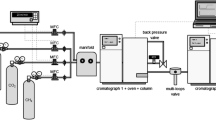
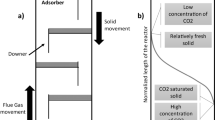
Abstract
Fixed bed adsorption is an economical method of removing harmful gases, such as SO2 and CO2, from industrial flue gas. It is possible to reduce the cost and environmental impact of fixed bed adsorption by repurposing waste materials to be used as adsorbents, such as semi-coke derived from oil shale, a possible alternative to fossil fuels. Fixed bed adsorption systems are difficult and time consuming to characterize experimentally, especially on large scales. Computational fluid dynamics (CFD) can expand researchers understaning of how these systems are affected by material selection and operating conditions. This study uses CFD to characterize fixed bed adsorption of SO2 and CO2. The research primarily focuses on SO2 adsorption on semi-coke, with an extension to CO2 adsorption on commercial carbon. The CFD modeling was able to describe the amount of the pollutants each material was able to adsorb over time based on a variety of inputs on a larger scale than experimental research. The model was further able to give more detailed comparisons of materials and operating conditions than the experiments, particularly the SO2 and semi-coke system.









Similar content being viewed by others
References
An, B., Wang, W., Ji, G., Gan, S., Gao, G., Xu, J., Li, G.: Preparation of nano-sized α-Al2O3 from oil shale ash. Energy 35, 45–49 (2010)
Dantas, T.L.P., Luna, F.M.T., Silva Jr., I.J., de Azevedo, D.C.S., Grande, C.A., Rodrigues, A.E., Moreira, R.F.P.M., Frias, R.: Carbon dioxide-nitrogen separation through adsorption on activated carbon in a fixed bed. Chem. Eng. J. 169, 11–19 (2011)
Ding, J., Gidaspow, D.: A bubbling fluidization model using kinetic theory of granular flow. AIChE J. 36, 523 (1990)
Dupre, K.R., Ryan, E.M., Suleimenov, A., Goldfarb, J.L.: Experimental and computational demonstration of a low-temperature waste to by-product conversion of U.S. oil shale semi-coke to a flue gas sorbent. Energies 11, 3195 (2018)
Dyni, J.R.: Geology and Resources of Some World Oil-Shale Deposits. Reston, Virginia (2006)
Ergun, S.: Fluid flow through packed columns. Chem. Eng. Prog. 48, 89–94 (1952)
Goldfarb, J.L., Buessing, L., Gunn, E., Lever, M., Billias, A., Casoliba, E., Schievano, A., Adani, F.: Novel integrated biorefinery for olive mill waste management: utilization of secondary waste for water treatment. ACS Sustain. Chem. Eng. 5(1), 876–884 (2016)
Goldfarb, J.L., D’Amico, A., Culin, C., Suuberg, E.M., Külaots, I.: Oxidation kinetics of oil shale semicokes: reactivity as a function of pyrolysis temperature and shale origin. Energy Fuels 27, 666–672 (2013)
Gregg, S.J., Sing, K.S.W.: Adsorption, Surface Area, and Porosity. Academic Press, London (1982)
Herzog, N., Schreiber, M., Egbers, C., Krautz, H.J.: A comparative study of different CFD-codes for numerical simulation of gas-solid fluidized bed hydrodynamics. Comput. Chem. Eng. 39, 41–46 (2012)
Hoomans, B.P.B., Kuipers, J.A.M., Briels, W.J., Van Swaaij, W.P.M.: Discrete particle simulation of bubble and slug formation in a two-dimensional gas-fluidised bed: a hard-sphere approach. Chem. Eng. Sci. 51(1), 99–118 (1996)
Isitan, S., Ceylan, S., Topcu, Y., Hintz, C., Tefft, J., Chellappa, T., Guo, J., Goldfarb, J.L.: Product quality optimization in an integrated biorefinery: conversion of pistachio nutshell biomass to biofuels and activated biochars via pyrolysis. Energy Conserv. Manag. 127, 576–588 (2016)
Karau, A., Benken, C., Thommes, J., Kula, M.-R.: The influence of particle size distribution and operating conditions on the adsorption performance in fluidized beds 1991. Biotechnol. Bioeng. 55, 54–64 (1997)
Külaots, I., Goldfarb, J.L., Suuberg, E.M.: Characterization of Chinese, American and Estonian oil shale semicokes and their sorptive potential. Fuel 89, 3300–3306 (2010)
Lane, W.A., Ryan, E.M.: Verification, validation, and uncertainty quantification of a sub-grid model for heat transfer in gas-particle flows with immersed horizontal cylinders. Chem. Eng. Sci. 176, 409–420 (2018)
Lane, W.A., Sarkar, A., Sundaresan, S., Ryan, E.M.: Sub-grid models for heat transfer in gas-particle flows with immersed horizontal cylinders. Chem. Eng. Sci. 151, 7–15 (2016)
Lane, W.A., Storlie, C.B., Montgomery, C.J., Ryan, E.M.: Numerical modeling and uncertainty quantification of a bubbling fluidized bed with immersed horizontal tubes. Powder Technol. 253, 733–743 (2014)
Leci, C.L.: Financial implications on power generation costs resulting from the parasitic effect of CO2 capture using liquid scrubbing technology from power station flue gases. Energy Conserv. Manage. 37, 210 (1996)
