Abstract
This work shows that magnetic adsorbents with different porosity characteristics can be produced by carbonization and steam activation of a mixture of a furfuryl alcohol and iron-containing compound, such as ferrocene (PFA/ferrocene), FeCl3 (PFA/FeCl3) and FeSO4 (PFA/FeSO4). The materials obtained in this work possess magnetic properties that are revealed in the neodymium magnet balance test as an increase in weight loss with an increase in iron content in the material. In order to verify whether the magnetic properties facilitate the removal of the used adsorbent, the sedimentation test was carried out. The porous texture characteristics obtained from the N2 adsorption isotherms at 77 K show a huge differences in porosities depending on the type of iron compound used. The presence of ferrocene promotes the formation of mesoporosity, whereas the presence of iron(II) sulphate induces microporosity. XRD measurements reveal the presence of magnetite and hematite in all magnetic adsorbents. The intensity of the XRD peak designated to magnetite increases with an increase in the saturation magnetization. Adsorption of Congo red (CR), phenol (P), atrazine (A) and isoproturon (I) from aqueous solutions was carried out at a temperature of 24 °C in a static system. The magnetic properties accelerated the sedimentation processes and materials demonstrated a high adsorption ability toward organic contamination. The adsorption capacity on PFA-based MAC is strongly related to adsorbent porosity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic fields and magnetic properties are widely used to separate substances in mining, metallurgy and solid waste utilization technologies or exhaust gases purification [40, 46]. These well-known separation technologies are considered environmentally friendly. For example, magnetic swing absorption in gas separation processes consumes less energy compared to conventional absorption [31]. Magnets can also be used for the collection of dust from subway or coal-fired power plants and for separation of fly ash from power plants in a certain regime of magnetic intensity [8, 20].
The magnetic properties are also very useful in processes of purification of liquids. Magnetic adsorbents have been shown to remove various common environmental pollutants, such as phenols, tetracycline, dyes, or heavy metals [3, 38, 41, 43, 51]. Because of their good adsorption performance, low toxicity, chemical stability, and easy separation and recovery, magnetic adsorbents are considered to be among the most promising adsorbents. Adsorbents with such properties have been produced from different raw materials [41, 43], many of them are carbon-containing materials [17, 38, 43, 51]. Nowadays also very popular graphene oxide [22], or carbon nanotubes [4] have been widely used as adsorbents. Nonetheless, but they are nonrenewable and relatively costly, hence are unfeasible for potential utilization in large-scale applications. Different carbon-containing waste materials are much more practical precursors [41, 43]. The adsorbents obtained from the waste are usually powder activated carbons (AC) [11, 47]. Although the powder ACs have many advantages like the lower cost of production and higher availability of the adsorption surface due to the small size of the particles, they also generate some problems during implementation. Their removal from the tanks and basins after application is often problematic due to the high dispersivity of their small particles in the solution. By imparting the magnetic properties to the adsorbent, post-process solutions can be more easily purified from the adsorbent. Placing a simple magnet at the bottom of tanks containing magnetic adsorbents will accelerate sedimentation. This will allow the elimination of processes such as filtration or centrifugation.
The most popular and easiest methods of producing waste-based magnetic adsorbents is chemical coprecipitation with paramagnetic or ferromagnetic precursors, ball milling, or impregnation [16, 32, 41, 43, 49]. In all of these methods, the magnetic iron compounds are loaded onto the surface of adsorbent. The biggest problem with the surface-loaded adsorbents is the rather weak binding of the iron compounds and consequently the loss of metal during the usage.
The aim of this work is to produce a magnetic adsorbent that will not lose its magnetic properties during use. To obtain this, an iron compound, which gives the magnetic properties, is introduced into the polymer matrix at the commencement of the polymerization. Then the pyrolysis and activation of the mixture of polymer and metal is applied to acquire the ACs. This approach is expected to have a few benefits, such as: (i) higher density of active sites; (ii) the presence of active sites not only on the surface but also in the bulk; and (iii) reduction of iron amount losses during usage. As a polymer, poly(furfuryl alcohol) (PFA) is used. PFA is a good precursor for fabrication of microporous adsorbents [34, 37], however, by introducing metals into polymer matrix, the porosity can be tailored. Generally, it is proven that metal particles gasify the carbon matrix in its immediate vicinity and create the porosity by forming holes in the carbon and depending on the metal introduced, pores with different sizes can be formed [33]. To introduce magnetism into the adsorbent, commonly, Fe containing compounds are used [3, 20, 38, 43, 51]. Iron is usually used to develop mesoporosity [23, 26], however, the activated carbons with magnetic properties (MACs) obtained from FeCl3 impregnated materials usually show microporosity [5, 44]. For this work, in order to obtain MACs with different surface porosities, three different iron-containing compounds i.e. ferrocene, FeCl3, and FeSO4 have been selected. The iron compounds have been selected for several reasons: (i) they have relatively low cost, (ii) are non-toxic, (iii) and are environmentally friendly. All of the inorganic Fe compounds have previously been used for the production of MAC [44], but there are no studies that show the comparison between MACs prepared from the same raw material treated with the addition of different iron sources. In this paper we compare the magnetic properties and porous textures of MACs depending on the Fe compound used to produce the adsorbent. Herein we present a simple balance-test that describes directly the positive effect of magnetization. We also give a deep insight into the magnitude of magnetization induced by the different iron compounds used. Additionally, the sorption ability toward common water contaminant representatives such as the Congo red, phenol, isoproturon, and atrazine have been tested. To confirm that MACs are easier to remove from the solution than the conventional ACs, the sedimentation test was performed. It was shown that all PFA-based MACs reveal magnetic properties that accelerate the sedimentation processes after the adsorption test. The work gives a deeper insight into the mechanisms of introducing magnetic properties by different iron compounds whereas novelty of this work is given by presenting a simple methods that allows evaluation of the utility of MAC and estimation of the magnetization force of MAC. Moreover it was demonstrated that the adsorption capacity was porous texture dependent.
