary differential equations was solved in time using a Differential–Algebraic solver (DASOLV) integrated in the gPROMS software, with a 10–5 tolerance.
The optimization of the Multifeed SMBR design variables was conducted with gPROMS, using the NLPSQP (Non-Linear Problem Sequential Quadratic Programming) solver included in the software, which solves nonlinear optimization problems by applying a sequential quadratic programming method. The convergence tolerance was set to 10–5. The objective function was the productivity maximization, limited by the minimum commercially required solketal purity in the raffinate and a water purity in the extract of 97%.
4 Optimization of the non-conventional SMBR
4.1 Multifeed SMBR
Considering the numerous variables that can be manipulated in the SMBR operation, the optimization is numerically complex and there are multiple optimal points that can lead to appropriate productivity and purity. This justifies the development of an equivalent Multifeed TMBR model, which enables the direct use of steady-state equations in the model, significantly decreasing the computational effort and the required time to complete this procedure.
According to the methodology proposed by Faria et al. and followed in the present study, the design of the SMBR may be simplified by approximating its behaviour to the TMBR. This approach, the so-called Equilibrium Theory, estimates the flow rate ratios between the liquid and the solid phases (γ) that guarantee the separation of the reaction products through simple analytical solutions, mathematically represented by Eqs. 33 and 34. This design strategy neglects the effect of axial dispersion and mass transport resistances. To account for the effects of these phenomena, safety factors (\({\beta }_{j}\)) are considered (Eqs. 35 and 36) [10, 13].
Following the Multifeed SMBR optimization proposed by Faria et al., to determine the best operating conditions, the first step was performing independent optimizations for different column configuration with the safety factor (β) of 30%, high enough to assure the solid and the desorbent proper regeneration. By specifying the β, one defines the limit flow rate ratios between the liquid and the solid phases in the desorbent and adsorbent regenerations sections (Sections I and IV, respectively), estimated by the mentioned Equilibrium Theory [10].
By setting the column configuration and the β for the regeneration sections, the decision variables to be optimized were reduced to solid velocity, extract flow rate and feed 1 and feed 2 flow rates. Afterwards, in the second step of the study, independent optimizations were performed by changing the β (and consequently the optimal feed and extract flow rates and the solid velocity) from 5 to 40% targeting maximum productivity (objective function), subjected to a minimum solketal purity in the raffinate and water purity in the extract of 97% (optimization constraints). Finally, in the third step of the optimization, a careful study on the equivalence between the TMBR and the SMBR was performed to assure the first can describe with sufficient accuracy the real unit.
4.2 ModiCon SMBR
The advantage of implementing ModiCon, namely, the flexibility provided by adding at least two more degrees of freedom, is precisely what increases the complexity of the optimization from the numerical perspective. Compared to the Multifeed SMBR, the ModiCon optimization is more demanding in terms of time and computational effort because it is a dynamic non-conventional operating strategy, thus the model equations must be dynamically solved, starting from the initial conditions given by Eq. (7) and successively simulating each cycle, with the bed conditions from the previous cycle being the initial conditions for the next, until CSS is reached [27].
The computational effort to solve CSS problems is extensive, which makes unfeasible to reproduce the optimization procedure proposed for the Conventional and the Multifeed SMBR [10]. Indeed, most of the literature on the ModiCon strategy concerns the separation of non-reactive mixtures under the optimal conditions of the Conventional SMB and the comparison of the performance of these units, rather than the rigorous optimization in a wide range of conditions [28].
Regarding the definition of the two new parameters, the duration of the subintervals of the time switch and the correspondent feed concentration of each subinterval, the concentration of the feed stream for the Conventional and the Multifeed SMBR was addressed in previous works, based on the fundamental study of Moreira et al. [10, 21]. The authors concluded that reducing the amount of reactants fed to the unit, or, in other words, diluting the feed stream in ethanol, negatively affected not only the reaction kinetics, but the equilibrium conversion [21]. It is inevitable to feed a diluted stream in the Conventional SMBR due to the miscibility issues of the reactants in the reactive mixture. For the Multifeed SMBR, on the other hand, there is no problem in the miscibility at any point of the unit even feeding pure acetone and pure glycerol [10]. The same behavior occurs in the ModiCon, therefore the feed stream was constituted of 100% glycerol in the first fraction of the time switch and 100% acetone in the second fraction. This reduces the number of decision variables in the simulation studies.
