Abstract
Two series of Pd–Pt/Al2O3 catalysts, characterized by high metal dispersions were prepared and investigated in the reaction of n-hexane conversion at the temperature < 300 °C. Incipient wetness co-impregnation of γ-alumina with the solutions of Pd(acac)2 and Pt(acac)2 led to relatively good Pd–Pt alloying. However, a similar catalyst preparation using solutions of PdCl2 and H2PtCl6 resulted in unsatisfactory homogenization; furthermore, significant amounts of chlorine were retained in the catalysts. The Pd–Pt alloy homogeneity has a significant effect on the relations of catalytic activities and product selectivities with Pd–Pt alloy composition. The relationship between the catalytic activity of chlorine-free Pd–Pt/Al2O3 catalysts and bulk Pd–Pt composition matches the accepted relation between the surface composition and bulk composition of Pd–Pt, reflecting a high surface enrichment in palladium. In contrast, for the chloride series of Pd–Pt/Al2O3, variations of the turnover frequency are directly correlated with the bulk composition, indicating that unalloyed, more active, Pt particles decide about the catalytic behavior. Pd/Al2O3 shows better than Pt/Al2O3 selectivity for isomerization, especially after reduction at higher temperatures, ≥ 500 °C. On alloying with Pt, for the Cl-free series the isomerization selectivity was very high for nearly all bimetallic catalysts and showed a synergistic effect for 20 at.% Pt. In contrast, for the Cl-containing series, the isomerization selectivity was proportional to the palladium content. The effect of reduction temperature on the isomerization propensity of Cl-containing Pd–Pt/Al2O3 catalysts was rather small, suggesting that possible variations in support acidity associated with chloride presence do not have large catalytic consequences, confirming the metal-only catalytic action in alkane isomerization carried out at the temperatures below 300 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Platinum is exceptional among the catalytic metals for its activity in alkane rearrangements, and as a consequence most fundamental work on these reactions has been done with this metal. Supported platinum catalysts are well known for their efficient performance in naphtha reforming, effectively catalyzing such reactions of alkanes as isomerization, hydrocracking and dehydrocyclization (Clarke and Rooney 1976; Leclercq et al. 1977; Gault 1981; Paál and Tétényi 1982; Galadima et al. 2009). Yet, modification of platinum catalysts by alloying with another metal may still result in an improvement of their catalytic performance, related to the increase of catalyst’s lifetime or the selectivity toward required products (Kluksdahl 1968; Dautzenberg and Kouwenhoven 1972; Sinfelt 1972, 1973, 1976). For example, several reports showed a superior sulfur tolerance of bimetallic Pt–Pd, compared to that of monometallic Pt catalysts, supported on acidic supports such as zeolite H-Beta (Lee and Rhee 1998). Previous works from our group dealt with alkane conversion reactions on Pd–Pt catalysts, either supported on silica (Karpiński and Kościelski 1980; Kościelski et al. 1982) or in the form of alloy films evaporated on Pyrex glass (Karpiński and Kościelski 1979). In those works synergistic effects were found for isomerization selectivity of neopentane, isomerization and C5-cyclization of n-pentane and n-hexane.
Although alumina is an important component of dual-function reforming catalysts, only very few studies have been published on the performance of alumina-supported Pt–Pd/Al2O3 in alkane conversions. Margitfalvi et al. (1981, 1982) showed that after calcination in oxygen, followed by hydrogen treatment, Pd–Pt/Al2O3 showed higher activity and better selectivity than the monometallic Pt/Al2O3 catalysts in n-hexane dehydrocyclization and isomerization, at relatively high reaction temperatures ≥ 450 °C. Contreras et al. (1977) reported the results of n-hexane conversion carried out on Pt–Pd/Al2O3 catalysts at much lower reaction temperatures, i.e.at 250–300 °C. At these temperatures, aromatization of n-hexane was practically not observed, so the selectivity changes were only related to hydrogenolysis and isomerization products. It appeared that the bimetallic Pd–Pt/Al2O3 catalysts exhibited a lower selectivity towards isomerization than the monometallic Pt/Al2O3 and Pd/Al2O3 counterparts. In addition, the overall catalytic activity was found high for the Pt/Al2O3 and small for Pd–Pt/Al2O3 samples and very small for the monometallic Pd/Al2O3 catalyst.
Other published works dealt with the conversion of other C6-alkanes, carried out on Pt–Pd/Al2O3 catalysts. Garin et al. (1984) reported results of the study on isomerization of 13C-labeled 2-methylpentane on well-characterized but poorly dispersed Pd–Pt/Al2O3 catalysts (metal crystallite size between 15 and 50 nm), prepared by co-impregnation of palladium and platinum chlorides. They found a synergistic effect for the cyclic mechanism of isomerization on an alloy with the surface concentration of 47% Pd. They also found that the catalytic behavior (overall activity and product selectivity) was controlled by the surface composition of Pd–Pt alloy particles, which, according to earlier reports (e.g. Kuijers et al. 1978) should be considerably enriched in palladium. The surface composition has also a big effect on the distribution of hydrogenolysis products: alkane demethylation (vs. internal splitting), characteristic for pure Pd, is largely maintained for a large range of Pd–Pt alloy composition manifesting again the surface enrichment in palladium of the Pd–Pt alloys. Similar results were reported for the Pd–Pt/Al2O3 catalysts prepared by hydrazine reduction of H2PtCl6 and PdCl2 dissolved in microemulsions consisting of water, hexadecane and pentaethylene glycol dodecyl ether (Touroude et al. 1992).