2 Materials and methods
2.1 Magnetic adsorbent synthesis
First, pure poly(furfuryl alcohol) (PFA) was synthesized. In order to achieve this, 100 cm3 of furfuryl alcohol (98%) was mixed with 0.60 cm3 of concentrated hydrochloric acid. The mixture of PFA and iron was prepared as follows: 100 cm3 furfuryl alcohol was mixed with one of the three iron precursors: ferrocene, FeCl3·6H2O, FeSO4·7H2O. Next 0.60 cm3 of concentrated hydrochloric acid was added. The amount of salt used corresponds to 3 wt% of Fe in pyrolyzed mixture. Naming of samples: polymer containing 3.0 wt.% iron from ferrocene: PFA/ferrocene, activated carbon: A-PFA/ferrocene. All polymers were dried at 65 °C for 20 h and next for 24 h at 140 °C. Polymers were pulverized into particles smaller than 0.5 mm. The samples were then carbonized in horizontal oven at a heating rate of 10 °C min−1 at 900 °C for 1 h, in a nitrogen atmosphere. The obtained char was steam activated at 850 °C up to 50% of burn off.
2.2 Characterization of the MACs
To evaluate the magnetic properties, magnetisation was measured in a function of applied magnetic field and a simple neodymium magnet-balance test was performed as well.
The magnetisation hysteresis curves were obtained using the MPMS squid magnetometer. The measurement was carried out at a constant temperature of 2 K and magnetic fields from – 7 to 7 T.
Simple neodymium magnet/balance test with neodymium magnet was performed. In this test, magnet was placed flat on the balance. Next, 0.2 g of sample was put in a glass vial and hanged over a magnet in a constant distance. The loss of mass caused by a magnet attraction by the sample was recorded. Fig. S.1. presents a test setup. The neodymium magnet/balance test was performed before and after washing the samples with water or acid.
The sedimentation test is based on observation of the behaviour of the suspension placed in a transparent graduated cylinder. Cylinder dimensions: diameter: 1 cm, height: 15 cm. The solution mixture was made of 15 cm3 deionized water and 0.5 g of AC. The samples were agitated by mechanical shaking for 10 min at a constant speed. Measurement starts immediately after the shaking was stopped. At regular intervals, the height of the top layer of the mixture and the height of the precipitate at the bottom of the vessel were measured. The measurement was complete when the thickness of the film at the bottom of the cylinder is comparable to the thickness of the sediment layer prior to stirring and the solution above the sediment is clear. In the case of examining the influence of magnetic properties on the sedimentation rate, immediately after stirring had been stopped, a neodymium magnet, with dimensions of 10 × 10 × 3 mm, was placed under the cylinder with the suspension.
The elemental analysis for C, H, N and S was performed using a Vario III Elemental Analyzer. The oxygen content was calculated by difference.
The porous texture was determined from nitrogen adsorption isotherms measured at 77 K on a NOVA 2200 (Quantachrome) device. Data were analyzed to obtain: (i) the specific surface area using the BET method at p/p0 < 0.15 (SBET); (ii) the amount of nitrogen adsorbed at a relative pressure (p/p0) of 0.98 to determine the total pore volume (VT); (iii) the micropore volume applying the Dubinin-Radushkevich equation up to p/p0, 0.15 (VDR); (iv) the mesopore volume (Vmez) calculated as the difference between the VT and VDR; and (v) the pore size distribution (PSD) in terms of microporosity by means of the density functional theory (DFT) method using Nova Quantachrome software.
The pHPZC (point of zero charge) of the ACs was determined by mixing 1 g of each AC with 20 ml of CO2-free distilled water, according to the procedure described by Moreno-Castilla et al. [35].
To verify the stability of iron particles introduced to polymer-based AC, washing in distilled water and 5% HCl was conducted. For that 0.5, g of MCA was placed in 50 ml of distilled water or 50 ml of 5% HCl. The closed flasks were agitated for 24 h at 30 °C. The acid was then washed with distilled water until there no chloride compounds were detected by AgNO3 test.
Before and after the treatment with water and HCl, the Fe content was calculated on the basis of the ash content. Ash was determined according to the ISO 1171:2010.
Scanning electron microscopy with energy dispersive X-ray analysis (SEM/Ga-FIB. FFI Helios NanoLab) was applied to monitor the metal distribution of Fe-loaded char and the surface morphology of activated carbons.
The crystalline properties of the materials were studied by X-ray diffraction (XRD) using an Ultima IV Rigaku analyzer equipped with a 2 kW X-ray tube (40 kV per 30 mA) using Cu Kα2 radiation.
2.3 Adsorption studies
The adsorption of Congo red (CR), phenol (P), atrazine (A), and isoproturon (I) from aqueous solutions was carried out at 24 °C in a static system. For CR, P and A a simple single-point adsorption (SPA) test was performed. For SPA adsorption, 0.01 g of AC and 0.10 dm3 of solution with concentration of: 150 mg/dm3 for P, 50 mg/dm3 for CR, and 15 mg/dm3 for A, was used. To obtain the equilibrium phase the sample in SPA was agitated for 7 days. Then, the concentrations and pH of the solutions after adsorption were measured. Full equilibrium and kinetic studies were conducted for selected MACs for A and I adsorbates. For this equilibrium adsorption test, 0.01–0.2 g of AC, whereas for kinetic studies 0.01 g of AC, was placed into Erlenmeyer flasks and 0.10 dm3 of adsorbate solutions with concentrations of 15 mg/dm3 for A and 20 mg/cm3 for I were added to each flask. The stoppered flasks were kept in a thermostat shaker bath and were agitated to reach equilibrium. Each set of flasks included two flasks containing blank solutions to check for sorbate volatilization and adsorption on the glass walls. The adsorption isotherms were determined without adding any buffer to control the pH to avoid the presence of a new electrolyte in the system. The pH of the solution was measured by a digital pH meter (Accumet Basic, Fisher Scientific) using a glass electrode. The concentration of each solute remaining in the water phase was quantified. The concentration was determined using a HITACHI U-2800A UV–Vis spectrophotometer at the wavelength given in Table 1, which additionally contains the basic characteristic of adsorbates used in this work.