As stated in the Introduction, the ModiCon operation is closer to the Multifeed than to the Conventional due to the better equivalence between the reactants feed methods and especially due to the countercurrent contact of the reactants that is typical of these operations. Therefore, the initial input of the ModiCon simulation studies is the result of the optimization of the Multifeed SMBR, namely optimal column configuration, combination of β, time switch and ratio of reactants fed to the unit.
By defining the β applied to Sections I and IV, one defines the flow rates of the desorbent and the recycle streams. Also, knowing the ratio of the reactants fed to the unit, it is possible to estimate the duration of each subinterval of the time switch. Since the time switch is one of the main decision variables of the process due to the reaction and mass transfer kinetics, a sensitivity analysis was performed to this variable. Also, the process is highly sensitive to the reactants’ excess, therefore the impact of this variable on the process was assessed through a sensitivity analysis to the duration of the subintervals of the time switch.
The optimization procedure followed in this work is based on the separation volume optimization. This procedure proposes finding the operation point in the \(({\gamma }_{2} \times {\gamma }_{3})\) plane that is further away from the diagonal within the required purity constraints [29, 30]. The optimization comprises two consecutive steps. In the first, a sensitivity analysis to flow rate rations Sections I and IV is performed by imposing high β to both sections and searching for the \(({\gamma }_{2}, {\gamma }_{3})\) combination that maximizes the productivity subjected to the purity constraints of 97% in the raffinate and extract streams. This value is stored, and the procedure is repeated for the same value of \({\gamma }_{1}\) and a lower value of \({\gamma }_{4}\) until there is no more operational point under the purity constraints. Then the procedure is repeated for a lower \({\gamma }_{1}\) until the purity constrains are no longer satisfied, demonstrating that the separation of the reaction products is unfeasible.
In the second step of the optimization, for the combination of SFs that perform better, a sensitivity analysis to the time switch is carried out. Similarly to the procedure followed in the previous step, the feed and extract flow rates are optimized, with the objective function of maximizing the productivity. Finally, following the same procedure, a sensitivity analysis to the duration of the subintervals of the time switch is performed.
5 Results and discussion
5.1 Multifeed SMBR
5.1.1 Sensitivity analysis of the unit’s configuration
As mentioned in the introduction, the number of columns on the reactive area of the SMBR has a considerable impact on thermodynamically limited reactions, therefore the unit’s configuration was the first variable analysed. The optimal flow rate ratios in each section, solid velocity and performance parameters for different column configurations are summarized in Table 2.
The results demonstrate the viability of producing solketal in a continuous chromatographic reactor using the non-conventional strategy once the configurations that performed better achieve productivities in line with the range of values reported in the open literature for similar systems [10, 31,32,33]. The results are even more remarkable when compared to the Conventional SMBR, using the 1-2-4-1 configuration to assure a similar residence time in each section (× mark on Fig. 3). The productivity increases more than 7.5 times, and the solid velocity increases by almost 30%, which combined with the increase in the feed flow rates contribute to an increment of nearly 6 times in the amount of the reactants fed to the unit. The Multifeed SMBR is a clear example of Process Intensification once the unit performance is exceedingly increased by the much more extensive use of the unit and the stationary phase.
The considerable increase in the reactor operational parameters and consequent improvement in the reactor performance was made possible by the countercurrent movement of the reactants inside the unit promoted by the strategy of feeding the reactants through different ports. This way, one of the main impairments of the Conventional SMBR, the different affinities of the reactants, was overcome because their contact time was appreciably extended. This can be confirmed by the analysis of the internal concentration profiles attained under the optimal operating conditions for an SMBR with a 1-1-1-4-1 configuration, as shown in Fig. 2.
Internal concentration profiles within the Multifeed SMBR, with a 1-1-1-4-1 configuration, at 303 K, feeding pure glycerol and acetone through two independent feed streams. (\({{\gamma }_{1}}^{in}\) = 6.00, \({{\gamma }_{2}}^{in}\) = 0.71, \({{\gamma }_{3\mathrm{A}}}^{in}\) = 1.53, \({{\gamma }_{3\mathrm{B}}}^{in}\) = 2.32, \({{\gamma }_{4}}^{out}\) = 0.48, \({u}_{s}\) = 0.61 \({\text{cm}} \, {\text{min}}^{-1}\))
Another noteworthy conclusion is the linear relation between the productivity and the number of columns in Section IIIB, as shown in Fig. 3.
