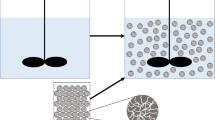
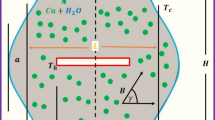
Abstract
The non-isothermal adsorption/desorption of gas in a cylindrical column filled with adsorbent particles has been considered theoretically. The model is based on Langmuir theory for equilibrium adsorption. Using the Heaviside operational method the analytical solutions for kinetics of changes of concentration of adsorbate in the inter- and intra-particle spaces were obtained. The numerical calculations of gas adsorption at different temperature of the gas flow (Ta = 273–373 K) followed by desorption by the flow of inert gas through the column at Td = 673 K were done. The results revealed the rather complex interrelations between spatial–temporal dependencies in distribution of concentrations of adsorbed gas inside adsorption column and temperatures of inlet gas.
Graphical Abstract




Similar content being viewed by others
Abbreviations
- a :
-
Concentration of adsorbate in the intra-particle space (nanopores) (ppmv)
- a m :
-
Maximum value of a (ppmv)
- c :
-
Concentration of adsorbate in the inter-particle space (ppmv)
- c m :
-
Maximum value of c (ppmv)
- D :
-
Effective diffusion coefficient in the interparticle space (m2/c)
- l :
-
Length of adsorption column (m)
- h g :
-
Heat capacity of gas; kJ /(kg·K)
- h t :
-
Effective heat capacity of gas and adsorbent kJ /(kg·K);
- ΔH :
-
Activation energy of adsorption/desorption (kJ/mol)
- Q ads :
-
Heat of adsorption in nanopores (kJ/kg)
- R :
-
Universal gas constant (= 8.314 4621(75) J/(K mol));
- r :
-
Radius of adsorption column (m)
- T :
-
Temperature (K)
- T a :
-
Temperature of the inlet gas flow for adsorption, Ta = 273–373 K (K)
- T d :
-
Temperature of the inlet inert gas flow for desorption, Td = 623 K, (K)
- T e :
-
Equilibrium temperature (K)
- t :
-
Time (s)
- u :
-
Linear velocity of the gas flow (m/c)
- z :
-
Distance along the column starting from the inlet (m)
- α h :
-
Coefficient of heat transfer from the gas-phase to the wall (W/(m2K))
- β :
-
total mass transfer coefficient (C−1)
- Λ :
-
Coefficient of thermal diffusion of fluid (kJ /(m2·K·s))
- ρ :
-
Bulk density –of particle packing in the adsorbent (kg/m3)
References
Feng, C., Jiaqiang, E., Han, W., Deng, Y., Zhang, B., Zhao, X., Han, D.: Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 144, 110954 (2021). https://doi.org/10.1016/j.rser.2021.110954
Rad, L.R., Anbia, M.: Zeolite-based composites for the adsorption of toxic matters from water: a review. J. Environ. Chem. Eng. 9, 106088 (2021). https://doi.org/10.1016/j.jece.2021.106088
Beckwée, E.J., Wittevrongel, G.R., Claessens, B.: Comparing column dynamics in the liquid and vapor phase adsorption of biobutanol on an activated carbon monolith. Adsorption (2022). https://doi.org/10.1007/s10450-022-00362-y
Nandanwar, S.U., Corbin, D.R., Shiflett, M.B.: A review of porous adsorbents for the separation of nitrogen from natural gas. Ind. \& Eng. Chem. Res. 59, 13355–13369 (2020). https://doi.org/10.1021/acs.iecr.0c02730
Qian, Z., Wei, L., Mingyue, W., Guansheng, Q.: Application of amine-modified porous materials for CO2 adsorption in mine confined spaces. Colloids Surf. A Physicochem. Eng. Asp. 629, 127483 (2021). https://doi.org/10.1016/j.colsurfa.2021.127483
Wang, Y., Wang, C., Wang, L., Wang, L., Xiao, F.-S.: Zeolite fixed metal nanoparticles: new perspective in catalysis. Acc. Chem. Res. 54, 2579–2590 (2021). https://doi.org/10.1021/acs.accounts.1c00074
Unger, N., Bond, T.C., Wang, J.S., Koch, D.M., Menon, S., Shindell, D.T., Bauer, S.: Attribution of climate forcing to economic sectors. Proc. Natl. Acad. Sci. 107, 3382–3387 (2010). https://doi.org/10.1073/pnas.0906548107
Niu, X., Bai, Y., Du, Y., Qi, H., Chen, Y.: Size controllable synthesis of ZSM-5 zeolite and its catalytic performance in the reaction of methanol conversion to aromatics. R. Soc. Open Sci. 9, 211284 (2022). https://doi.org/10.1098/rsos.211284