3 Results and discussion
3.1 Surface characteristic of MACs
Adsorption processes are dependent on many factors. One of the most important is the porosity due to the fact that the adsorption of compounds with different dimensions is pore size dependent. Pyrolysis of the polymer material usually gives an adsorbent with a low pore volume; however, further physical activation leads to porosity development. In our previous work [30] we demonstrated that ferrocene/PFA-based AC are adsorbents with a high mesopore ratio. In the case of magnetic adsorbents, there was no work in which the ferrocene was selected as Fe sources. For this application, usually different inorganic iron salts are used. The most popular is FeCl3 [41, 44]. In this work, in terms of magnetic properties development, the polymer-based magnetic adsorbents have been obtained by steam activation of char obtained from pyrolysis of the mixture of polymer and iron complex like ferrocene, FeCl3 and FeSO4. As mentioned above, this approach, compared to metal loading on the surface by impregnation, allows the introduction of active sites on both the bulk and on the surface of the material. For comparison, polyfurfuryl-based AC was also produced with no metal loading. Table 2 shows that iron loading does not have a significant effect on char yield. The char yield for PFA is 52%. It should be emphasized that in the case of char production with iron (III) chloride, the pyrolysis process shifts to gas production and the char yield is only 42% whereas the char yield for A-PFA/ferrocene and A-PFA/FeSO4 is comparable to the A-PFA char yield. However, the presence of iron influences the reactivity of char for steam activation. In our previous work, iron introduced in the form of ferrocene was shown to increase the reactivity of the obtained char [30]. In these studies, the same phenomenon is observed. The activation time necessary to reach 50% burn-off during steam activation in PFA/Ferrocene is only 27 min. For A-PFA 50% burn-off is received after 96 min. Iron from FeCl3 has also strong influence on the course of activation, as the required 50% burn-off was reached after 19 min. Similarly to pyrolysis, catalytic activity toward gasification processes is observed in the case of FeCl3. Iron introduced into MCA from iron sulfate is inactive in steam activation. It does not catalyze gasification since the activation time is almost three times greater compared to A-PFA. Iron is generally considered as a catalyst that promotes mesopore development [23, 27, 30]. However, as can be concluded based on of nitrogen sorption (Fig. 1, Table 2), iron introduced into polyfurfuryl alcohol from different compounds produces activated carbons with a completely different porous texture. All nitrogen sorption isotherms show shapes typical for activated carbons, but they differ significantly depending on the type of iron salt used. In the case of A-PFA/ferrocene and A-PFA/FeCl3, the isotherms are of type IV according to the BDDT classification [13]. The isotherms reveal a pronounced hysteresis loop that signals the mesoporosity development. The shape of the hysteresis loop indicates the formation of pores in the slit shape.
As expected, the ferrocene-based AC reveals a high mesopore ratio of 0.81 with a mesopore volume as high as 0.530 cm3 g−1. A consequence of the small micropore volume is the relatively low SBET of only 329 m2 g−1. This value is c.a. 1000 m2 g−1 smaller than the surface area for A-PFA which is characterized by SBET of 1324 m2 g−1. Still, the total pore volume of A-PFA and A-PFA/ferrocene is comparable. Likewise, both ferrocene, and FeCl3 have shown the activity toward mesopore development [2] in pitch based activated carbon fibers or in PFA based membrane [48]. However, in the case of magnetic adsorbents, when FeCl3 was used to produce the MAC, the porosity of the adsorbents was strongly differentiated but usually not well developed. Herein, the A-PFA/FeCl3 surface area (SBET) is twice larger than that of A-PFA/ferrocene and twice smaller than that of A-PFA but the total pore volume of this sample is the lowest and is only 0.403 cm3 g−1. Only slightly larger total pore volume is observed in A-PFA/FeSO4. Surprisingly, the iron sulfate-based adsorbent exhibits a high microporosity ratio, which is due to the very low mesopore volume. Iron in A-PFA/FeSO4 does not show any catalytic activity. Iron from ferrocene and FeCl3 reveals the catalytic activity that leads not only to the mesopore formation but also to ordering of the carbon layers that increases the graphitization of the sample [2, 48]. This fact is also confirmed in these studies.
The XRD analysis (Fig. 2) shows that the reflexes at 2Ɵ of c.a. 25° and 43° that are typical peaks of the (002) and (100) planes of carbon, change their shapes depending of iron precursors of the studied MACs. We can observe that the peaks become sharper and narrower, in the diffractograms of iron chloride—and ferrocene-based ACs, while in the diffractogram of A-PFA/FeSO4 as well as in that of A-PFA, the peak is wide, indicating the randomly oriented carbon layers. It should be noted that the amount of regularly oriented layers, expressed as the height or sharpness of the aforementioned peak, changes in the following order: A-PFA < A-PFA/FeSO4 < A-PFA/FeCl3 < A-PFA/ferrocene. In the same sequence, the surface area, SBET, decreases, so the more oriented the carbon layers, the lower the surface area is.