This reveals the productivity limitation by the length of this section, which is explained by the fact that most of the reaction occurs between the second feed port and the raffinate collection port (Fig. 2). Also, there is no significant difference in the unit productivity for configurations with the same number of columns in Section IIIB, revealing that the unit performance is driven by this parameter. Since the maximum number of columns in Section IIIB is four, the only possible configuration is 1–1-1–4-1.
5.1.2 Optimization of the Multifeed SMBR
The sensitivity analysis of the productivity (a) and the desorbent consumption (b) with variations in the flow rate ratios are presented in Fig. 4.
The safety factors (β) are used in Sections I and IV to assure the solid and liquid phases are properly regenerated before being recycled into the unit. Therefore, the regeneration will be more effective as higher β (higher \({\gamma }_{1}\) and lower \({\gamma }_{4}\)), and consequently, the productivity will also be enhanced, until a maximum value when there is no more improvement because the solid and liquid phases are completely regenerated. Indeed the unit reaches a maximum productivity plateau that is limited by the catalysts activity and the adsorbent capacity for these operational conditions. Yet, the higher productivity expected for a lower \({\gamma }_{4}\) is not observed due to the simultaneous increase in the solid velocity and consequent ability of the unit of processing higher flowrates when a higher \({\gamma }_{4}\) is applied.
For lower β applied to Section IV (higher \({\gamma }_{4}\)) the optimum solid velocity and the operational flow rates are higher, and the unit reaches higher productivity. It would be expected, then, a decrease in the DC, once this performance parameter is inversely proportional to the productivity. However, the DC is directly proportional to the difference between \({\gamma }_{1}\) and \({\gamma }_{4}\), so to keep \({\gamma }_{1}\) constant, the desorbent flow rate must also increase. This also justifies the increase in the DC by increasing the SF applied to Section I (consequently \({\gamma }_{1}\)), considering a constant \({\gamma }_{4}\). Besides, when the liquid phase is not properly regenerated, the unit runs with lower operational parameters and the performance is severely affected.
The conversion is strongly affected by the β applied to \({\gamma }_{4}\), while it remains almost unchanged with \({\gamma }_{1}\). By relying on a higher \({\gamma }_{4}\), the recycle flow rate, which is directly related to the flow rate ratio in Section IV, increased, together with the solid phase velocity. The flow rates in all the other sections only experience minor changes. The increment in these two variables, recycle flow rate and solid velocity, was enough to hinder the conversion by reducing the contact time of the reactants with the stationary phase. Due to the strong thermodynamic limitation of solketal synthesis, it is crucial to guarantee that the reactants have sufficient contact time with the catalyst to reach good conversion. The increase in \({\gamma }_{1}\) drives the increase in the solid velocity and all the operational flow rates. The reduced contact time resulting from the increase in the solid velocity was compensated by the higher amount of reactants fed to the unit, which fostered the direct reaction. This justifies the unchanged behaviour of the conversion with variations in \({\gamma }_{1}\).
A noteworthy conclusion of the sensitivity analysis to the safety factor is the approximately constant ratio between the reactants regardless of the \({\gamma }_{1}\) and \({\gamma }_{4}\) values considered. The ratio between the feed stream flow rates is 1.03, which results in a molar excess of acetone fed to the unit of approximately 5%. This small excess of acetone was removed together with water by the extract stream, and, due to the distance between acetone and the raffinate port, one of the key issues of the Conventional SMBR was avoided, the contamination of the raffinate stream with this reactant. The fact that the feed flow rates can be independently optimized demonstrates another major advantage of the Multifeed SMBR.
In the range of SF studied, the maximum productivity was attained for β of 40% in Section I and 10% in Section IV; however, when using the rigorous SMBR model, the desorbent is not sufficiently regenerated, and solketal ends up being recycled from Section IV to Section I and contaminating the extract stream. Therefore, for this combination of β, there is no operational condition under the extract and raffinate purity constraints. Increasing the β applied to Section IV to 25% provides comparable performance parameters, as exhibited in Table 3.
5.2 ModiCon SMBR
The effect of SFs variations on the productivity (a) and the desorbent consumption (b) for the ModiCon SMBR are exhibited in Fig. 5. It is noteworthy that no RSR was identified for a β lower than 30% in Section I. Also, the unit performance is severely affected when a β of 15% in Section IV is used, therefore this was set as the minimum β for this section. To assure a sufficiently large optimization area, the maximum β applied to Section I is 70% and to Section IV, 35%.
