Abstract
A series of new carbonaceous adsorbents has been obtained by means of direct and physical activation of Polish brown coal, characterised by high mineral matter content. The influence of activation procedure on the porous structure development, acidic and basic surface groups generation as well as the sorptive properties of the adsorbents prepared toward liquid and gas pollutants was tested. Additionally the effect of mineral matter presence on the physicochemical and sorption properties of materials prepared was studied. The final products were micro/mesoporous activated carbons of medium developed surface area ranging from 407 to 674 m2/g, showing strongly basic or intermediate acidic-basic character of the surface. The results obtained during this study showed that direct and physical activation of low quality brown coal led to activated carbons with very good sorption capacity both toward gas contamination of acidic character (especially nitrogen dioxide) as well as toward methylene blue and inorganic pollutants of molecules of size similar to that of iodine molecules. It was also proved that demineralization of prepared activated carbons by hydrochloric acid significantly reduced their ability to toxic gases sorption, but simultaneously increased the efficiency of removing impurities from the liquid phase.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, adsorption processes are applied in many modern industrial and households technologies. Incessant progress in this field stimulates the search for new and effective but first of all low-cost adsorbents. From among the various materials used for this purpose (Goscianska et al. 2013; Wiśniewska et al. 2007, 2013, 2014; Kierys et al. 2013; Wiśniewska 2010, 2012; Krysztafkiewicz et al. 2002; Thomas and Syres 2012; De Smedt et al. 2015; Qian et al. 2015) the most popular and promising are the carbonaceous sorbents, especially activated carbons (Jiang et al. 2015; Deng et al. 2015; Sharma and Upadhyay 2009; Goscianska and Pietrzak 2015; Jiang and Chen 2011). Such materials could be prepared in a simple way by physical or chemical activation of variety of organic substances, including wood (Wang et al. 2009; Nowicki et al. 2015a), peat (Khadiran et al. 2015), fossil coals (Nowicki and Pietrzak 2011; Maroto-Valer et al. 2005; Teng et al. 1998) as well as many biodegradable (Karagöz et al. 2008; Soleimani and Kaghazchi 2008; Kazmierczak et al. 2013, 2015; Nowicki et al. 2015b) and industrial waste (Nakagawa et al. 2003; Hofman and Pietrzak 2011; Nowicki et al. 2013; Lin and Teng 2002). Taking into account economic and ecological aspects, particularly suited for this purpose are fossil coals of low quality, the use of which in a chemical industry (e.g. for degassing, gasification and liquefaction) or power generation is not very cost-effective, due to high nitrogen, sulfur or mineral matter content.
Many previous studies have shown that by thermo-chemical processing of brown coals, it is possible to obtain a wide range of activated carbons characterized by well-developed porous structure and good sorption properties to different kinds of pollution (Pokonova 1996; Toles et al. 1996; Burg et al. 2002a; Burg et al. 2002b; Bimer et al. 1998). However, the vast majority of the research made has been focused on the production of activated carbons from the precursors with a relatively low mineral substance content and even deliberately deprived of mineral admixtures (Starck et al. 2004; Pietrzak et al. 2006; Jurewicz et al. 2008; Pietrzak et al. 2008).
Therefore, the main objective of this study was to prepare a series of carbonaceous adsorbents by means of direct activation (simultaneous pyrolysis and activation of carbonaceous material) as well as physical activation of low quality brown coal and to investigate the effect of mineral matter presence on their physicochemical and sorption properties toward gaseous pollutants represented by nitrogen dioxide and hydrogen sulfide as well toward liquid impurities represented by methylene blue and iodine.
2 Experimental
2.1 Preparation of activated carbons
The starting raw sample was prepared from a Polish brown coal (Konin colliery), characterised by high ash content ~18 wt%. The precursor (B) was milled and sieved to the grain size of 2–4 mm, divided into two parts and subjected to two different treatments: (1) direct activation of starting coal with carbon dioxide (BA sample) and (2) pyrolysis of raw material followed by physical activation with carbon dioxide (BPA sample).
Direct activation of the precursor was carried out in a quartz tubular reactor heated by a resistance furnace at temperature of 850 °C, under a stream of carbon dioxide at the flow rate of 250 ml/min, for 45 min. Pyrolysis of starting materials was conducted under a stream of argon at the flow rate of 170 ml/min. A portion of precursor (about 15 g) was heated (10 °C/min) from room temperature to the final pyrolysis temperature of 700 °C and maintained for 30 min. After that, the gas flowing through the reactor was switched to carbon dioxide and the obtained char was subjected to physical activation at 900 °C, under a stream of carbon dioxide at the flow rate of 250 ml/min, for 45 min.