As was presented, three different iron precursor have a completely divergent impact on the porous texture development of the product MACs. The impregnation with inorganic Fe salt was commonly used to introduce the magnetic properties; however, no studies present the results of ferrocene use for this purpose. The easiest test to check if the material possesses any magnetic properties is to put a magnet in its vicinity and check if any magnetic attraction can be observed. Here, to evaluate the strengths of the magnetization, a simple magnet-balance test was performed. Activated carbons, activated carbons after washing with water and after washing with 5% hydrochloric acid were all examined by this technique. The result presented as mass losses in a function of ash content are shown in Fig. 3. In our case, because A-PFA does not contain mineral impurities, the ash content corresponds directly to the iron content as under applied conditions all iron compounds convert into Fe2O3 [2]. As can be observed, the A-PFA, that does not contain any Fe, does not react to the magnetic field generated by the applied neodymium magnet and consequently, does not give any weight loss. Mass loss is observed for all of the Fe-doped PFA-based activated carbons produced in this work. Additionally, the higher the ash content, the greater the observed mass loss. To examine the magnetic properties, magnetization hysteresis curves were obtained. Magnetic hysteresis curves for selected MAC is shown in Fig. 4 whereas Table 3 contains the characteristic parameters obtained from magnetization curves for all MACs produced in this work. The magnetization reaches the highest values in the fields of 2–4 T, when the ferromagnetic component is saturated. In the stronger fields, a slight decrease in magnetization can be seen, which reflects the diamagnetism of the other components of the samples. On the basis of the hysteresis loop, which is narrow, and the value of coercivity and retentivity (Table 3), it can be deduced that all PFA-based MACs are soft ferromagnets. Such ‘soft’ materials, like annealed iron, can be easily magnetized but do not tend to stay magnetized. We can also observe that the saturation of magnetization changes in the following order: A-PFA/ferrocene > A-PFA/FeSO4 > A-PFA/FeCl3. This tendency cannot be strictly explained on the basis of iron content. To understand this tendency, the form of iron found in MACs should be compared.
The XRD diffraction patterns of the PFA-based MACs are not entirely similar (Fig. 2.). This suggests the samples vary in Fe species in their composition. Usually crystalline phase of hematite, magnetite and α-Fe can be discern easily in magnetic adsorbents [5, 15, 50] For A-PFA/FeCl3 we can observe the peaks at 2Ɵ of 24.3; 33.4; 41.0; 49.6; 57.7°; corresponding to (012); (104); (113); (024); (122) of the basal plane of hematite, while the reflexes at 2Ɵ of 30.2; 35.6; 43.3; 52.6; 57. 6° indicating the cubic spinel structure of the magnetite peak index (220); (311); (400); (511); (440), is found in all MACs obtained in this work (Fig. 2.). It should be emphasized that the height of the peak designated to Fe2O3 change in the following order: A-PFA/ferrocene > A-PFA/FeSO4 > A-PFA/FeCl3 that matches the increase in saturation point found on the magnetic hysteresis curve (Table 3). The higher amount of magnetite, which has stronger magnetic properties, explains why the saturation point of magnetization is the highest for ferrocene-based MAC although this sample does not have the highest iron content.
In the case of adsorption from solution, the ash content should be as low as possible to eliminate the possibility of water recontamination. Therefore, to check if the iron oxides, that are present on the surface of MACs, do not leach to the solution, the MACs were treated with water; similarly in order to evaluate the amount of iron on the surface, the acid treatment was used. The ash content after the water treatment did not change (Table 4), similarly, nor did its magnetic properties manifested as a mass loss on a neodymium magnet test. After acid washing a small decrease in ash content was observed for all MACs, however, the biggest drop was found in the A-PFA/FeCl3. This indicates a higher deposition of iron oxides on the surface that can give an explanation for the higher gasification activity revealed by this sample, in pyrolysis and activation processes. Although the magnetic properties observed in acid treated MACs are still present, there are much smaller compared to those observed in raw MACs.
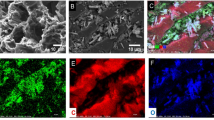
The SEM analysis, Fig. 5, confirms that A-PFA/FeCl3 and A-PFA/FeSO4 have a higher metal loading on the surface. In addition, metal particles originating from inorganic iron salts have a tendency to agglomerate. In the A-PFA/ferrocene, the iron-based particles are relatively homogenously dispersed, and their size is in the nanoscale range. The SEM–EDS analyses of A-PFA/FeCl3 and A-PFA/FeSO4 are given in Table 5 and Fig. 6. The analyses support the notion that the FeCl3 based adsorbent has higher iron surface coverage compared to the FeSO4 based adsorbent. This conclusion is consistent with previous results reported based on the behavior of the MAC under washing with 5% HCl. The EDS mapping, presented in Fig. 6, proves that the small agglomerates that can be seen in SEM image of iron-based MAC, are rich in evenly distributed iron. Similarly to the results of the CHNS analysis (Table 4), the EDS analysis of A-PFA/FeSO4 (Table 5) shows a higher content of oxygen compared to A-PFA/FeCl3. Furthermore, the oxygen concentration is higher in the EDS than in the CHNS analysis, while the carbon amount has a reverse tendency. This can be explained by a fact that the high concentration of oxygen is observed in the region of the presence of the iron. It can also be observed that sulfur is present only in the A-PFA/FeSO4 both in the regions of presence of Fe and in the activated carbon matrix. Furthermore, in the A-PFA/FeCl3 sample, iron is evenly distributed in big agglomerates.
3.2 Adsorption processes
Adsorption processes were carried out on particles of size between 0.2 and 0.5 nm. It should be emphasized that the PFA-based activated carbons are rather flat and resemble petals. Additionally, due to the high carbon content and low heteroatom content (Table 4.), the surface of AC is rather hydrophobic with the pHPZC between 7 and 7.8. The lowest pHPZC is observed for A-PFA/FeSO4, this can be explained on the basis of a slightly higher oxygen content and the presence of sulfur. The sedimentation test was performed to evaluate the effectiveness of the removal of the spent MACs. In this test, the height of sedimentation boundary versus time was measured. The Fig. 7. shows that the A-PFA requires the longest time to sediment. By applying the magnetic properties the sedimentation processes were indeed accelerated. The sedimentation time for all MACs is at least 3 time shorter compared to A-PFA and changes from 29 s for A-PFA to 5 s for A-PFA/FeCl3.