In some already cited works on Pt–Pd/Al2O3 catalysts (Contreras et al. 1977; Garin et al. 1984; Margitfalvi et al. 1982) the chloride-containing precursors were used for catalyst preparation. Common knowledge about the industrial performance of Pt(Cl)/Al2O3 catalysts in catalytic isomerization of alkanes (Galadima et al. 2009) must raise the question if the presence of residual chloride originating from the precursor on the surface of alumina would enhance the acidic function of Pt–Pd/Al2O3 catalysts and create an additional reaction pathway to the metal-only catalytic action (Ollendorff et al. 1990). The present work includes the comparison of the catalytic performance of two series platinum–palladium catalysts supported on chloride-free alumina. One series was prepared using chloride-containing precursors, the other-metal acetylacetonates. The use of the latter precursors was shown to result in the preparation of well mixed Pd–Pt catalysts, supported on alumina (Micheaud et al. 1998; Bazin et al. 2005), silica (Renouprez et al. 2000) and magnesia (Dossi et al. 2003). Easy formation of well-defined mixed acetyloacetonates precursors depositing on a support followed by calcination and reduction results in the preparation of Pd–Pt nanoparticles of homogeneous composition, so this preparation method would be considered as a sort of recommendation.
2 Experimental
2.1 Catalyst preparation and characterization
Two series of Pd–Pt/γ-alumina catalysts were prepared and tested. The support was γ-alumina, Sasol Puralox Scca, 150–200 mesh, surface area ~ 200 m2/g. One series of Pd–Pt catalysts was prepared by an incipient wetness impregnation of alumina with a mixture Pd and Pt bis-acetylacetonate precursors (Pd(acac)2 and Pt(acac)2 from Sigma-Aldrich, 99%) dissolved in toluene (analytical reagent from Chempur, Piekary Śląskie, Poland). After impregnation, the wet material was dried at 120 °C, calcined in a flow of air at 530 °C, and stored in a desiccator. The total metal loading was 1 wt%. The other series of Pd–Pt/Al2O3 catalysts were prepared by incipient wetness impregnation of γ-Al2O3 with aqueous solutions of palladium chloride (analytical regent from POCh, Gliwice, Poland) and chloroplatinic acid (obtained from a Pt wire, Johnson-Matthey, grade 1 after dissolving it in a hot mixture of HCl and HNO3, both of analytical purity from POCh, Gliwice, Poland). After impregnation, the wet materials were dried at 120 °C for overnight and stored in a desiccator. In further text, both catalyst series will be referred as to the ‘acac’ and the ‘chloride’ series, whereas particular catalysts will be designed as PdXPtY(acac) or PdXPtY(Cl), respectively. X and Y stand for atomic percentages of Pd and Pt in the metal phase.
A 10% H2/Ar mixture (25 cm3/min) was used in the temperature-programmed reduction (TPR) runs of Pd–Pt/Al2O3 catalysts. The sample temperature was ramped from RT to 550 °C at 10 °C/min. The effluent gas was passed through a moisture trap (to remove H2O released from precalcined metal acetylacetonates) or a bath with ethanol-liquid nitrogen slurry (to remove HCl released from non-calcined chloride catalyst precursors), and detected on-line by a Gow-Mac thermal conductivity detector, kept at RT. The consumed amounts of H2 were generally 10–15% lower than expected from calculated amounts of PdO and PtO2. This deficit appears acceptable because some small part of noble metal (Pd, Pt) could be reduced before starting a TPR run at RT.
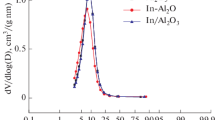
The same reaction system was used for performing H2 chemisorption in a pulse-flow fashion. Prior to chemisorption, the prepared catalysts were reduced in flowing 10% H2/Ar (25 cm3/min), ramping the temperature from RT to a specified temperature (400, 500 or 600 °C) at 10 °C/min, and kept at this temperature for 1 or 17 h. All the gases (H2, Ar and 10% H2/Ar mixture, all 99.9999%) were further purified by passing through zeolite 4A (drying) and MnO/SiO2 (deoxo) traps. The results of chemisorption studies together with catalyst designation are shown in Tables 1 and 2.
XRD diffraction studies (a standard Rigaku Denki diffractometer) of all reduced catalysts did not show any metal (Pt, Pd) reflections but only intense reflections originating from γ-alumina (diffractograms not presented).
TEM investigations of differently pretreated Pd/Al2O3 samples were performed in a probe Cs-corrected S/TEM Titan 80e300 FEI microscope equipped with EDAX EDS. The images were recorded in STEM mode, using the high-angle annular dark field (HAADF) detector. A 300 keV electron beam with a convergence semi-angle of 17 and 27 mrad was used. The condensed beam analyzed using X-ray energy-dispersive spectrometer in TEM mode and EDS with energy resolution 134 eV scanning in HAADF-STEM mode using EDAX detector allowed to estimate the local concentration of chemical elements. Moreover, surface-weighted average diameter of metal particles determined as
where ni is the amount of counted particles of average diameter di, allowed to compare particle sizes determined by hydrogen chemisorption.