The 1-2-4-1 configuration of the ModiCon SMBR is the closest equivalent to the 1-1-1-4-1 configuration of the Multifeed. Most of the reaction takes place in Section III of the ModiCon and in Section IIIB of the Multifeed, therefore assigning more columns to these sections leads to better performance. Besides, it is where the most difficult separation occurs, between solketal and acetone.
As mentioned, a relatively large β in Section I (30%) is necessary to find a set of operational parameters under the purity constraints. From the analysis of the compound’s concentration profiles, it is verified that water concentration is high in Section I when lower β are used, therefore a minimum of 30% is necessary to assure the sufficient regeneration of the solid.
As mentioned in the optimization of the Multifeed SMBR, higher safety factors typically lead to better performances due to the better regeneration of the solid phase and eluent. Indeed, this behavior is observed in the ModiCon SMBR when a sufficient β is applied to \({\gamma }_{4}\) to assure the regeneration of the liquid phase. When \({\gamma }_{4}\) is close to the value of the equilibrium theory, however, the productivity does not improve even if \({\gamma }_{1}\) enhances (more fresh desorbent fed to the unit). In fact, the productivity decreases due to the higher solid velocities imposed. As mentioned previously, such increase in the solid velocity is to compensate the displacement of the concentration bands caused by the higher velocity of Section I. Consequently, the overall flowrates in the entire unit increase. For the ModiCon SMBR the reduced contact time of the species with the stationary phase limits the unit capacity, and the feed flowrate reduces, which affects the productivity. Consequently, the DC increases significantly, once it is inversely proportional to the productivity. It was also observed that the displacement of acetone concentration band leads to the contamination of the raffinate stream.
It was also mentioned in the Multifeed SMBR optimization that the maximum productivity reaches a plateau after which there is no point in adding more eluent or reducing the recycle flow rate because both eluent and solid are properly regenerated. In the ModiCon SMBR, such plateau was not reached within the range of β explored. This is related to the simultaneous decrease in the time switch, which, as mentioned, is linked with the higher flowrates processed in the unit. This indicates that the maximum capacity of the unit was not reached for β of 70% applied to Section I and 35% to Section IV.
As it was observed for the Multifeed SMBR, the productivity behavior with variations in \({\gamma }_{4}\) is counterintuitive once it increases for higher flow rates in Section IV, while it would be expected a decrease due to the worse regeneration of the eluent. The reason is the simultaneous decrease in time switch, which increases the overall flowrates and consequently the SMBR processing capacity.
The effect of variations in the β on the desorbent consumption must be carefully analyzed once this variable is inversely proportional to the productivity and directly proportional to the difference between \({\gamma }_{1}\) and \({\gamma }_{4}\). For a constant \({\gamma }_{1}\), DC is kept almost unchanged with an increase in \({\gamma }_{4}\) because it compensates the reduction that would be consequence of the increased productivity. For high flow rates in Section IV, the insufficient regeneration of the liquid phase affects the productivity and consequently the DC. The effect of \({\gamma }_{1}\) in this performance parameter is much more significative than \({\gamma }_{4}\) due to the larger absolute value of the first variable compared to the second, even considering the increased productivity for higher flow rates in Section I.
Considering the objective function of maximizing the productivity, the optimum combination of β is 70% in Section I and 20% in Section IV, resulting in a productivity of 9.48 \({\text{kg}}_{{{\text{Solk}}}} \, {\text{L}}_{{{\text{Ads}}}}^{{ - {1}}} \, {\text{day}}^{{ - {1}}}\) with a DC of 5.88 \({\text{L}}_{{{\text{Desorbent}}}} \,{\text{kg}}_{{{\text{Prod}}}}^{ - 1}\). It is noteworthy that at this point a higher feed flowrate is processed compared to the optimal point of the Multifeed SMBR. The following steps of the optimization were performed considering this combination of β.
The impact of the time switch in the unit performance, namely on the productivity and on the desorbent consumption, is evaluated by varying it between 80 and 105% of the optimal from the Multifeed SMBR. For that, the separation region was determined for constant β in Sections I and IV (70% and 20%, respectively) to find the (\({\gamma }_{2}, {\gamma }_{3}\)) pair that reaches the highest productivity. Figure 6 represents the results of this study.
