Abstract
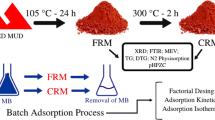
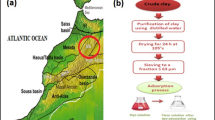
The effluents from the textile industry, which not properly treated or discharged directly into water bodies, are a serious environmental problem. In order to find options to solve this problem, in this study, it was evaluate two alternative adsorbents: red mud and smectite clay, to remove the dye Indosol dark-blue SF-BL SGR 240. Assays were performed in batch using the experimental design to optimize the process and evaluate the influence of pH, mass and agitation speed on the adsorption capacity of dye by each material. The results showed that the adsorption process was influenced by the amount of mass of the adsorbent, by the pH and the by interaction between pH and mass, thereby obtaining a removal of approximately 0.110 and 0.119 mg g−1 with red mud and smectite clay respectively.








Similar content being viewed by others
References
Barres, O., Ghanbaja, J., Samrani, A.G.E., Kacha, S., Ouhib, R., Lartiges, B.S., Bouyakoub, A.Z.: MnCl2 and MgCl2 for the removal of reactive dye Levafix Brilliant Blue EBRA from synthetic textile wastewaters: an adsorption/aggregation mechanism. J. Hazard. Mater. 187, 264–273 (2011)
Carbonea, C., Tomaselloa, B., Ruozi, B., Renis, M., Puglis, G.: Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur. J. Med. Chem. 49, 110–117 (2012)
Chaari, I., Feki, M., Medhioub, M., Bouzid, J., Fakhfakh, E., Jamoussi, F.: Adsorption of a textile dye “Indanthrene Blue RS (C.I. Vat Blue 4)” from aqueous solutions onto smectite-rich clayey rock. J. Hazard. Mater. 172, 1623–1628 (2009)
Chiou, M.S., Ho, P.Y., Li, H.Y.: Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 60, 69–84 (2004)
De Souza, K.C., Antunes, M.L., Couperthwaite, S.J., da Conceicao, F.T., de Barros, T.R., Frost, R.: Adsorption of reactive dye on seawater-neutralised bauxite refinery residue. J. Colloid Interface Sci. 396, 210–214 (2013)
Elemen, S., Kumbasar, E.P.A., Yapar, S.: Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dyes Pigments 95, 102–111 (2012)
Eren, E., Cubuk, O., Ciftci, H., Eren, B., Caglar, B.: Adsorption of basic dye from aqueous solutions by modified sepiolite: equilibrium, kinetics and thermodynamics study. Desalination 252, 88–96 (2010)
Feza, G., Erdal, K., Semra, Ç., Sermin, E.: Modelling of lead adsorption from industrial sludge leachate on red mud by using RSM and ANN. Dyes Pigments 183, 53–59 (2012)
Geyikc, F., Kılıc, E., Çoruh, S., Elevli, S.: Modelling os lead adsorption from industrial sludge leachate on red mud by using RSM and ANN. Chem. Eng. J. 183, 53–59 (2012)
Gil, A., Assis, F.C.C., Albeniz, S., Korili, S.A.: Removal of dyes from wastewaters by adsorption on pillared clays. Chem. Eng. J. 168, 1032–1040 (2011)
Gil, L.F., Melo, T.M.S., Gurgel, L.V.A., Gusmão, K.A.G.: Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions e Kinetic and equilibrium studies. Dyes Pigments 92, 967–974 (2012)
González, Joaquín A., Villanueva, María E., Piehl, Lidia L., Copello, Guillermo J.: Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: adsorption and desorption study. Chem. Eng. J. 280, 41–48 (2015)
Jovicic, N.J., Nikolic, A.M., Bankovic, P., Mojovic, Z., Zunic, M., Grzetic, I., Jovanovic, D.: Organo-inorganic bentonite for simultaneous adsorption of Acid Orange 10 and lead ions. Appl. Clay Sci. 47, 452–456 (2010)
Khenifi, A., Zohra, B., Kahina, B., Houari, H., Zoubir, D.: Removal of 2,4-DCP from wastewater by CTAB/bentonite using one-step and two-step methods: a comparative study. Chem. Eng. J. 146, 345–354 (2009)
Li, Q., Yue, Q.Y., Su, Y., Gao, B.Y., Fu, L.: Cationic polyelectrolyte/bentonite prepared by ultrasonic technique and its use as adsorbent for Reactive Blue K-GL dye. J. Hazard. Mater. 147, 370–380 (2007)
Li, Q., Yue, Q.Y., Su, Y., Gao, B.Y., Li, J.: Two-step kinetic study on the adsorption and desorption of reactive dyes at cationic polymer/bentonite. J. Hazard. Mater. 165, 1170–1178 (2009)
Liu, Y., Naidu, R., Ming, H.: Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 163, 1–12 (2011)
Marques, J.J., Costa, A.S., Araújo, B.R., Romão, L.P.C., Jesus, A.M.D.: Use of humin as an alternative material for adsorption/desorption of reactive dyes. Desalination 274, 13–21 (2011)
Montgomery, D.C.: Design and analysis of experiments, 5th edn. Wiley, New York (2001)
Neto, B.B., Scarminio, I.S., Bruns, R.E.: Como fazer experimentos: pesquisa e desenvolvimento na ciência e na indústria, 4th edn, p. 406p. Coleção Livros-Textos UNICAMP, Campinas (2007)
Ozcan, A.S., Oncu, E.M., Ozcan, A.: Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A Physicochem. 277, 90–97 (2006)
Reis, E.L., Lázaro, E., Reis, C., Silva, C.J., Moreira, G.C., Neta, J.J.S.: Use of polyurethane foams for the removal of the Direct Red 80 and Reactive Blue 21 dyes in aqueous medium. Desalination 281, 55–60 (2011)
Samal, Sneha, Ray, Ajoy K., Bandopadhyay, Amitava: Proposal for resources, utilization and processes of red mud in—a review. Int. J. Miner. Process. 118, 43–55 (2013)
Wang, S., Ang, H.M., Tadé, M.O.: Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72, 1621–1635 (2008)
Wang, Q., Luan, Z., Wei, N., Li, J., Liu, C.: The color removal of dye wastewater by magnesium chloride/red mud (MRM) from aqueous solution. J. Hazard. Mater. 170, 690–698 (2009)
Yagub, M.T., Sen, T.K., Afroze, S., Ang, H.M.: Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014)
Yue, Q., Zhao, Y., Li, Q., Li, W., Gao, B., Han, S., Qi, Y., Yu, H.: Research on the characteristics of red mud granular adsorbents (RMGA) for phosphate removal. J. Hazard. Mater. 176, 741–748 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, E.H.C., Mendonça, É.T.R., Barauna, O.S. et al. Study of variables for optimization of the dye indosol adsorption process using red mud and clay as adsorbents. Adsorption 22, 59–69 (2016). https://doi.org/10.1007/s10450-015-9742-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9742-0