Because of the huge differences in the porous texture of the PFA-based magnetic adsorbents, for the preliminary adsorption test, three completely different organic compounds have been selected, as representatives for water contaminations. The single-point adsorption tests were carried out for phenol, Congo red, and atrazine. The results are given in Fig. 8a. As can be observed the amount adsorbed varies significantly depending on adsorbent and adsorbate type. The highest adsorption capacity for phenol and atrazine was found in A-PFA, while for Congo red, the highest removal is in A-PFA/ferrocene. In the case of adsorption from water, the surface characteristics as well as the porous textures are considered very important factors [9]. Because of the very similar pHPZC values (Table 4) but completely different porosities, the relationships between the single-point adsorption capacity and the pore volume are given in Fig. 8b, c.
As can be seen, the adsorption for phenol and atrazine increases with micropore volume increase (VDR). Quite opposite relation can be observed for CR. The amount of CR adsorbed increases with the mesopore volume. This is consistent with our earlier studies [30].
Special attention should be paid to the adsorption of phenol and atrazine. Although the adsorption of both compounds increases with an increase in the volume of the micropore, for a given pore volumes, a greater adsorption of atrazine is observed. Atrazine is a heavier and possesses larger molecule than phenol, so smaller adsorption could be expected. To explain this difference, factors other than porous texture should be taken into account. An important factor that influences the sorption capacity is the pH of the solution and the electrostatic interactions. However, by taking into account the pHPZC of the tested PFA-based ACs and the pH of the solution after adsorption (Fig. 8a.), the influence of electrostatic interaction on the sorption capacity can be excluded. Based on the pHPZC values of the PFA-based ACs (Table 4) the ACs surface charge of the studied MACs and A-PFA is around zero. Under that condition the phenol molecules remain undissociated, while in the system CR/PFA-based ACs or A/PFA-based, the CR and A molecules will be in anionic form as the pH of the solution (Fig. 8a) is higher than pKa (Table 1). Despite the occurrence in ionic form no electrostatic forces are expected to appear, due to the fact that the charge on the ACs surface is close to zero.
Another important parameter that has an impact on adsorption from solution is the solubility of the removed compounds [10]. In the case of physical adsorption, which is a spontaneous adsorption, compounds that are less soluble in solvents better adsorb from a given solution. In our case, the solubility of phenol is significantly greater (82.7 g dm−3) than the solubility of atrazine (0.035 g dm−3). This explains the higher adsorption of atrazine on all of the adsorbents tested in the study despite the larger size of the compound. Furthermore, the pore size distribution has an impact on the amount of adsorbed. In our previous work [24, 28], a correlation was reported between the adsorption capacity of AC for phenol compounds and the volume of pores with sizes smaller than 1.4 nm. Also in other works [19, 21, 39], the relationship between adsorption capacity and volume of pores with sizes 1.5–2.0 times bigger than the molecules size was given. If we assume that the smallest pores into which molecules can penetrate are limited by the second largest size for a three-dimensional molecule, it turns out that the limiting pore size for phenol and atrazine are pores with a very similar width. However, the greatest attraction occurs in pores with a width of 1.5–2 times larger. Thus, in the case of atrazine, a strong attraction will occur in the pore with a greater width. Therefore, taking into account the lower limit of the pore available for adsorption and the pore size giving the highest affinity, it can be concluded that the adsorption of atrazine will also occur effectively in pores with a width of about 1.8–2.0 nm, which, if we take into account the pore volume (Fig. 1b), explains the greater adsorption of atrazine compared to phenol. However, one should be aware that the single-point capacity is not the equilibrium capacity so the result cannot be so strictly related to pore volume.
3.3 Pesticides adsorption
A single-point adsorption test revealed that PFA-based magnetic adsorbents are efficient adsorbents toward different organic contaminants. Continuous development of agriculture causes contamination of groundwater and river waters with compounds such as pesticides. Therefore, complete adsorption processes were carried out for two compounds of agricultural origin on A-PFA/FeSO4. Atrazine and isoproturon have been selected as the representatives of herbicides, respectively. The adsorption of A and I as a function of time is given in Fig. 9a. As can be seen, it takes a long time to achieve the equilibrium phase. For the A-PFA/atrazine, it takes 260 h while for A-PFA/FeSO4/isoproturon 330 h to obtain the equilibrium. Such a long time necessary to reach the balance is a result of a very small mesopore volume of only 0.056 cm3 g−1. However, it should be emphasized that despite the small mesopore volume, 50% of the removal is obtained after about 24 h.
Usually, to describe the kinetics, the pseudo-first (Lagergren) (PFO) and pseudo-second order (PSO) model or the Elovitch equation (E) are the mostly used (Table S1), however, they do not give an information on the mechanism that rules the adsorption. Additionally, if there is no chemisorption, the PSO model usually better fits the result [15, 18, 32]. Similarly, here (Table S2), based on the correlation coefficient (0.927; 0.951 for PFO, 0.999; 0.998 for PSO and 0.975; 0.970 for E, it can be stated that PSO is favorable for the adsorption systems studied in this work.
To gain further insight into the mechanism controlling the rate of adsorption of A and I, an intramolecular diffusion model was used. A graph showing a Weber-Moris model can be a curve with three ranges of linearity. Such a "multilinearity" proves that more than one process determines the rate of adsorption. The transport of molecules from the bulk phase to the adsorption sites can be controlled by boundary film diffusion or intramolecular diffusion, or both will affect the transport of the molecules. The slowest will be the rate controlling factor. As can be seen in Fig. 9b, the curve shows multilinearity. The multiple nature of these plots can be explained in relation to processes such as, i.e., boundary layer diffusion, which is described by the intercept of the plot (CI) and the intraparticle diffusion, which gives the two linear parts (described as I and II). The larger the intercept, the grater is the boundary layer [25, 28, 29]. For adsorption on A-PFA/FeSO4 we can observe that the CI values, although similar (CI = 17.649 for Isoproturon and CI = 15.527 for Atrazine), are not the same. This reflects the higher affinity of larger molecules toward the adsorbent surface, as atrazine has a molecular mass bigger than isoproturon (Table 1). The second part, which describes the adsorption on active sites, is almost comparable for both adsorbates. It can be concluded that, because of the similarity in molecular size of adsorbed pesticides, they were adsorbed in pores with a comparable size.