The overall loadings of platinum and palladium in the prepared catalysts (1 wt%) resulted from the inherent character of incipient wetness impregnation of metal salts were confirmed by X-ray fluorescence (Epsilon 3XLE Panalytical instrument equipped with rhodium X-ray tube of 15 W, data analyzed using an Omnian 1.4.G. software). This method also served for determination of chlorine content in prereduced Pd–Pt/Al2O3 catalysts prepared from metal chloride-precursors.
2.2 Catalytic conversion of n-hexane
The catalytic conversion of n-hexane (>99%, analytical grade from Chempur, Piekary Śląskie, Poland, with 3-metylpentane and methylcyclopentane as main impurities) in excess H2 (> 99.9999% from hydrogen generator), additionally purified using a MnO/SiO2 trap, was conducted in a continuous-flow reaction glass system under atmospheric pressure. After reduction at the conditions specified in Tables 1 and 2, the catalysts (~ 0.2 g) were contacted with the reaction mixture (flow of hydrogen at 13.6 cm3/min and n-hexane, provided from a saturator kept at 0 °C), at the highest reaction temperature, i.e. 291 °C. The partial pressure of n-hexane was 6.0 kPa, resulting in H2-to-hexane ratio ~ 16:1. A tubular fused silica reactor equipped with a fritted disk was loaded with 1 wt% Pd–Pt/Al2O3 catalyst.
The Pd–Pt/Al2O3 catalysts deactivated with time on stream loosing ~ 20–30% of the initial activity, and steady-state conversions were achieved after ~ 2 h on stream. Then, a usual catalyst screening was continued, i.e. collection of experimental points at lower reaction temperature, i.e. at 272 and 252 °C. The reaction was followed by gas chromatography (Bruker SCION456-GC, with FID, 6 m squalane/Chromosorb P column, controlled by Compass software from Bruker). Blank experiments with differently pretreated metal-free γ-Al2O3 showed no reaction at temperatures up to 300 °C. Turnover frequencies (TOFs) were calculated on the basis of the metal fraction exposed measured by H2 chemisorption. Product selectivities were calculated as the carbon percentage of n-hexane consumed in the formation of a designated product.
3 Results and discussion
3.1 Catalyst characterization
The temperature programmed reduction of all precalcined Pd–Pt/Al2O3 catalysts prepared from metal acetylacetonates showed a common course, characterized by two TPR peaks: one at ~ 120 °C and the other, with maximum at 320–370 °C (Fig. 1). Adopting earlier recommendations as to the assignment of these peaks (Matam et al. 2012; Esteves et al. 2016) we ascribe the low temperature and high temperature TPR peaks to reduction of more easily and strongly with support interacting PdO species, respectively. However, for reduction of the monometallic, Pd100(acac), the first TPR peak (at ~ 120 °C) was distorted by a negative feature, most probably associated with the decomposition of a palladium hydride phase (Fig. 1, upper profile). TPR profiles for other PdXPtY(acac) samples did not reveal such a feature, in a good agreement with Noh et al. (1995), who showed that the introduction of only 5 at.% Pt to Pd stops the formation of the β-hydride phase at the hydrogen pressure below 12 kPa (Fig. 7 in Noh et al. (1995)). Therefore, the TPR profiles for Pd-richer bimetallic samples indicate that we have already dealt with well-mixed Pd–Pt precursors deposited on alumina.
Pd–Pt/Al2O3 catalysts prepared from non-calcined chloride precursors were not subjected to detailed TPR studies. It is well known that the alumina-supported Pd and Pt chloride precursors are fully reduced at a relatively low temperature (Kennedy et al. 2004), well below 400 °C, i.e. the lowest temperature level of catalyst’s reduction used in this study prior to kinetic and chemisorption experiments. Representative TPR experiments with three Pd–Pt/Al2O3(Cl) catalysts (Pd100, Pt100 and Pd90Pt10) confirmed that these catalysts are fully reduced at relatively low temperatures, in our case below 200 °C (TPR profiles not shown).
Tables 1 and 2 present basic data obtained for the characterization of two series of Pd–Pt/Al2O3 catalysts subjected to different reduction conditions. As expected, the more severe reduction conditions resulted in the decrease of metal dispersion. Some illustrative data from transmission electron microscopy confirm this metal sintering. In general, the reduced catalysts were characterized by quite small metal particles ranged from ~ 2 to ~ 6 nm (mean size, Tables 1 and 2), however larger metal particles (~ 10–20 nm) were occasionally also detected. The catalysts prepared from metal chlorides show considerable amounts of chlorine after reduction at 400 °C for 17 h (Table 2).
As mentioned in the Introduction, the previous reports (Micheaud et al. 1998; Bazin et al. 2005) provided evidence that preparation of Pd–Pt/Al2O3 catalysts by co-impregnation using solutions of metal acetylacetonates resulted in good Pd–Pt alloying. Figure 2 shows an exemplary TEM-EDS image which confirms a reasonably good (although not complete) extent of Pd–Pt alloying. On the other hand, the ex-chlorides catalysts are not as good. Figure 3 reveals that the Pt80Pd20(Cl) catalyst was poorly alloyed, where practically unmixed Pd and Pt particles co-existed. Inhomogeneous distribution of both metals on alumina surface obtained by impregnation of their chloride-containing precursors was also observed and discussed by Cho and Regalbuto (2015). They demonstrated that, in contrast to the incipient wetness impregnation, the electrostatic adsorption of mixed metal precursors results in well dispersed, homogeneously alloyed nanoparticles.