In order to check the influence of mineral matter present in the structure of the activated products on their physicochemical and sorption properties some part of the activated carbons was subjected to demineralization (D) with hot concentrated hydrochloric acid for 3 h. After demineralisation stage, the samples were washed with hot distilled water until free of chloride ions and dried at 110 °C for 24 h.
2.2 Sample characterization
Elemental analysis of the all samples under investigation was carried out using the Elementar Analysensysteme instrument, model Vario EL III. The ash content was determined according to the ISO 1171:2002 standard: the dried sample was burned in a microwave oven at temperature 850 °C, for 60 min.
Nitrogen adsorption/desorption isotherms were measured at −196 °C using the Quantachrome Autosorb iQ surface area analyser. Prior to the isotherm measurements, the samples were outgassed at 150 °C for 8 h. BET specific surface area (SBET) was evaluated in the range of relative pressures p/p0 of 0.05–0.30. Total pore volume (Vt) was calculated by converting the amount adsorbed at p/p0 ~ 0.99 to the volume of liquid adsorbate. Average pore diameter was calculated (d) was calculated from equation d = 4V t /S BET. Pore size distribution was calculated from the adsorption branches of isotherms using the BJH method. Additionally, micropore volume and area were determined by the t plot method.
The acid–base surface properties were evaluated according to the Boehm method (Boehm et al. 1964; Boehm 1994). Volumetric standard HCl (0.1 M) and NaOH (0.1 M) were used as the titrants. The pH of activated carbons was measured using the following procedure: a portion of 0.4 g the sample of dry powder was added to 20 ml of demineralised water and the suspension was stirred overnight to reach equilibrium. After that time, pH of the suspension was measured.
SEM images of the activated carbons were obtained using a scanning electron microscope (SEM) made by PHILIPS (Netherlands) in the following conditions: working distance of 14 mm, accelerating voltage of 15 kV and digital image recording by DISS.
2.3 Adsorption studies
2.3.1 Evaluation of H2S and NO2 sorption capacity
The adsorption tests were performed in dry (D) and wet (W, 70 % moisture content) conditions. Moreover, additional variant was applied: the sample was moistened by air with 70 % moisture content for about 30 min, and then the sorption capacity was determined in dry (MD) or wet (MW) conditions.
The samples sieved to a particle size between 0.75 and 1.5 mm were packed into a glass column (bed volume 3 ml). Dry or moist air with 0.1 % of H2S or NO2 was passed through the dry or moistened bed of the adsorbent at flow 450 ml/min, at room temperature. The breakthrough of H2S or NO2 were monitored using Q-RAE PLUS PGM-2000/2020 with electrochemical sensors. The tests were stopped at the breakthrough concentration of 100 ppm (in case of H2S) or 20 ppm (for NO2) because of the electrochemical sensor limits. The interaction capacities of each sorbent in terms of milligram of H2S or NO2 per gram of adsorbent were calculated by integration of the area above the breakthrough curves, and from the toxic gas concentration in the inlet gas, flow rate, breakthrough time and mass of sorbent.
2.3.2 Adsorption from liquid phase
Determination of the iodine adsorption was performed according to the ASTM D4607-94(2006) standard. In a brief: samples of the prepared activated carbons (of particle size below 0.09 mm) of equal portion of 0.2 g were added to 20 ml of 0.1 M iodine solution and 5 ml of 5 % HCl. Next, the mixture was shaken for 4 min, filtered through filter paper and washed 50 ml of water. The resulting solution was titrated with 0.1 M sodium thiosulphate until the solution become colourless (1 % starch solution was used as an indicator). Determination of the methylene blue adsorption was performed using the following procedure: samples of the prepared activated carbons (of particle size below 0.09 mm) of equal portion of 0.0025 g were added to 50 ml of the methylene blue solution with initial concentrations from 30 to 120 mg/l and the suspension was stirred for 24 h to reach equilibrium. After the adsorption equilibrium had been achieved, the solution was separated from the adsorbent by filtration on syringe filters ABLUO™–CAMEO™ (pore size: 1.2 μm).