Equilibrium adsorption isotherms are useful for investigating the relationship and interaction between the adsorbent and pesticides. Figure 9c shows that the equilibrium adsorption curves for atrazine and isoproturon have a typical Langmuir shape. This is the case when no strong competition, to occupy the adsorption sites, between the adsorbate and the solvent is observed. The equilibrium adsorption isotherm has been interpreted with the simplest and most often used models, i.e., Langmuir and Freundlich (Table S1). They directly give information on the adsorption capacity or heterogeneity factor, that allow us to study the mechanism of adsorption of organic compounds. The calculated values of the parameters of the Langmuir and Freundlich equation are given in Table 6. Generally, based on the isotherm L-shape, it could be predicted that the Langmuir model fits the experimental data better. The correlation coefficient supports this statement. The R2 for that equation is 0.99, while for Freundlich R2 is only 0.89 and 0.93. The calculated Langmuir adsorption capacity is 278 mg g−1 for isoproturon and 250 mg g−1 for atrazine. The obtained for A-PFA/FeSO4 Langmuir adsorption capacity is quite high. The amount of pesticides, such as atrazine or isoproturon, adsorbed on different low-cost or non-conventional usually does not exceed 50 mg g−1 [1, 6, 12, 42] whereas the adsorption capacity of commercial ACs or ACs obtained from waste biomass and polymers usually slightly above 100 mg/g [14, 42, 45]. However, in the case of atrazine adsorption, there is a report where the adsorption capacity is incredibly high. Cheng et al. [7] adsorbed over 1000 mg g−1 of atrazine on graphene-based adsorbents. The mechanism of atrazine adsorption on the sorbents was explained on the basis of hydrogen bond formation and ππ interaction. Nevertheless, no reasonable explanation was provided how such high adsorption was reached while porous texture characteristic of studied graphene-based materials, was quite moderate. There must have been other factors that influenced the adsorption capacity. It seems impossible that adsorbent with SBET = 26 m2 g−1 and pore volume of VBJH = 0.18 cm3 g−1 adsorbed QDR = 1306 mg g−1 of atrazine while the other graphene-based material with surface characteristic of SBET = 247 m2 g−1 and VBJH = 1.6 cm3 g−1 adsorbed a comparable amount of atrazine QDR = 1273 mg g−1.
In this work, the processes of pesticide adsorption were carried out in an unbuffered condition. Taking into account the pH of the solutions after adsorption (5.7–6.2 for A and 5.8–6.1) in both cases it was around 6, so the electrostatic forces do not influence significantly the adsorption processes in carbon A-PFA/FeSO4. The L-type isotherm reveals no interaction between the adsorbate/solvent/adsorbent; however, under that conditions some pesticides molecule might exist in an ionic form that might insignificantly enhance the adsorption. The Langmuir adsorption capacity is slightly larger for isoproturon compared to atrazine adsorption. This can be easily explained by the assumption that the mechanism that rules the adsorption of organic compounds from water is the pore-filling mechanism. The total three-dimensional molecules size of isoproturon is larger than atrazine; hence, more isoproturon molecule can be adsorbed in the volume of pore with size close to pesticides molecule size.
4 Conclusions
This work shows that magnetic adsorbents with high adsorption capacity toward different organic compound can be produce from polymer. All types of iron compounds used here give products with magnetic properties, but under the pyrolysis and subsequent steam activation conditions, different Fe salts promote development of completely different porous textures. Iron(III) chloride has the strongest catalytic properties and increases the intensity of gasification processes. Both ferrocene and FeCl3 induced development or mesoporosity in MACs, while the FeSO4 preserved the microporosity observed in A-PFA adsorbent. The magnetic properties were induced by iron in hematite and magnetite from. The higher magnetite content in MAC leads to stronger magnetic saturation. A simple test with neodymium magnet and balance revealed that the higher the total iron oxide found in PFA-based magnetic adsorbent the bigger was the mass loss in the neodymium-balance test. Additionally conducted sedimentation test has shown that indeed, the magnetic properties accelerate the process of adsorbent separation from the solution, which facilitates the MACs removal from the sorption system. Adsorption capacity of MAC toward Congo red, phenol, atrazine and isoproturon was pore size dependent.
5 Availability of data and materials.
Not applicable.
Data availability
Not applicable.