The prepared two series of Pd–Pt/Al2O3 catalysts differed not only by a very different degree of Pd–Pt alloying but also by the fact that the ‘chloride’ series contained large amounts of chlorine left after reduction in hydrogen (Table 2). A possible effect of both these issues will be assessed in interpretation of catalytic data.
3.2 Catalytic behavior of Pd–Pt/Al2O3 in n-hexane conversion
In order to diagnose the effect of Pd–Pt alloy composition on the catalytic behavior in n-hexane conversion, the following discussion of kinetic results will be based on their graphical presentation. Extents of conversions, turnover frequencies, apparent activation energies and product selectivities are presented in Figs. 4, 5, 6, 7, 8. For the detailed source material the Reader is referred to Tables 1 (ESM)–6(ESM) located in Electronic Supplementary Material.
n-Hexane conversion over Pd–Pt/Al2O3 catalysts subjected to different reduction conditions. specified in the graphs. The effect of nominal bimetal composition on turnover frequency at 291 °C: a The ‘acac’ series. b The ‘chloride’ series. In b all, but one (open red circle), experimental points contribute to a linear relationship characterized by a correlation coefficient close to 0.95. Inset in a: the relation between surface and bulk composition of Pd–Pt alloys adopted from Rousset et al. (1996) (Color figure online)
Very low degrees of conversion (except for Pt-rich catalysts) indicate that most products are considered as primary products. At relatively low reaction temperatures (< 300 °C) the reaction of n-hexane conversion is confined mainly to isomerization and hydrogenolysis. In agreement with an earlier work (Contreras et al. 1977). Dehydrocyclization (methylcyclopentane and benzene formation) was found in minor amounts and is not discussed here.
3.2.1 Catalytic activity versus Pd–Pt alloy composition
Figure 4a, b presents the relation of the catalytic activity of Pt–Pd/Al2O3 catalysts versus alloy composition for the reaction of n-hexane conversion. Platinum is the most active catalyst, while palladium and palladium-rich alloys are roughly 20–50 times less active. However, the character of the relation between TOF and Pd–Pt alloy composition relation is different for both series of Pd–Pt/Al2O3 catalysts. For the ‘acac’ series this relation shows a concave shape, characterized by a rather sharp decrease of TOF for Pt-rich alloys, and much milder changes for the rest of Pd–Pt alloys (Fig. 4a). On the other hand, the respective TOF-alloy composition relations for the catalysts prepared from metal chlorides, shows a linear decrease of the catalytic activity with the Pt content, characterized by the correlation coefficient close to 0.95 (Fig. 4b). It should be stressed that the activity of chloride-free Pt/Al2O3 catalyst is generally a few times higher than that of the ‘chloride’ series. This relatively small difference (i.e. within one order of magnitude) cannot be rationalized by blocking of the metal surface by residual chloride species. The reaction of alkane hydroconversion was carried out under high excess of hydrogen (hexane-to-hydrogen ratio ≈ 16) at the temperature close to 300 °C, therefore the surface of Pd(Pt) should be fully reduced and got rid of chlorine. The residual chlorine in Pd–Pt/Al2O3(Cl) catalysts after reduction at 400 °C (Table 2) is rather strongly attached to the support and is removable with difficulty by high thermal treatment (Arena et al. 1992). Alumina modification by chlorine should result in the formation of acid sites which play a role in alkane hydroconversion occuring via a dual-function mechanism. The fact that the activity of the “chloride” series is even slightly lower that that of the “acac” series demonstrates that at the reaction conditions used in this study (< 300 °C) the n-hexane conversion is attributable to the metal-only catalytic action. This conclusion is in line with earlier reports from the Strasbourg group (Maire et al. 1971; Dartigues et al. 1976), who proved that observed changes in mechanisms of alkane isomerization carried out at < 350 °C would be explained by a platinum particle size effect and not by a bifunctional effect due to the presence of chloride in alumina.
Hardly rationalized small differences between the catalytic activity of both catalyst series recall earlier results of Le Normand et al. (1993) who investigated the hydroconversion of saturated hydrocarbons on Pd/Al2O3 catalysts prepared from different palladium salts. Their attention was focused at a big difference in the behavior of ex-nitrate Pd/Al2O3 catalyst compared to chloride and (acac)2 derived counterparts. However, a simple recalculation of data presented in Tables 1 and 2 of the cited paper demonstrates that the catalyst prepared from the acetylacetonate precursor (I-1) was ~ 2.3 times more active than the catalyst prepared from the chloride precursor (V-1).
As mentioned earlier, preparation of Pd–Pt/Al2O3 catalysts from metal acetylacetonates led to a reasonable degree of Pd–Pt alloying. Because for the reduction of catalysts rather high temperatures and prolonged periods were employed (resulting in high values of diffusion coefficients), it seems reasonable to apply existing thermodynamic data on the surface composition of Pd–Pt alloys. Theoretical predictions (Rousset et al. 1996, 2001) for the surface segregation in Pt–Pd alloys, confirmed by experimental data (Bazin et al. 2005; Van Den Oetelaar et al. 1998) are inserted in Fig. 4a. It is seen that the catalytic activity follows very similar trend as the surface composition. This similarity implies that the overall catalytic activity is determined by platinum sites which are more active that the palladium ones. On the other hand, the approximately straight relation exhibited by the ‘chloride’ series (which as mentioned earlier, were rather poorly homogenized) practically corresponds to a linearly decreasing amount of platinum in the catalysts. Therefore, these data confirm an absence of significant Pd–Pt interaction in the ‘chloride’ series of Pd–Pt/Al2O3 catalysts, and the system behaves like a mechanical mixture of Pd100(Cl) and Pt100(Cl).