Li, Z., Liu, Y., Wang, H., Tsai, C.-J., Yang, X., Xing, Y., Zhang, C., Xiao, P., Webley, P.A.: A numerical modelling study of SO2 adsorption on activated carbons with new rate equations. Chem. Eng. J. 353, 858–866 (2018)
Lizzio, A.A., DeBarr, J.A.: Effect of surface area and chemisorbed oxygen on the SO2 adsorption capacity of activated char. Fuel 75, 1515–1522 (1996)
Lua, A.C., Yang, T.: Theoretical and experimental SO2 adsorption onto pistachio-nut-shell activated carbon for a fixed-bed column. Chem. Eng. J. 155, 175–183 (2009)
Luan, J., Li, A., Su, T., Cui, X.: Synthesis of nucleated glass-ceramics using oil shale fly ash. J. Hazard. Mater. 173, 427–432 (2010)
Miller, D.C., Syamlal, M., Mebane, D.S., Storlie, C., Bhattacharyya, D., Sahinidis, N.V., Agarwal, D., Tong, C., Zitney, S.E., Sarkar, A., Sun, X., Sundaresan, S., Ryan, E.M., Engel, D., Dale, C.: Carbon capture simulation initiative: a case study in multiscale modeling and new challenges. Annu. Rev. Chem. Biomol. Eng. 5, 301–323 (2014)
Mulgundmath, V.P., Jones, R.A., Tezel, F.H., Thibault, J.: Fixed bed adsorption for the removal of carbon dioxide from nitrogen: breakthrough behaviour and modelling for heat and mass transfer. Sep. Purif. Technol. 85, 17–27 (2012)
Qiang, T., Zhigang, Z., Wenpei, Z., Zidong, C.: SO2 and NO selective adsorption properties of coal-based activated carbons. Fuel 84, 461–465 (2004)
Raukas, A., Punning, J.-M.: Environmental problems in the Estonian oil shale industry. Energy Environ. Sci. 2, 723–728 (2009)
Rios, R.B., Correia, L.S., Bastos-Neto, M., Torres, A.E.B., Hatimondi, S.A., Ribeiro, A.M., Rodrigues, A.E., Cavalcante Jr., C.L., de Azevedo, D.C.S.: Evaluation of carbon dioxide-nitrogen separation through fixed bed measurements and simulations. Adsorption 20, 945–957 (2014)
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Sage, P.W., Ford, N.W.J.: Review of sorbent injection processes for low-cost sulphur dioxide control. Proc. Inst. Mech. Eng. Part A J. Power Energy. 210, 183–190 (1996)
Shafeeyan, M.S., Daud, W.M.A.W., Shamiri, A.: A review of mathematical modeling of fixed-bed columns for carbon dioxide adsorption. Chem. Eng. Res. Des. 92, 961–988 (2014)
Sumathi, S., Bhatia, S., Lee, K.T., Mohamed, A.R.: Optimization of microporous palm shell activated carbon production for flue gas desulphurization: experimental and statistical studies. Biosour. Technol. 100, 1614–1621 (2009)
Taghipour, F., Ellis, N., Wong, C.: Experimental and computational study of gas-solid fluidized bed hydrodynamics. Chem. Eng. Sci. 60, 6857–6867 (2005)
Taulbee, D.N., Graham, U.M., Carter, S.D., Robl, T.L., Derbyshire, F.: Examination of eastern US oil shale by-products and their markets. Fuel 74, 1118–1124 (1995)
Ueyama, K.: A study of two-fluid model equations. J. Fluid Mech. 690, 474–498 (2012)
Weller, H., Greenshields, C., de Rouvray, C.: OpenFOAM, https://openfoam.org/
Williams, P.T., Ahmad, N.: Influence of process conditions on the pyrolysis of Pakistani oil shales. Fuel 78, 653–662 (1999)
Yaghoobi-Khankhajeh, S., Alizadeh, R., Zarghami, R.: Adsorption modeling of CO2 in fluidized bed reactor. Chem. Eng. Res. Des. 129, 111–121 (2018)
Yang, H., Xu, Z., Fan, M., Gupta, R., Slimane, R.B., Bland, A.E., Wright, I.: Progress in carbon dioxide separation and capture: a review. J. Environ. Sci. 20, 14–27 (2008)
Acknowledgements
Partial funding for this research was provided by a Clare Booth Luce Foundation Graduate Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 Semi-coke experiment details
1.1.1 Semi-coke preparation
The oil shale was ground in a planetary ball mill and sieved to a particle size between 0.5 and 1 mm. To prepare the “semi-coke,” the fractionated oil shale was placed in a porcelain boat and pyrolyzed in a Thermo-Scientific Lindberg Blue 1” tube furnace under high-purity nitrogen gas to mimic ex situ retorting conditions. Samples were heated at a rate of 10 °C and held at 110 °C for 30 min to drive off moisture, then heated to 600 °C at the same rate and held at this temperature for 1 h before cooling under \(\text {N}_{2}\). To activate the semi-coke, 2.5 g was mixed in a 2:1 (weight) ratio of concentrated \(\text {H}_{2}\text {SO}_{4}\):semi-coke and an additional 50 mL of deionized water was added, then stirred at 80 °C for 2 h. The mixture was filtered, then mixed in a 1:1 ratio with NaOH and 50 mL of deionized water, again stirred at 80 °C for 2 h. The resulting solid was filtered, dried in a laboratory oven at 80 °C overnight, and then pyrolyzed in the tube furnace at 600 °C using the same program as before.