The highest productivity is attained for a time switch of 85% of the Multifeed SMBR optimal. It is 15.42 min and results in a productivity of 9.69 \({\text{kg}}_{{{\text{Solk}}}} \, {\text{L}}_{{{\text{Ads}}}}^{{ - {1}}} \, {\text{day}}^{{ - {1}}}\) with a DC of 6.76 \({\text{L}}\,{{\text{kg}}_{{{\text{Solk}}}}}^{{ - {1}}}\). The increase in the DC is consequence of the need for a higher desorbent flowrate to regenerate the solid phase when higher time switches are used. Also, from the analysis of the internal concentration profiles, when using the time switch from the Multifeed SMBR, the compounds concentration fronts are displaced in the direction of the fluid flow. This is due to the increased capacity of the unit of operating with higher flow rates. The SMBR performance improves by using a lower time switch (higher solid velocity) because it compensates the displacement caused by operating with higher flow rates.
Comparing with the internal concentration profile of the Multifeed SMBR at its optimal (βs of 40% and 25%), it is verified that the extract stream in the Multifeed is highly susceptible to contamination with acetone, since there is only one column between acetone feed port and the extract collection port. Indeed, acetone is the main contaminant of the extract stream on the Multifeed SMBR and such contamination may limit the amount of reactants fed to the unit and, consequently, the productivity.
The following steps of the ModiCon SMBR simulation studies were performed using time switches of 85% of the Multifeed SMBR optimal.
From the Multifeed SMBR optimization, the molar excess of acetone fed to the unit is approximately 5%. To keep such reactant ratio in the ModiCon SMBR, it is necessary to feed glycerol during 49% of the time switch and acetone in the remaining time. The sensitivity analysis to the duration of each subinterval of the time switch reveals that the operation is highly limited by the reaction equilibrium since there is no RSR when varying this variable even slightly. When operating with the same combination of safety factors from the Multifeed optimal (70% and 20%) and 85% of the optimal time switch, but feeding glycerol during 48% of the time switch, the raffinate stream purity is impaired by the acetone contamination. As for feeding each reactant during half of the time switch, glycerol is present in high enough amount near the raffinate port to hinder this stream purity.
At the optimal point, where the maximum productivity is achieved, the productivity increases 38% compared to the Multifeed SMBR, against a DC increase of nearly 35%. As for the reactant’s consumption, it decreases by 12%. This is due to the higher conversion attained for the ModiCon and to the need of the unit of operating with lower time switches. Similar results of achieving higher production rates despite reducing the amount of reactants fed are reported in the literature when comparing the ModiCon with other SMBR operating strategies [19].
The increase in the productivity despite the decrease in the reactants consumption clearly demonstrates that the ModiCon SMBR is a Process Intensification strategy that enhances even more the efficiency of an already intensified strategy, the Multifeed SMBR.
6 Conclusions
The SMBR is still much behind the SMB in terms of development of special units and alternative operations, and there is plenty of room for investigations on the non-conventional operations for this multifunctional reactor. The lower energy requirements, the large number of stationary phases already used and produced at industrial scale and the fact that its precursor (the SMB) is such a well-established technology are the main advantages compared to other multifunctional reactors used to produce solketal.
In the present work, two different non-conventional SMBR operation strategies were proposed for solketal production from the reaction between glycerol and acetone. The Multifeed and the ModiCon SMBR improvements are based on the same principle of promoting the countercurrent contact between the reactants to improve the separation and keeping acetone concentration front further apart from the raffinate collection port. From previous studies, 303 K was selected as the best operational temperature and Amberlyst-35 and ethanol are used as stationary phase and eluent, respectively. The process was modelled considering a pilot scale unit available at the LSRE-LCM. Both processes were optimized based on the “Reactive-Separation Volumes” methodology due to being a well-established procedure.
The maximum productivity achieved by the Multifeed SMBR was 7.03 \({\text{kg}}_{{{\text{Solk}}}} \, {\text{L}}_{{{\text{Ads}}}}^{{ - {1}}} \, {\text{day}}^{{ - {1}}}\) with a DC of 5.02 \({\text{L}}_{{{\text{Desorbent}}}} \,{\text{kg}}_{{{\text{Prod}}}}^{ - 1}\), when using a SF of 40% to Section I and 25% to Section IV. Since the optimization of the ModiCon SMBR is more limited due to the much higher computational effort needed, in the first step the time switch values used were prevenient from the Multifeed optimization. It was verified that a larger β in Section I is needed to achieve the productivity plateau due to the capacity of the unit of processing higher flowrates. The sensitivity analysis to the time switch indicates that higher productivities are achieved when using a slightly lower time switch. As for the sensitivity to the amount of reactants fed, it was revealed that the process is highly limited by the reaction equilibrium, once no RSR was found when the reactants ration deviates from an excess of acetone of 5%.