3.2.2 Catalytic activity versus catalyst pretreatment
Figure 4a shows the effect of reduction temperature of the catalysts prepared from metal acetylacetonates. At mentioned in subsection 3.2.1. The Pt/Al2O3 is more active than all Pd-containing catalysts. However, after the high temperature reduction (at 500 and 600 °C) one observes a considerable weakening of this difference: Pt/Al2O3 becomes less active whereas Pd100, palladium rich (Pd80Pt20 and Pd60Pt40) catalysts turn out to be more active. This effect is not observed for the ‘chloride’ series (Fig. 4b). It appears that the presence of residual chloride must neutralize changes in alumina-supported Pd–Pt catalysts generated by high temperature reduction. Our previous work (Juszczyk et al. 1995) showing the possibility of Pd–Al formation from Pd/Al2O3 catalyst subjected to reduction at 600 °C, indicated that such a transformation is not observed for the catalyst containing chloride (ex-PdCl2).
The activation energies for Pt/Al2O3 catalysts (Tables 1 (ESM)–6 (ESM)) were found somewhat lower than those for Pd/Al2O3, in agreement with earlier findings (Skotak and Karpiński 2002). However, because this difference is not large, the relation between Ea and Pd–Pt alloy concentration relation should be regarded rather weak (Fig. 5a), especially because the Ea’s values are subjected to certain inaccuracies specified in Tables 1 (ESM)–6 (ESM). However, two results are worth of comment. First, one observes the unexpectedly high values of activation energies Ea for Pt100(Cl) and Pd20Pt80(Cl) catalysts after reduction at 400 °C for 17 h (Fig. 5b). Interestingly, a more severe reduction conditions (500 °C, 17 h) regain a “normal” activation energy level (Fig. 5b). Second, the activation energies for palladium and palladium-rich alloys decrease with the increase of reduction temperature, attaining the level characteristic for Pt100/Al2O3. Such variations with the severity of catalyst’s pretreatment are hardly ventured, however one can analyze them in a qualitative way by referring to earlier results for two series of Pt100/Al2O3 catalysts characterized by very different metal loadings (0.2 vs. 10 wt%), and, in effect, by very different metal particle sizes (Maire and Garin 1985). Table 2 in the cited review article presents kinetic data (reaction orders vs. H2 and apparent energies of activation) for isomerization and hydrogenolysis of C5 alkanes. It is seen that, for a variety of reaction routes, the low Pt loaded, highly dispersed catalyst exhibited much higher activation energies than the high metal loaded, poorly dispersed, counterpart (by ~ 30–60 kJ/mol). Now one adopts the fact that larger metal particles possess much higher proportion of face atoms, compared to very small metal particles which contain a higher proportion of highly unsaturated metal sites (e.g. corner and edge atoms). Therefore, a dominant presence of highly unsaturated metal sites appears responsible for a higher level of the activation energy in alkane conversion. Recently, we demonstrated by HRTEM that the high temperature reduction of alumina-supported Pd catalyst (at 600 °C) leads to a considerable smoothing of metal particles (Fig. 8 (SM) in Radlik et al. 2019). This effect would be responsible for lowering of the activation energy.
3.2.3 Isomerization selectivity in n-hexane conversion on Pd–Pt catalysts
Variations of the isomerization selectivity versus the Pt–Pd alloy composition after different catalyst pretreatments, based on the data presented in Tables 1 (ESM)–6 (ESM), are shown in Fig. 6 for the “acac” series of Pd–Pt/Al2O3 catalysts. Interestingly, the selectivity toward isomerization is always higher for palladium than for pure platinum. This difference is small for the catalysts reduced at 400 °C for 1 h, but it is significantly increased after 17 h-reduction at 400 °C, 500 °C, and 600 °C. One can observe that the increase of reduction temperature (400 °C → 500 °C/600 °C) leads to a gradual increase of the isomerization selectivity by the samples containing increasingly higher amounts of platinum. The Pd80Pt20/Al2O3 after reduction at 500 °C shows the best selectivity for isomerization, nearly 90%. We suggest that such synergistic effect, not observed after reduction at 400 °C, for 1 h, must result from the interaction of all catalyst components: palladium, platinum and alumina. More severe reduction conditions (increased temperature and prolonged time) should improve the degree of metal alloying. In addition, as mentioned in the previous subsection, palladium interacts with alumina during H2 reduction at higher temperatures to generate Pd–Al alloys (Kępiński et al. 1989; Penner et al. 2007), which showed an enhanced selectivity to isomerization in neopentane conversion (Juszczyk et al. 1995).
Interestingly enough, calculation of turnover frequencies for isomerization [TOFis = overall TOF ×isomerization selectivity (%)/100] for the ‘acac’ series shows that the high activity of platinum catalysts is reduced after high temperature reduction (500–600 °C), whereas the TOFis values for Pd and a majority of Pd–Pt alloys is increased, making the TOFis-alloy composition highly flattened (Fig. 7a). After reductions at 500 and 600 °C, the isomerization activity of platinum is only roughly two times higher than the activity of palladium and Pd–Pt alloys. It is recalled that the starting catalytic behavior of Pd–Pt/Al2O3 catalysts showed nearly two orders of magnitude higher TOF level of the monometallic platinum compared to palladium catalysts.