References
Akpinar, I., Yazaydin, A.O.: Adsorption of atrazine from water in metal-organic framework materials. J. Chem Eng. Data. (2018). https://doi.org/10.1021/acs.jced.7b00930
Alcañiz-Monge, J., Lillo-Ródenas, M.Á., Bueno-López, A., Illán-Gómez, M.J.: The influence of iron chloride addition to the precursor pitch on the formation of activated carbon fibers. Microporous Mesoporous Mater. (2007). https://doi.org/10.1016/J.MICROMESO.2006.10.043
Bastami, T.R., Entezari, M.H.: Activated carbon from carrot dross combined with magnetite nanoparticles for the efficient removal of p-nitrophenol from aqueous solution. Chem. Eng. J. (2012). https://doi.org/10.1016/J.CEJ.2012.08.011
Bayazit, ŞS., Kerkez, Ö.: Hexavalent chromium adsorption on superparamagnetic multi-wall carbon nanotubes and activated carbon composites. Chem. Eng. Res. Des. (2014). https://doi.org/10.1016/J.CHERD.2014.02.007
Bedia, J., Peñas-Garzón, M., Gómez-Avilés, A., Rodriguez, J.J., Belver, C.: Review on activated carbons by chemical activation with FeCl3. C6 (2020). https://doi.org/10.3390/c6020021
Boudesocque, S., Guillon, E., Aplincourt, M., Martel, F., Noël, S.: Use of a low-cost biosorbent to remove pesticides from wastewater. J. Environ. Qual. (2008). https://doi.org/10.2134/JEQ2007.0332
Cheng, C., Shi, X., Yin, G., Peng, F., Hou, W., Zhang, W., Lin, X., Li, J., Wang, X.: Atrazine adsorption by graphene-based materials: Interaction mechanism and application in real samples. Environ. Technol. Innov. (2022). https://doi.org/10.1016/J.ETI.2022.102823
Choi, S.I., Feng, J., Kim, S.B., Jo, Y.M.: Magnetization of metal mesh for fine dust capture. Aerosol. Air Qual. Res. 18, 1932–1943 (2018). https://doi.org/10.4209/aaqr.2017.11.0491
Da̧browski, A., Podkościelny, P., Hubicki, Z., Barczak, M.: Adsorption of phenolic compounds by activated carbon—a critical review. Chemosphere (2005). https://doi.org/10.1016/J.CHEMOSPHERE.2004.09.067
Daifullah, A.A.M., Girgis, B.S.: Removal of some substituted phenols by activated carbon obtained from agricultural waste. Water Res. (1998). https://doi.org/10.1016/S0043-1354(97)00310-2
Dias, J.M., Alvim-Ferraz, M.C.M., Almeida, M.F., Rivera-Utrilla, J., Sánchez-Polo, M.: Waste materials for activated carbon preparation and its use in aqueous-phase treatment: a review. J. Environ. Manag. (2007). https://doi.org/10.1016/J.JENVMAN.2007.07.031
Fernández-López, J.A., Miñarro, M.D., Angosto, J.M., Fernández-Lledó, J., Obón, J.M.: Adsorptive and surface characterization of Mediterranean agrifood processing wastes: Prospection for pesticide removal. Agronomy (2021). https://doi.org/10.3390/agronomy11030561
Gregg, S.J.K.S.W.S.: Adsorption, Surface Area and Porosity. Academic Press, London (1882)
Gupta, V.K., Gupta, B., Rastogi, A., Agarwal, S., Nayak, A.: Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res. (2011). https://doi.org/10.1016/J.WATRES.2011.05.016
Han, Y., Cao, X., Ouyang, X., Sohi, S.P., Chen, J.: Adsorption kinetics of magnetic biochar derived from peanut hull on removal of Cr (VI) from aqueous solution: Effects of production conditions and particle size. Chemosphere (2016). https://doi.org/10.1016/J.CHEMOSPHERE.2015.11.050
Hao, W., Bjçrkman, E., Yun, Y., Lilliestråle, M., Hedin, N.: Iron oxide nanoparticles embedded in activated carbons prepared from hydrothermally treated waste biomass. Chemsuschem (2014). https://doi.org/10.1002/cssc.201300912
Hao, W., Bjo, F., Trushkina, Y., Oregui Bengoechea, M., Barth, T., Hedin, N.: Correction to "High-Performance Magnetic Activated Carbon from Solid Waste from Lignin Conversion Processes. 1. Their Use As Adsorbents for CO2 ". ACS Sustain. Chem. Eng. (2017). https://doi.org/10.1021/acssuschemeng.6b02795
Hariani, P.L., Faizal, M., Ridwan, Marsi, Setiabudidaya, D.: Removal of Procion Red MX-5B from songket’s industrial wastewater in South Sumatra Indonesia using activated carbon-Fe3O4 composite. Sustain. Environ. Res. (2018). https://doi.org/10.1016/J.SERJ.2018.01.004
Juang, R.S., Tseng, R.L., Wu, F.C.: Role of microporosity of activated carbons on their adsorption abilities for phenols and dyes. Adsorption (2001). https://doi.org/10.1023/A:1011225001324
Jung, H.J., Kim, B.W., Malek, M.A., Koo, Y.S., Jung, J.H., Son, Y.S., Kim, J.C., Kim, H.K., Ro, C.U.: Chemical speciation of size-segregated floor dusts and airborne magnetic particles collected at underground subway stations in Seoul, Korea. J Hazard Mater. (2012). https://doi.org/10.1016/J.JHAZMAT.2012.02.006
Li, L., Quinlivan, P.A., Knappe, D.R.U.: Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon N Y. (2002). https://doi.org/10.1016/S0008-6223(02)00069-6
Lin, Y., Xu, S., Li, J.: Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. (2013). https://doi.org/10.1016/j.cej.2013.03.104
Liu, Z., Ling, L., Qiao, W., Liu, L.: Preparation of pitch-based spherical activated carbon with developed mesopore by the aid of ferrocene. Carbon N Y. (1999). https://doi.org/10.1016/S0008-6223(98)00242-5
Lorenc-Grabowska, E.: Effect of micropore size distribution on phenol adsorption on steam activated carbons. Adsorption (2016). https://doi.org/10.1007/S10450-015-9737-X
Lorenc-Grabowska, E., Diez, M.A., Gryglewicz, G.: Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J Colloid Interface Sci. (2016). https://doi.org/10.1016/j.jcis.2016.02.007
Lorenc-Grabowska, E., Gryglewicz, G.: Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dyes Pigm. (2006). https://doi.org/10.1016/j.dyepig.2006.01.027
Lorenc-Grabowska, E., Gryglewicz, G., Gryglewicz, S.: Development of mesoporosity in activated carbons via coal modification using Ca- And Fe-exchange. Microporous Mesoporous Mater. (2004). https://doi.org/10.1016/j.micromeso.2004.08.012