The optimal combination of β is 70% in Section I and 20% to Section IV with a time switch of 15.42 min (85% from the optimum of the Multifeed SMBR). At this optimal point, the productivity is 9.69 \({\text{kg}}_{{{\text{Solk}}}} \, {\text{L}}_{{{\text{Ads}}}}^{{ - {1}}} \, {\text{day}}^{{ - {1}}}\) with a DC of 6.76 \({\text{L}}_{{{\text{Desorbent}}}} \,{\text{kg}}_{{{\text{Prod}}}}^{ - 1}\). This indicates an increase in the productivity of 38% compared to the Multifeed, with a cost of an increase of 35% in the desorbent consumption. The most promising result is that the reactants consumption decreases by 12%. These results demonstrate it is more advantageous to operate using the ModiCon than the Multifeed, once the first strategy intensifies even more an equipment that by itself is an example of Process Intensification.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
Abbreviations
- \({a}_{i}\) :
-
Activity coefficient of compound \(i\) (–)
- \({C}_{i,j}\) :
-
Liquid phase concentration of component \(i\) in column \(j\) (mol L−1)
- \({\overline{C} }_{p,i,j}\) :
-
Average concentration in the particle pores of component \(i\) in column \(j\) (mol L−1)
- \({C}_{i,j,0}\) :
-
Initial concentration of compound \(i\) in the liquid phase (mol L−1)
- \({C}_{in,i,j}\) :
-
Inlet concentration of compound \(i\) (mol L−1)
- \({D}_{ax,j}\) :
-
Axial dispersion coefficient in column \(j\) (cm2 s−1)
- \({D}_{eff,i}\) :
-
Effective diffusion coefficient (cm2 s−1)
- \({D}_{i,m}\) :
-
Diffusion coefficient of component \(i\) in a mixture (cm2 s−1)
- \({D}_{i,j}^{0}\) :
-
Diffusion coefficient for a dilute solute \(i\) in a solvent \(j\) (cm2 s−1)
- \({E}_{a}\) :
-
Reaction activation energy (kJ mol−1)
- \({k}_{{c}_{0}}\) :
-
Reaction pre-exponential factor (mol kg−1 s−1)
- \({K}_{eq}\) :
-
Reaction thermodynamic equilibrium constant (–)
- \({k}_{ext,i,j}\) :
-
External mass transfer coefficient of compound \(i\) in column \(j\) (cm s−1)
- \({K}_{i}\) :
-
Langmuir adsorption equilibrium constant for compound \(i\) (L mol−1)
- \({k}_{int,i,j}\) :
-
Internal mass transfer coefficient of compound \(i\) (cm s−1)
- \({k}_{L,i,j}\) :
-
Global mass transfer coefficient of compound \(i\) (cm s−1)
- \({K}_{S,W}\) :
-
Adsorption equilibrium constant for water (rate law) (–)
- \({L}_{j}\) :
-
Length of column \(j\) (cm)
- \({M}_{i}\) :
-
Molecular mass of compound \(i\) (kg mol−1)
- n :
-
Number of the column
- \(Pe\) :
-
Peclet number (–)
- \(Prod\) :
-
SMBR productivity (kg LAds−1 day−1)
- \(PuR\) :
-
Product purity in the raffinate stream (–)
- \(PuX\) :
-
Product purity in the extract stream (–)
- \(Q\) :
-
Flow rate (L min−1)
- \({Q}_{i}\) :
-
Langmuir maximum capacity for compound \(i\) (mol LAds−1)
- \({q}_{i,j,0}\) :
-
Initial concentration of compound \(i\) in the liquid phase (mol LAds−1)
- \({q}_{in,i,j}\) :
-
Adsorbed concentration of compound \(i\) in equilibrium with the feed concentration (mol LAds−1)
- \(\overline{{q}_{i,j}}\) :
-
Average adsorbed concentration of compound \(i\) in column \(j\) (mol LAds−1)
- \(\mathfrak{R}\) :
-
Reaction rate (mol kg−1 s−1)
- \(R\) :
-
Gas constant (J mol−1 K−1)
- \({Re}_{p}\) :
-
Reynolds number (particle) (–)
- \({r}_{p}\) :
-
Particle radius (cm)
- \(Sc\) :
-
Schmidt number (–)
- \({Sh}_{p}\) :
-
Sherwood number (particle) (–)
- \(T\) :
-
Temperature (K)
- \({T}_{ref}\) :
-
Reference temperature (K)
- \(t\) :
-
Time (s)
- \({t}^{*}\) :
-
Time switch (min)
- \({u}_{j}\) :
-
Liquid phase interstitial velocity (cm s−1)