The concentrations of the methylene blue in the solution before and after adsorption were determined using a double beam UV–Vis spectrophotometer (Cary Bio 100, Varian) at a wavelength of 665 nm. The equilibrium adsorption amounts (qe, mg/g) were calculated according to the following formula:
where ci and ce (mg/l) are the initial and equilibrium concentrations of the methylene blue, V (l) is the volume of the solution, and m (g) is the mass of adsorbent used, respectively.
3 Results and discussion
3.1 Elemental composition of the activated carbons prepared
According to the data presented in Table 1, the precursor used for the study is characterised by relatively high content of mineral substance as well as organic non-carbon impurities, especially oxygen. Both pyrolysis and activation of the starting brown coal cause significant changes in its structure. Thermo-chemical treatment brings a substantial increase in the content of Cdaf, accompanied by a considerable decrease in the content of the other elements, with the exception of sulphur. These changes are certainly related to the high temperature of the process, which is responsible for breaking of the least stable chemical bonds present in the carbonaceous matrix and consequently, for the removal of heteroatoms in the form of simple gas or liquid compounds. High temperature treatment of the precursor (independent on the variant) causes also a significant increase in the ash content, as evidenced by the fact that the activation products are characterized by almost twice higher content of mineral substances than the starting material.
As follows from further analysis of the data presented in Table 1 and in Fig. 1, activated carbons treatment with hydrochloric acid results in a significant decrease in ash content, especially in case of BPAD sample, which contains threefold less mineral ballast than the respective BPA sample, untreated by HCl. Partial demineralisation of the samples brings also some changes in their elemental composition. Samples BAD and BPAD show a slightly higher content of carbon than BA and BPA and at the same time a lower content of hydrogen, sulphur and in particular oxygen.
3.2 Textural parameters of activated carbons
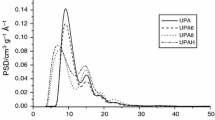
Analysis of the data presented in Table 2 has shown that both the direct and two-stage activation of brown coal, do not allow efficient development of surface area and porous structure. The surface area of the activated carbon prepared varies between 407 and 436 m2/g, whereas the total pore volume varies between 0.34 and 0.39 cm3/g. The main reason behind so poor textural parameters of the materials prepared probably is a very high content of inorganic substance, which can be deposited in the pores and consequently block the access of the adsorbate molecules to smaller pores. The porous structure of both activated carbons includes micropores with high contribution of mesopores as follows from low micropores contribution in the total pore volume, relatively high average pore diameter (Table 2), as well as from nitrogen adsorption isotherms and pore size distribution presented in Figs. 2 and 3, respectively. According to the IUPAC classification, the isotherms obtained for BA and BPA samples are close to type I, characteristic of microporous and mesoporous materials with pore size close to the micropores range. However, broad hysteresis loops (H4 type) prove the presence of pores of greater diameters. As follows from the course of pore size distribution curves, these are mainly mesopores with diameters ranging from 2 to 15 nm.
As follows from further analysis of the data presented in Table 2 and in Figs. 2 and 3, activated carbons treated by hydrochloric acid show much more beneficial textural parameters than un-modified samples, which is most probably a result of removal of a significant part of the ash. This assumption is confirmed by the fact that the BAD and BPAD samples are characterized by a much higher total pore volume than the corresponding samples not treated with hydrochloric acid. What is more, much wider hysteresis loops observed in the course of nitrogen adsorption isotherms for samples BAD and BPAD, confirm the earlier assumption that the mineral substance can block a significant part of pores present in the structure of the materials obtained. It is particularly well seen for sample BA subjected to direct activation. After removal of a considerable portion of ash, there was a nearly threefold increase in the total pore volume, and more importantly, an increase in the contribution of mesopores from 46 to 78 %. On the basis of this observation, it can be also assumed that the conditions of direct activation process were too drastic for the precursor applied, leading to partial combustion of organic substance and formation of wider pores, as confirmed by a greater average pore diameter for samples BA and BAD. However, this issue requires further study.