The respective relation for the ‘chloride’ series is quite different (Fig. 7b). Here, the increase of the reduction temperature results only in small changes. Again, as in the previous Sect. (3.2.2), one considers that the presence of chloride in alumina-supported metal catalysts must inhibit the transformation of Pd–Al2O3 catalysts into Pd–Al alloys.
n-Hexane isomerization carried out under very low conversion level leads mainly to 2- and 3-methylpentanes (2MP and 3MP), the presence of other isomers (like 2,2-dimethylbutane) is marginal. The ratio of 2MP/3MP would result from the mechanism of isomerization (C5-cyclic or bond-shift), so variations of this factor with Pd–Pt alloy composition and catalyst pretreatment may also serve for diagnosing the structure of active sites. Earlier studies on alkane isomerization on Al2O3-supported catalysts with the use of 13C-labelled compounds showed that the isomerization on platinum proceeds mainly via C5-cyclic mechanism, and the contribution of the bond-shift mechanism becomes important only for larger particles (Gault 1981; Maire et al. 1971; Dartigues et al. 1976). On the other hand, palladium catalysts appear to isomerize via the C5-cyclic route (Hajek et al. 1974; O’Cinneide and Gault 1975; Le Normand et al. 1993). Unselective cyclic mechanism should result in the 2MP/3MP ratio close to 2. After reduction at 400 °C for 1 h of the ‘acac’ series, the 2MP/3MP values only mildly changes with Pd–Pt alloy composition. Any departure from the 2MP/3MP ratio = 2 should result either from the change to a more selective cyclic route or from the contribution of the bond-shift (this is actually marked for Pt-rich samples). This may also suggest that the mechanism of isomerization changes to more ‘acidic’, involving carbenium ions (Surjo and Christoffel 1979; Smirniotis and Ruckenstein 1993). Without using 13C-labelled compounds it is impossible to distinguish between these possibilities, nevertheless variations of the 2MP/3MP ratio and Pd–Pt alloy composition would be used for catalytic probing. Figure 8 shows this relations for both series of Pd–Pt/Al2O3 catalysts subjected to different reduction conditions. For the ‘acac’ series the 2MP/3MP ratio is very similar for the Pd-containing catalysts (up to Pd50Pt50(acac)), confirming our earlier findings which indicate high surface enrichment in palladium (Fig. 8a).
4 Conclusions
-
1.
Incipient wetness co-impregnation of γ-alumina with the solution of Pd(acac)2 and Pt(acac)2 leads to preparation of a relatively well metal mixed Pd–Pt/Al2O3 catalysts. However, co-impregnation of aqueous solutions of PdCl2 and H2PtCl6 results in unsatisfactory Pd–Pt alloying.
-
2.
The relation of the overall catalytic activity in n-hexane conversion of the ‘acac’ series of Pd–Pt/Al2O3 catalysts versus Pd–Pt composition follows a similar trend as the relation of surface composition-bulk composition of Pd–Pt, i.e. exhibits high surface enrichment in palladium, which is much less catalytically active than platinum.
-
3.
For the chloride-free Pd–Pt catalysts, the Pd100 is more selective toward isomerization products than the Pt100. More severe reduction conditions (500–600 °C) make this effect even stronger. The synergistic effect, observed as the highest selectivity for Pd80Pt20/Al2O3, is interpreted in the light of interaction between palladium, platinum and aluminum (from alumina support).
-
4.
The effect of reduction temperature on the isomerization selectivity for chloride-free Pd–Pt/Al2O3 catalysts was significantly positive, whereas it was rather negligible (or even somewhat negative) for the ‘chloride’ series. It is concluded that potential variations in support acidity associated with chloride presence and dehydroxylation in alumina do not have large effect on the isomerization behavior of highly reduced Pd–Pt/Al2O3 catalysts, tested in the reaction temperatures below 300 °C. Instead, strong metal support interactions inferred as generation of a Pd–Al alloy, are suggested for the explanation of observed catalytic variations.