- \({u}_{s}\) :
-
Solid phase velocity (cm s−1)
- \({V}_{SMBR}\) :
-
SMBR volume (L)
- \({V}_{M,i}\) :
-
Molar volume of compound \(i\) (L mol−1)
- \({X}_{lim}\) :
-
Limiting reactant conversion (–)
- \({x}_{i}\) :
-
Molar fraction of compound \(i\) (–)
- \(z\) :
-
Axial coordinate (cm)
- \({\gamma }_{j}\) :
-
Flow rate ratio in section \(j\) of the SMBR (–)
- \({\varepsilon }_{b}\) :
-
Bed porosity (–)
- \({\varepsilon }_{p}\) :
-
Particle porosity (–)
- \({\eta }_{j}\) :
-
Viscosity of solvent \(j\) (cP)
- \({\eta }_{m}\) :
-
Mixture viscosity (cP)
- \({v}_{i}\) :
-
Stoichiometric coefficient of compound \(i\) (–)
- \(\rho\) :
-
Fluid phase density (kg L−1)
- \({\rho }_{b}\) :
-
Bulk density (kg L−1)
- \({\tau }_{p}\) :
-
Particle tortuosity (–)
References
Knobloch, F., et al.: Net emission reductions from electric cars and heat pumps in 59 world regions over time. Nat. Sustain. 3(6), 437–447 (2020)
Guillot, J.D.: How to Increase the Use of Alternative Fuels for Cars. European Parliament (2022)
Köhler, J., et al.: Leaving fossil fuels behind? An innovation system analysis of low carbon cars. J. Clean. Prod. 48, 176–186 (2013)
Seabra, J.E.A.: Roadmap to 2050: The land-water-energy nexus of biofuels. In: Laird, F. (ed.) Sustainable Development Solutions Network (SDSN) and Fondazione Eni Enrico Mattei (FEEM), p. 164. New York (2021)
Corrêa, I., Faria, R.P.V., Rodrigues, A.E.: Continuous valorization of glycerol into solketal: recent advances on catalysts, processes, and industrial perspectives. Sustain. Chem. 2(2), 286–324 (2021)
Mota, C.J.A., et al.: Glycerin derivatives as fuel additives: the addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 24(4), 2733–2736 (2010)
Moreira, M.N., et al.: Solketal production in a fixed bed adsorptive reactor through the ketalization of glycerol. Ind. Eng. Chem. Res. 59(7), 2805–2816 (2020)
Li, X., et al.: Toward sustainable and eco-efficient novel catalytic distillation process for production of solketal using seepage catalytic packing internal. Catal. Today 388–389, 92–108 (2022)
Roldán, L., et al.: Glycerol upgrading by ketalization in a zeolite membrane reactor. Asia-Pac. J. Chem. Eng. 4(3), 279–284 (2009)
Faria, R.P.V., et al.: Improving the performance of a simulated moving bed reactor for the synthesis of solketal by implementing multifeed strategy. Ind. Eng. Chem. Res. 61(39), 14531–14545 (2022)
Corrêa, I., Faria, R.P.V., Rodrigues, A.E.: Continuous valorization of glycerol into solketal: from the fixed-bed adsorptive reactor to the simulated moving-bed reactor. Ind. Eng. Chem. Res. 61(11), 4017–4030 (2022)
Rodrigues, A.E., et al.: Simulated Moving Bed Technology: Principles, Design and Process Applications. Elsevier Science, Amsterdam (2015)
Rodrigues, A.E., Madeira, L.M., Wu, Y.-J., Faria, R.: Sorption Enhanced Reaction Processes. Sorption Enhanced Reaction Processes (2017)
Migliorini, C., et al.: Analysis of simulated moving-bed reactors. Chem. Eng. Sci. 54(13), 2475–2480 (1999)
Faria, R.P., Rodrigues, A.E.: Instrumental aspects of simulated moving bed chromatography. J. Chromatogr. A 1421, 82–102 (2015)
Kim, K.-M., et al.: Advanced operating strategies to extend the applications of simulated moving bed chromatography. Chem. Eng. Technol. 40(12), 2163–2178 (2017)
Aniceto, J.P.S., Silva, C.M.: Simulated moving bed strategies and designs: from established systems to the latest developments. Sep. Purif. Rev. 44(1), 41–73 (2015)