3.3 Acid–base properties of activated carbons
According to the data presented in Table 3, the materials obtained show a diversity of acid–base properties as can be concluded from the content of oxygen functional groups varying in the range 1.21–5.77 mmol/g and pH values varying from 3.94 to 12.36. As seen, the content and type of the oxygen functional groups depend on the variant of activation as well as treatment of the resulting carbons by hydrochloric acid. As far as the unmodified samples are concerned (BA and BPA), a strongly basic character of the surface is observed (pH > 11.5). It is of course a consequence of the high content of mineral substance in the structure of the precursor, which undergoes transformations during pyrolysis or activation processes and remains in the structure of the products. As regards the samples treated with hydrochloric acid (BAD and BPAD), they show completely different acid–base properties of the surface. The total amount of the surface oxygen groups (1.21–2.19 mmol/g) as well as pH value (~4) are much lower in samples BAD and BPAD and, in contrast to the samples BA and BPA, a domination of functional groups of acidic character is observed in them. It is most probably the effect of removal of a significant part of mineral substance present in the activated carbons structure, during the acid washing step.
3.4 Sorption abilities of the activated carbons toward nitrogen dioxide and hydrogen sulphide
The main premises in favor of undertaking the adsorption study toward gas pollutants of acidic nature were the high content of mineral substance in the activated carbons structure as well as the presence of a high number of basic surface functional groups, which, according to previous literature reports, have a positive impact on the effectiveness of removal of this type of pollution (Yuan and Bandosz 2007; Feng et al. 2005; Nowicki et al. 2013, 2014; Kante et al. 2012). To verify this supposition, all the materials prepared were subjected to adsorption tests in four variants. The results of relevant measurements are given in Tables 4 and 5.
The results clearly illustrate a significant effect of the variant of activation, post-activation treatment with hydrochloric acid as well as conditions of the adsorption tests on the sorption capacity towards H2S and NO2. Moreover, each of the prepared materials shows a definitely higher sorption capacity towards o nitrogen dioxide, therefore, the results regarding this pollutant will be discussed first. As the results obtained for individual samples vary significantly depending on the adsorption conditions, it is difficult to point out a single material of the best adsorptive performance. The most effective adsorbent in dry conditions (46.0 mg/g) was sample BA, obtained by direct activation with CO2 at 850 °C, whereas from among the samples subjected to pre-humidification (MD conditions), the most efficient proved sample BPA obtained by physical activation, which adsorbed 101.2 mg NO2. The least effective sorbent of nitrogen dioxide during adsorption both in dry and mix-dry conditions was sample BAD whose sorption capacity was 14.4 and 20.4 mg/g, respectively. The sorption capacity of the second sample subjected to hydrochloric acid treatment (BPAD) also decreased, but to a lesser extent. These results clearly indicate a significant impact of mineral matter on the adsorption capacity of nitrogen dioxide.
Also the presence of water in the system has a significant effect on the efficiency of NO2 removal. Pre-humidification of the adsorbent bed by using a stream of moist air, appreciably enhance the sorption capacity of the materials, especially for samples not subjected to demineralization. It is particularly well seen for sample BPA, for which almost a threefold increase in the amount of adsorbed gas is observed. Much more beneficial impact on the efficiency of NO2 removal brings a change in the conditions of sorption, from dry to wet.
The most effective adsorbent in wet conditions (87.3 mg/g) was the sample obtained by physical activation, whereas in mix-wet conditions the best sorption ability showed sample BA, which adsorbed up to 192.5 mg/g. Similarly as in dry conditions, the results obtained for carbons subjected to demineralization (BAD and BPAD) are much less satisfactory, especially in mix-wet conditions, in which differences between samples treated and untreated by hydrochloric acid reach from 159 to 175 mg/g. Moreover, the effect of the pre-humidification of the adsorbent bed prior to the measurement under wet conditions is much greater than in dry conditions. However, it should be noted that for the samples subjected to demineralization this procedure brings a negative result.
As was mentioned above, all the activated carbons under investigation exhibit significantly less favourable adsorption capacity towards hydrogen sulphide (Table 5). Similarly as for NO2, the ability of H2S sorption from gas flux is to a high degree determined by the method of activation, post-activation treatment as well as the conditions of adsorption test. The highest sorption capacities were found for the BA sample obtained by direct activation of the precursor. Slightly less satisfactory results were also obtained for the BPA sample obtained by physical activation. Unfortunately, both samples treated by hydrochloric acid showed very poor sorption abilities towards hydrogen sulphide (< 3 mg/g), irrespective of the adsorption conditions.