Lorenc-Grabowska, E., Rutkowski, P.: High basicity adsorbents from solid residue of cellulose and synthetic polymer co-pyrolysis for phenol removal: kinetics and mechanism. Appl. Surf. Sci. (2014). https://doi.org/10.1016/j.apsusc.2014.08.024
Lorenc-Grabowska, E., Rutkowski, P.: High adsorption capacity carbons from biomass and synthetic polymers for the removal of organic compounds from water. Water Air Soil Pollut. (2014). https://doi.org/10.1007/s11270-014-2082-y
Lorenc-Grabowska, E., Rutkowski, P.: Tailoring mesoporosity of poly(furfuryl alcohol)-based activated carbons and their ability to adsorb organic compounds from water. J. Mater. Cycles Waste Manag. (2018). https://doi.org/10.1007/s10163-018-0733-6
Maiorov, M.M., Zablotsky, D., Blums, E., Lickrastina, A.: Magnetic field control of gas-liquid mass transfer in ferrofluids. J. Magn. Magn. Mater. (2020). https://doi.org/10.1016/j.jmmm.2019.165958
de Marco, C., Mauler, R.S., Daitx, T.S., Krindges, I., Cemin, A., Bonetto, L.R., Crespo, J.S., Guégan, R., Carli, L.N., Giovanela, M.: Removal of malachite green dye from aqueous solutions by a magnetic adsorbent. Sep. Sci. Technol. (2020). https://doi.org/10.1080/01496395.2019.1585455
Marsh, H., Heintz, E.A., Rodrguez-Reinoso, F.: Introduction to Carbon Technologies. Universidad de Alicante (1997)
Moreira, R.F.P.M., Rodrigues, A.E.: Polyfurfuryl alcohol-derived carbon molecular sieves. Latin Am. Appl. Res. 31, 391–396 (2001)
Moreno-Castilla, C., López-Ramón, M.V., Carrasco-Marín, F.: Changes in surface chemistry of activated carbons by wet oxidation. Carbon N Y. (2000). https://doi.org/10.1016/S0008-6223(00)00048-8
Oliveira, L.C.A., Rios, R.V.R.A., Fabris, J.D., Garg, V., Sapag, K., Lago, R.M.: Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon N Y. (2002). https://doi.org/10.1016/S0008-6223(02)00076-3
Qajar, A., Peer, M., Rajagopalan, R., Liu, Y., Brown, C., Foley, H.C.: Surface compression of light adsorbates inside microporous PFA-derived carbons. Carbon N Y. (2013). https://doi.org/10.1016/J.CARBON.2013.04.001
Salem, S., Teimouri, Z., Salem, A.: Fabrication of magnetic activated carbon by carbothermal functionalization of agriculture waste via microwave-assisted technique for cationic dye adsorption. Adv. Powder Technol. (2020). https://doi.org/10.1016/J.APT.2020.09.007
Seredych, M., Hulicova-Jurcakova, D., Lu, G.Q., Bandosz, T.J.: Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance. Carbon N Y. (2008). https://doi.org/10.1016/J.CARBON.2008.06.027
Svoboda, J.: Magnetic Methods for the Treatment of Minerals in Development in Mineral Processing, vol. 8. Elsevier (1987)
Tamjidi, S., Esmaeili, H., Kamyab Moghadas, B.: Application of magnetic adsorbents for removal of heavy metals from wastewater: a review study. Mater. Res. Express (2019). https://doi.org/10.1088/2053-1591/ab3ffb
Tang, W.W., Zeng, G.M., Gong, J.L., Liu, Y., Wang, X.Y., Liu, Y.Y., Liu, Z.F., Chen, L., Zhang, X.R., Tu, D.Z.: Simultaneous adsorption of atrazine and Cu (II) from wastewater by magnetic multi-walled carbon nanotube. Chem. Eng. J. (2012). https://doi.org/10.1016/J.CEJ.2012.09.102
Tee, G.T., Gok, X.Y., Yong, W.F.: Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: a review. Environ. Res. (2022). https://doi.org/10.1016/j.envres.2022.113248
Thines, K.R., Abdullah, E.C., Mubarak, N.M., Ruthiraan, M.: Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: a review. Re. Sustain. Energy Rev. (2017). https://doi.org/10.1016/J.RSER.2016.09.057
Torrellas, S.A., Rodriguez, A.R., Escudero, G.O., Martin, J.M.G., Rodriguez, J.G.: Comparative evaluation of adsorption kinetics of diclofenac and isoproturon by activated carbon. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. (2015). https://doi.org/10.1080/10934529.2015.1055149
Xie, S., Hu, Z., Lu, D., Zhao, Y.: Dry PERMANENT MAGNETIC SEPARATOR: PRESENT STATUS AND FUTURE PROSPECTS. Minerals. (2022). https://doi.org/10.3390/MIN12101251
Yunus, Z.M., Yashni, G., Al-Gheethi, A., Othman, N., Hamdan, R., Ruslan, N.N.: Advanced methods for activated carbon from agriculture wastes; a comprehensive review. Int. J. Environm. Anal. Chem. (2022). https://doi.org/10.1080/03067319.2020.1717477
Zaharopoulou, A., Yannopoulos, S.N., Ioannides, T.: Carbon membranes prepared from poly (Furfuryl Alcohol–Furfural) precursors: effect of FeCl3 additive. C. J. Carbon Res. (2020). https://doi.org/10.3390/c6030053
Zhang, G., Qu, J., Liu, H., Cooper, A.T., Wu, R.: CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration. Chemosphere (2007). https://doi.org/10.1016/J.CHEMOSPHERE.2007.01.081
Zhang, M., Gao, B., Varnoosfaderani, S., Hebard, A., Yao, Y., Inyang, M.: Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol. (2013). https://doi.org/10.1016/J.BIORTECH.2012.11.132
Zhu, X., Liu, Y., Qian, F., Zhou, C., Zhang, S., Chen, J.: Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour Technol. (2014). https://doi.org/10.1016/j.biortech.2013.12.019
Funding
The work received funding from a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Science and Technology.
Author information
Authors and Affiliations
Contributions
EL-G has made a substantial contribution to the concept and design of the article. She carried out the adsorption processes and performed the analysis and interpretation of the data for the article. She has written the manuscript. OS was responsible for the preparation of activated carbon. KK-K conducted the SEM and XRD analysis and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Nothing to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lorenc-Grabowska, E., Stasiak, O. & Kordek-Khalil, K. Polymer-based porous carbon doped with iron nanoparticles for enhanced organic compounds removal. Adsorption 30, 279–291 (2024). https://doi.org/10.1007/s10450-023-00412-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00412-z