The surface area was analyzed on a Quantachrome Autosorb iQ gas sorption analyzer. The sample was outgassed for a minimum of 6 h at 180 °C and weighed pre- and post-degas on a Sartorius semi-microbalance to the 0.1 mg. Data were fitted to the BET isotherm to find total surface area over a partial pressure range of 0–0.30 P/P0, a range suitable for microporous samples (Gregg and Sing 1982). Proximate analysis was conducted on a Mettler–Toledo Thermogravimetric Analyzer-Differential Scanning Calorimeter (TGA-DSC). Approximately 10 mg of sample was loaded into a 70 \(\upmu\)L alumina crucible. The sample was heated under 50 mL/min of high-purity \(\text {N}_{2}\) to 110 °C and held for 30 min to establish a dry baseline. Because carbonate mineral matter begins to decompose around 620 °C, the dry sample was heated at 10 °C to 610 °C and held for 60 min; mass loss in this period was attributed to volatile matter (Williams and Ahmad 1999). The sample was further heated to 900 °C under air (50 mL/min) and held for 60 min; loss over this regime is considered to be fixed carbon. Remaining mass is termed “ash” and consists of non-oxidizable mineral matter. Scanning electron microscopy (SEM; Zeiss Supra 55VP) provided qualitative insight into the effect of the acid-base activation.
1.1.2 Experimental characterization
During pyrolysis, the kerogen contained in the oil shale devolatilizes, leaving behind a semi-carbonaceous rock with moderately enhanced porosity but lower carbon content. The White River Mine oil shale had an initial volatile matter (VM) content of \(41.3 \pm 1.5\) wt% and fixed carbon (FC) of \(28.0 \pm 1.4\) wt% (balance ash). Upon pyrolysis, the VM of the semi-coke drops to \(9.56 \pm 0.7\) wt%, FC to \(8.4 \pm 0.4\) wt%. After activation, the VM content increases to \(14.22 \pm 0.8\) wt% and FC drops to \(7.5 \pm 0.6\) wt%, suggesting that the acid + base treatment removes both some carbonate minerals and some of the silica preset in the inorganic ash (Gregg and Sing 1982; 2007). This is supported by the SEM images of Fig. 10, where we see upon activation a considerable reduction of the smooth amorphous regions and loss of the white semi-crystalline silica (identified via EDX) as the porous nature of the material expands. The particles, originally 0.5–1.0 mm in diameter, experienced shrinkage upon pyrolysis and activation, and after treatment ranged from approximately 0.05 to 0.4 mm in diameter as measured by optical microscopy (and were subsequently modeled as having a diameter of 0.2 mm).
Across the literature we see semi-coke samples with surface areas up to \(100\text { m}^{2}/\text {g}\); however, these are usual for finely ground (\(\text {d}_{p}<150\,\upmu \text { m}\)) samples (An et al. 2010; Luan et al. 2010; Williams and Ahmad 1999). The as-produced semi-coke sample, given its larger, more commercially viable particle size, had a surface area of only \(18.420 \pm 0.553\text { m}^{2}/\text {g}\). The combined acid/base activation treatment yielded an activated semi-coke sample with a surface area of \(59.196 \pm 1.776\text { m}^{2}/\text {g}\). This is considerably higher than a typical class F coal fly ash at \(5\text { m}^{2}/\text {g}\), which has long been used as a sorbent material for flue gas control (Külaots et al. 2010).
Rights and permissions
About this article
Cite this article
Dupre, K.R., Vyas, A., Goldfarb, J.L. et al. Investigation of computational upscaling of adsorption of SO2 and CO2 in fixed bed columns. Adsorption 25, 773–782 (2019). https://doi.org/10.1007/s10450-019-00050-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00050-4