All samples showed the lowest H2S sorption capacities on adsorption in dry conditions (D). This result means that strongly basic surface character, high content of mineral substance (in the samples untreated by HCl) or medium-developed surface area (the samples treated by HCl) are not sufficient for effective removal of H2S from the flux of gases. According to the data collected in Table 6, the most important in the process is the presence of steam in the system. As seen, the wetting of adsorbent bed with moist air (MD conditions) improves sorption capacities of the activated carbons, but mainly for samples not subjected to demineralisation, for which four or fivefold increase was observed. Most probably it is a result of generation of a thin film of water on the surface of carbon matrix as it is conducive to H2S bonding. More impressive effect was obtained when the process of adsorption was performed in wet conditions (W). A continuous presence of steam in the flux of gases flowing through the adsorbent bed is evidently favourable for H2S removal as the sorption capacities towards H2S are much higher than those in dry as well as mix-dry conditions.
The greatest increase in the sorption capacity (about 25 and 35 times, respectively) with respect to that measured in dry and mix-dry conditions was observed for sample BA, obtained by direct activation of starting coal. The sorption capacities obtained for sample BPA were approximately by 50 % lower. Unfortunately, for the samples treated with hydrochloric acid this improvement was minimal, as indicated by the sorption capacities not exceeding 3 mg/g. On the basis of these results it can be definitely concluded that the presence of mineral substance is conducive to effective removal of H2S from the flux of gases, especially in the presence of water.
So attractive sorption capacities of the activated carbons not subjected to demineralisation are most probably a consequence of chemisorption of NO2 and H2S on the adsorbents surface, that occurs according to the mechanism proposed earlier by Bandosz research group (Pietrzak and Bandosz 2007; Bagreev et al. 2001), assuming formation of the corresponding nitrates and sulphides, in the reaction between the molecules of the adsorbed gas and metal oxides present in the mineral substance. However, a detail explanation of this issue needs further studies.
3.5 Sorption abilities toward iodine and methylene blue
The data presented in Fig. 4 and in Table 6 clearly illustrate a significant effect of the method of activation as well as post-activation on the sorption abilities towards the liquid pollutants studied. However, in contrast to the gas pollutants removal, more effective adsorbents toward liquid impurities are samples subjected to demineralisation. Most probably it is a consequence of better developed surface area and porous structure of these samples.
From among the samples untreated by hydrochloric acid, more effective adsorbent toward both adsorbates proved to be sample BPA (obtained by two-stage activation), whose sorption capacity was 702 mg/g for iodine and 156.25 mg/g for methylene blue, respectively. As mentioned above, partial demineralisation of the activated carbon samples significantly improves their sorption properties. Iodine number of samples BAD and BPAD is by 120 and 77 mg/g higher than that of the corresponding samples untreated by HCl. Towards methylene blue, an increase in the sorption capacity of the samples is much lower, from 2.41 mg/g for sample BPAD to 46.3 mg/g for sample BAD. A much better result obtained for sample BAD sample is most probably related to a considerable higher mesopores contribution in its porous structure (Table 2). It should be also noted that sorption capacities of most of the samples prepared are similar to those achieved for commercial micro/mesoporous activated carbon—Norit® SX2, which is very often used in practice, for water purification.
According to the equilibrium adsorption isotherms presented in Fig. 5, the amount of adsorbed methylene blue increases significantly with increasing initial dye concentration, up to saturation. As the shape of isotherms is single and smooth, it suggests a monolayer coverage of the adsorbents surface with methylene blue molecules. R2 values ranging from 0.989 to 0.999 (Table 6) show that the adsorption of methylene blue onto the activated carbons prepared is described by the Langmuir model. In addition, the 1/n value in the range between 0 and 1 indicated that the adsorption conditions were favourable and methylene blue molecules had free access to the pores present in the activated carbons structure.
4 Conclusions
The above discussed results have confirmed that brown coals with a high mineral matter content can be successfully applied as precursors of cheap activated carbons, showing very good sorption capacity towards gas contaminants of acidic character (especially nitrogen dioxide) as well as toward methylene blue and inorganic pollutants of molecules whose size is similar to that of iodine molecules. As shown by the results, the effectiveness of NO2 and H2S removal from the flux of gases, depends first of all on the conditions of adsorption. It has been proved that preliminary wetting of the adsorbent bed as well as the presence of steam in the mixture of gases passed through the adsorbent, significantly increase the amount of the pollutants removed. Moreover, demineralization of prepared activated carbons by hydrochloric acid significantly reduced their ability to toxic gases sorption, but simultaneously increased the efficiency of removing impurities from the liquid phase.