References
Arena, F., Frusteri, F., Mondello, N., Giordano, N., Parmaliana, A.: Interaction pathway of chloride ions with γ-Al2O3,: surface acidity and thermal stability of the Cl/γ-AI2O3 system. J. Chem. Soc. Faraday Trans. 88, 3353–3356 (1992)
Bazin, D., Guillaume, D., Pichon, Ch., Uzio, D., Lopez, S.: Structure and size of bimetallic palladium–platinum clusters in an hydrotreatment catalyst. Oil Gas Sci. Technol. 60, 801–813 (2005)
Cho, H.-R., Regalbuto, J.R.: The rational synthesis of Pt-Pd bimetallic catalysts by electrostatic adsorption. Catal. Today 246, 143–153 (2015)
Clarke, J.K.A., Rooney, J.J.: Stereochemical approaches to mechanisms of hydrocarbon reactions on metal catalysts. Adv. Catal. 25, 125–183 (1976)
Contreras, J.J., Ferreira, J.M., Fuentes, S., Gómez, R.: n-Hexane hydrogenolysis on bimetallic platinum-palladium catalysts. React. Kinet. Catal. Lett. 7, 373–378 (1977)
Dartigues, J.-M., Chambellan, A., Gault, F.G.: Isomerization on metals. Correlation between metal particle size and reaction mechanisms. J. Am. Chem. Soc. 98, 856–857 (1976)
Dautzenberg, F.M., Kouwenhoven, H.W.: Ger. Offen. 2,153,891 (1972)
Dossi, C., Pozzi, A., Recchia, S., Fusi, A., Psaro, R., Dal Santo, V.: An organometallic route to mono and bimetallic Pt and Pt-Pd catalysts supported on magnesium oxide: thermoanalytical investigation and catalytic behavior in MCP conversion. J. Mol. Catal. A 204–205, 465–472 (2003)
Esteves, L.M., Brijaldo, M.H., Passos, F.B.: Decomposition of acetic acid for hydrogen production over Pd/Al2O3and Pd/TiO2: influence of metal precursor. J. Mol. Catal. A 422, 275–288 (2016)
Galadima, A., Anderson, J.A., Wells, R.P.K.: Solid acid catalysts in heterogeneous n-alkanes hydroisomerization for increasing octane number of gasolines. Sci. World J. 4, 1–22 (2009)
Garin, F., Girard, P., Chaqroune, A., Weisang, F., Maire, G.: Activity and selectivity in isomerization of 13C-labeled hexanes on supported palladium-platinum alloys. In: Proceedings of the 8th International Congress on Catalysis, vol. III, pp. 405–415. Verlag Chemie, Weinheim (1984)
Gault, F.G.: Mechanisms of skeletal isomerization of hydrocarbons on metals. Adv. Catal. 30, 1–95 (1981)
Hajek, M., Corolleur, S., Corolleur, C., Maire, G., O’Cinneide, A., Gault, F.G.: Mécanismes d’isomérisation et d’hydrogénolyse des hexanes sur palladium-alumine. J. Chim. Phys. 71, 1329–1336 (1974)
Ichikawa, S., Poppa, H., Boudart, M.: Disproportionation of CO on small particles of silica-supported palladium. J. Catal. 91, 1–10 (1985)
Juszczyk, W., Łomot, D., Karpiński, Z., Pielaszek, J.: Neopentane Conversion over Pd/γ-Al2O3. Catal. Lett. 31, 37–45 (1995)
Karpiński, Z., Kościelski, T.: Catalytic reactions of hydrocarbons over Pt-Pd alloys. I. Skeletal reactions of C5 and C6 alkanes over Pt–Pd alloy films. J. Catal. 56, 430–437 (1979)
Karpiński, Z., Kościelski, T.: Catalytic reactions of hydrocarbons over Pt-Pd alloys. III. Skeletal reactions of n-pentane and n-hexane over Pt-Pd/SiO2 catalysts. J. Catal. 63, 313–328 (1980)
Kennedy, D.R., Webb, G., Jackson, S.D., Lennon, D.: Propyne hydrogenation over alumina-supported palladium and platinum catalysts. Appl. Catal. A 259, 109–120 (2004)
Kępiński, L., Wołcyrz, M., Jabłoński, J.M.: Effect of high-temperature reduction on carburization of alumina-supported palladium: evidence for palladium-aluminium alloy formation. Appl. Catal. 54, 267–276 (1989)
Kluksdahl, H.E.: U.S. Patent 3,415,737 (1968)
Kościelski, T., Karpiński, Z., Paál, Z.: Catalytic reactions of hydrocarbons over Pt-Pd alloys. IV. Hydrogen effects in the conversion of saturated C6 hydrocarbons over Pt-Pd/SiO2 catalysts. J. Catal. 77, 539–549 (1982)
Kuijers, F.J., Tieman, B.M., Ponec, V.: The surface composition of platinum-palladium alloys determined by auger electron spectroscopy. Surf. Sci. 75, 657–680 (1978)
Le Normand, F., Kili, K., Schmitt, J.L.: Mechanisms of reforming reactions on Pd/Al2O3 catalysts. J. Catal. 139, 234–255 (1993)
Leclercq, G., Leclercq, L., Maurel, R.: Hydrogenolysis of saturated hydrocarbons. III. Selectivity in hydrogenolysis of various aliphatic hydrocarbons on platinum/alumina. J. Catal. 50, 87–97 (1977)
Lee, J.-K., Rhee, H.K.: Sulfur tolerance of zeolite beta-supported Pd-Pt catalysts for the isomerization of n-Hexane. J. Catal. 177, 208–216 (1998)
Maire, G.L.C., Garin, F.G.: Metal catalyzed skeletal reactions of hydrocarbons on metal catalysts. In: Anderson, J.R., Boudart, M. (eds.) Catalysis—Science and Technology, Chap. 3, vol. 6, pp. 161–226. Akademie-Verlag, Berlin (1985)