Puertolas, B., Navarro, M.V., Lopez, J.M., Murillo, R., Mastral, A.M., Garcia, T.: Modelling the heat and mass transfers of propane onto a ZSM-5 zeolite. Sep. Purif. Technol. 86, 127–136 (2012). https://doi.org/10.1016/j.seppur.2011.10.036
Sanchez-Varretti, F.O., Bulnes, F.M., Ramirez-Pastor, A.J.: Adsorption of interacting binary mixtures on heterogeneous surfaces: theory, Monte Carlo simulations and experimental results. Adsorption 25, 1317–1328 (2019). https://doi.org/10.1007/s10450-019-00093-7
Van Assche, T.R.C., Baron, G.V., Denayer, J.F.M.: An explicit multicomponent adsorption isotherm model: accounting for the size-effect for components with Langmuir adsorption behavior. Adsorption 24, 517–530 (2018). https://doi.org/10.1007/s10450-018-9962-1
Wilkins, N.S., Rajendran, A.: Measurement of competitive CO2 and N2 adsorption on Zeolite 13X for post-combustion CO2 capture. Adsorption 25, 115–133 (2019). https://doi.org/10.1007/s10450-018-00004-2
Santander, J.E., Conner, W.C., Jr., Jobic, H., Auerbach, S.M.: Simulating microwave-heated open systems: tuning competitive sorption in zeolites. J. Phys. Chem. B 113, 13776–13781 (2009). https://doi.org/10.1021/jp902946g
Hammond, K.D., Conner, W.C., Jr.: Analysis of Catalyst Surface Structure by Physical Sorption. In: Gates, B.C., Jentoft, F.C. (eds.) Advances in Catalysis, pp. 1–101. Elsevier, Amsterdam (2013)
Kärger, J., Ruthven, D.M., Theodorou, D.N.: Diffusion in nanoporous materials. Wiley, Hoboken (2012)
Krishna, R.: Thermodynamically consistent methodology for estimation of diffusivities of mixtures of guest molecules in microporous materials. ACS Omega 4, 13520–13529 (2019). https://doi.org/10.1021/acsomega.9b01873
Leclerc, S., Petryk, M., Canet, D., Fraissard, J.: Competitive diffusion of gases in a zeolite using proton NMR and a slice selection procedure. Catal. Today 187, 104–107 (2012). https://doi.org/10.1016/j.cattod.2011.09.007
Petryk, M., Leclerc, S., Canet, D., Deineka, S.I.V., V.S., Fraissard, J.: The competitive diffusion of gases in a zeolite bed: NMR and slice procedure, modelling ANMD identification of parameters. J. Phys. Chem. C ACS 119, 26519–26525 (2015). https://doi.org/10.1021/acs.jpcc.5b07974
Petryk, M.R., Khimich, A., Petryk, M.M., Fraissard, J.: Experimental and computer simulation studies of dehydration on microporous adsorbent of natural gas used as motor fuel. Fuel 239, 1324–1330 (2019). https://doi.org/10.1016/j.fuel.2018.10.134
Petryk, M.R., Khimich, A.N., Petryk, M.M.: Simulation of adsorption and desorption of hydrocarbons in nanoporous catalysts of neutralization systems of exhaust gases using nonlinear Langmuir isotherm. J. Autom. Inf. Sci. 50, 18–33 (2018). https://doi.org/10.1615/JAutomatInfScien.v50.i10.20
Doetsch, G.: Handbuch der Laplace-Transformation: Band I: Theorie der Laplace-Transformation. Springer, Basel (1950)
Staines, J.: The Heaviside Operational Calculus: The Laplace Transform for Electrical Engineers. CreateSpace Independent Publishing Platform, Scotts Valley (2013)
Landau, L.D.: On the theory of phase transitions. I. Phys. Zeitschrift der Sowjetunion. 11, 26 (1937)
Bogoliubov, N.N., Mitropolsky, Y.A.: Asymptotic methods in the theory of non-linear oscillations. Gordon and Breach Science Publisher, Philadelphia (1961)
Acknowledgements
The research results mentioned in this work were partly supported by Grant SSHN Campus France, 2021, funding from the Ministry of Education and Science of Ukraine, Project # DI 247-22, 0122U001859, and Projects KPKVK # 6541230, # 23BF05101.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petryk, M., Boyko, I., Fraissard, J. et al. Modelling of non-isothermal adsorption of gases in nanoporous adsorbent based on Langmuir equilibrium. Adsorption 29, 141–150 (2023). https://doi.org/10.1007/s10450-023-00389-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00389-9