Yu, Y., Wood, K.R., Liu, Y.A.: Simulation and comparison of operational modes in simulated moving bed chromatography. Ind. Eng. Chem. Res. 54(46), 11576–11591 (2015)
Subramani, H.J., Hidajat, K., Ray, A.K.: Optimization of reactive SMB and Varicol systems. Comput. Chem. Eng. 27(12), 1883–1901 (2003)
Agrawal, G., et al.: Optimization of reactive simulated moving bed systems with modulation of feed concentration for production of glycol ether ester. J. Chromatogr. A 1360, 196–208 (2014)
Moreira, M.N., et al.: Solketal production from glycerol ketalization with acetone: catalyst selection and thermodynamic and kinetic reaction study. Ind. Eng. Chem. Res. 58(38), 17746–17759 (2019)
Glueckauf, E.: Theory of chromatography. Part 10. Formulae for diffusion into spheres and their application to chromatography. Trans. Faraday Soc. 51, 1540–1551 (1955)
Wilson, E.J., Geankoplis, C.J.: Liquid mass transfer at very low Reynolds numbers in packed beds. Ind. Eng. Chem. 5(1), 9–14 (1966)
Scheibel, E.G.: Correspondence. Liquid diffusivities. Viscosity of gases. Ind. Eng. Chem. 46(9), 2007–2008 (1954)
Perkins, L.R., Geankoplis, C.J.: Molecular diffusion in a ternary liquid system with the diffusing component dilute. Chem. Eng. Sci. 24(7), 1035–1042 (1969)
Calderón Supelano, R., Barreto, A.G., Secchi, A.R.: Optimal performance comparison of the simulated moving bed process variants based on the modulation of the length of zones and the feed concentration. J. Chromatogr. A 1651, 462280 (2021)
Minceva, M., Pais, L.S., Rodrigues, A.E.: Cyclic steady state of simulated moving bed processes for enantiomers separation. Chem. Eng. Process. 42(2), 93–104 (2003)
Schramm, H., et al.: Improving simulated moving bed processes by cyclic modulation of the feed concentration. Chem. Eng. Technol. 25(12), 1151–1155 (2002)
Minceva, M., Rodrigues, A.E.: Two-level optimization of an existing SMB for p-xylene separation. Comput. Chem. Eng. 29(10), 2215–2228 (2005)
Mazzotti, M., Storti, G., Morbidelli, M.: Optimal operation of simulated moving bed units for nonlinear chromatographic separations. J. Chromatogr. A 769(1), 3–24 (1997)
Silva, V.M.T.M., Rodrigues, A.E.: Novel process for diethylacetal synthesis. AIChE J. 51(10), 2752–2768 (2005)
Pereira, C.S.M., et al.: Multifunctional reactor for the synthesis of dimethylacetal. Ind. Eng. Chem. Res. 47(10), 3515–3524 (2008)
Graça, N.S., et al.: Analysis of the synthesis of 1,1-dibutoxyethane in a simulated moving-bed adsorptive reactor. Chem. Eng. Process. 50(11–12), 1214–1225 (2011)
Acknowledgements
This work was financially supported by LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC). Isabella Corrêa gratefully thanks the FCT – Fundação para a Ciência e a Tecnologia for the Doctoral Grant (2020.07258.BD) through NORTE2020 – Programa Operacional Regional do Norte from the FSE – Fundo Social Europeu of the UE.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was financially supported by LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC). Isabella Corrêa gratefully thanks the FCT – Fundação para a Ciência e a Tecnologia for the Doctoral Grant (2020.07258.BD) through NORTE2020 – Programa Operacional Regional do Norte from the FSE – Fundo Social Europeu of the UE.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corrêa, I., Faria, R.P.V. & Rodrigues, A.E. Enhanced simulated moving bed reactor performance for the synthesis of solketal by implementing the ModiCon strategy. Adsorption 30, 79–93 (2024). https://doi.org/10.1007/s10450-023-00427-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00427-6