Maire, G., Corolleur, C., Juttard, D., Gault, F.G.: Comments on a dispersion effect in hydrogenolysis of methylcyclopentane and isomerization of hexanes over supported platinum catalysts. J. Catal. 21, 250–253 (1971)
Margitfalvi, J., Hegedüs, M., Szabó, S., Nagy, F.: Investigation of n-hexane dehydrocyclization over supported Pt catalyst modified by adsorbed Pd. React. Kinet. Catal. Lett. 18, 89–93 (1981)
Margitfalvi, J.S., Szabó, S., Nagy, F., Göbölös, S., Hegedüs, M.: Bimetallic supported catalysts prepared via metal adsorption. Preparation and catalytic activity of Pd-Pt/Al2O3 catalysts. In: Poncelet, G., Grange, P., Jacobs, P.A. (eds.) Preparation of Catalysts III, pp. 473–482. Elsevier, Amsterdam (1982)
Matam, S.K., Otal, O.H., Aguirre, M.H., Winkler, A., Ulrich, A., Rentsch, D., Weidenkaff, A., Ferri, D.: Thermal and chemical aging of model three-way catalyst Pd/Al2O3 and its impact on the conversion of CNG vehicle exhaust. Catal. Today 184, 237–244 (2012)
Micheaud, C., Marécot, P., Guérin, M., Barbier, J.: Preparation of alumina supported palladium-platinum catalysts by surface redox reactions. Activity for complete hydrocarbon oxidation. Appl. Catal. A 171, 229–239 (1998)
Noh, H., Flanagan, T.B., Sonoda, T., Sakamoto, Y.: Solubility and thermodynamics of hydrogen in homogeneous f.c.c. PdPt alloys. J. Alloys Compd. 228, 164–171 (1995)
O’Cinneide, A., Gault, F.G.: Reactions of hexanes, unlabeled and labeled with 13C, on alumina-supported palladium-gold and platinum-gold alloys. J. Catal. 37, 311–323 (1975)
Ollendorff, R.L., Bošković, G., Butt, J.B.: Platinum/alumina. III. Influence of chloride on methylcyclopropane hydrogenolysis at moderate temperatures. Appl. Catal. 62, 85–103 (1990)
Paál, Z., Tétényi, P.: Reactions of hydrocarbons on metallic catalysts. In: Bond, G.C., Webb, G. (eds.) Catalysis. Specialists periodical reports, vol. 5, pp. 80–126. The Royal Society of Chemistry, London (1982)
Penner, S., Jenewein, B., Hayek, K.: Pd-Al interaction at elevated temperatures: a TEM and SAED study. Catal. Lett. 113, 65–71 (2007)
Rachmady, V., Vannice, M.A.: Acetic acid hydrogenation over supported platinum catalysts. J. Catal. 192, 322–334 (2000)
Radlik, M., Małolepszy, A., Matus, K., Śrębowata, A., Juszczyk, W., Dłużewski, P., Karpiński, Z.: Alkane isomerization on highly reduced Pd/Al2O3 catalysts. The crucial role of Pd-Al species. Catal. Commun. 123, 17–22 (2019)
Renouprez, A.J., Malhomme, A., Massardier, J., Cattenot, M., Bergeret, G.: Sulphur resistant palladium-platinum catalysts prepared from mixed acetylacetonates. Stud. Surf. Sci. Catal. 130C, 2579–2584 (2000)
Rousset, J.L., Bertolini, J.C., Miegge, P.: Theory of segregation using the equivalent-medium approximation and bond-strength modifications at surfaces: Application to fcc Pd-X alloys. Phys. Rev. B 53, 4947–4957 (1996)
Rousset, J.L., Stievano, L., Cadete Santos Aires, F.J., Geantet, C., Renouprez, A.J., Pellarin, M.: Hydrogenation of toluene over γ-Al2O3-supported Pt, Pd, and Pd-Pt model catalysts obtained by laser vaporization of bulk metals. J. Catal. 197, 335–343 (2001)
Sinfelt, J.H.: U.S. Patent 3 634 697 (1972)
Sinfelt, J.H. Ger. Offen. 2,153,475 (1973)
Sinfelt, J.H.:U.S. Patent 3,953,368 (1976)
Skotak, M., Karpiński, Z.: C6-alkane conversion over γ-alumina supported palladium catalysts. Chem. Eng. J. 90, 89–96 (2002)
Smirniotis, P.G., Ruckenstein, E.: Comparison between Zeolite β and γ-Al2O3 supported Pt for reforming reactions. J. Catal. 140, 526–542 (1993)
Surjo, I., Christoffel, E.G.: Kinetics of simultaneous isomerization and cracking of n-hexane over Pt Al2O3. J. Catal. 60, 133–139 (1979)
Touroude, R., Girard, P., Maire, G., Kizling, J., Boutonnet-Kizling, M., Stenius, P.: Preparation of colloidal platinum/palladium alloy particles from non-ionic microemulsions: characterization and catalytic behavior. Colloids Surf. 67, 9–19 (1992)
Van Den Oetelaar, L.C.A., Nooij, O.W., Oerlemans, S., Denier Van Der Gon, A.W., Brongersma, H.H., Lefferts, L., Roosenbrand, A.G., Van Veen, J.A.R.: Surface segregation in supported Pd-Pt nanoclusters and alloys. J. Phys. Chem. B 102, 3445–3455 (1998)
Acknowledgements
This work was carried out within Research Project #2016/21/B/ST4/03686 from the National Science Centre (NCN), Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to S.I. ISSHAC10, but it reach the press at the time the special issue was published.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Radlik, M., Śrębowata, A., Juszczyk, W. et al. n-Hexane conversion on γ-alumina supported palladium–platinum catalysts. Adsorption 25, 843–853 (2019). https://doi.org/10.1007/s10450-019-00083-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00083-9