References
Bagreev, A., Bashkova, S., Locke, D.C., Bandosz, T.J.: Sewage sludge-derived materials as efficient adsorbents for removal of hydrogen sulfide. Environ. Sci. Technol. 35, 1537–1543 (2001)
Bimer, J., Sałbut, P.D., Berłożecki, S., Boudou, J.P., Broniek, E., Siemieniewska, T.: Modified active carbons from precursors enriched with nitrogen functions: sulfur removal capabilities. Fuel 77, 519–525 (1998)
Boehm, H.P., Diehl, E., Heck, W., Sappok, R.: Surface oxides of carbon. Angew. Chem. Int. Ed. Engl. 3, 669–677 (1964)
Boehm, H.P.: Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 32(5), 759–769 (1994)
Burg, P., Cagniant, D., Fydrych, P., Magri, P., Gruber, R., Bimer, J., Nanse, G., Jankowska, A.: Characterization of surface properties of nitrogen-enriched activated lignites by spectroscopic and chromatographic coupled with LSER modelling measurements. Fuel Process. Technol. 79, 233–237 (2002a)
Burg, P., Fydrych, P., Cagniant, D., Nanse, G., Bimer, J., Jankowska, A.: The characterization of nitrogen-enriched activated carbons by IR, XPS and LSER methods. Carbon 40, 1521–1531 (2002b)
Deng, S., Hu, B., Chen, T., Wang, B., Huang, J., Wang, Y., Yu, G.: Activated carbons prepared from peanut shell and sunflower seed shell for high CO2 adsorption. Adsorption 21(1), 125–133 (2015)
De Smedt, C., Spanoghe, P., Biswas, S., Leus, K., Van der Voort, P.: Comparison of different solid adsorbents for the removal of mobile pesticides from aqueous solutions. Adsorption 21(5), 243–254 (2015)
Feng, W., Kwon, S., Borguet, E., Vidic, R.: Adsorption of hydrogen sulfide onto activated carbon fibers: effect of pore structure and surface chemistry. Environ. Sci. Technol. 39(24), 9744–9749 (2005)
Goscianska, J., Olejnik, A., Pietrzak, R.: Adsorption of l-phenylalanine onto mesoporous silica. Mater. Chem. Phys. 142, 586–593 (2013)
Goscianska, J., Pietrzak, R.: Removal of tartrazine from aqueous solution by carbon nanotubes decorated with silver nanoparticles. Catal. Today 249, 259–264 (2015)
Hofman, M., Pietrzak, R.: Adsorbents obtained from waste tires for NO2 removal under dry conditions at room temperature. Chem. Eng. J. 170, 202–208 (2011)
Jiang, H., Chen, H.: Research on preparation of activated carbon from agricultural wastes and analysis of pore structure. Energy Procedia 11, 3629–3633 (2011)
Jiang, M., Bai, Y., Ning, P., Huang, X., Liu, H., Fu, J.: Adsorption removal of arsine by modified activated carbon. Adsorption 21(1), 135–141 (2015)
Jurewicz, K., Pietrzak, R., Nowicki, P., Wachowska, H.: Capacitance behaviour of brown coal based active carbon modified through chemical reaction with urea. Electochim. Acta 53, 5469–5475 (2008)
Kante, K., Nieto-Delgado, C., Rangel-Mendez, J.R., Bandosz, T.J.: Spent coffee-based activated carbon: specific surface features and their importance for H2S separation process. J. Hazard. Mater. 201–202, 141–147 (2012)
Karagöz, S., Tay, T., Ucar, S., Erdem, M.: Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 99, 6214–6222 (2008)
Kazmierczak, J., Nowicki, P., Pietrzak, R.: Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption 19, 273–281 (2013)
Kazmierczak, J., Nowicki, P., Pietrzak, R.: The use of microwave radiation for obtaining activated carbons from sawdust and their potential application in removal of NO2 and H2S. Chem. Eng. J. 269, 352–358 (2015)
Khadiran, T., Hussein, M.Z., Zainal, Z., Rusli, R.: Textural and chemical properties of activated carbon prepared from tropical peat soil by chemical activation method. Bioresources 10(1), 986–1007 (2015)
Kierys, A., Zaleski, R., Gorgol, M., Goworek, J.: n-Heptane adsorption in periodic mesoporous silica by in situ positron annihilation lifetime spectroscopy. Microporous Mesoporous Mater. 179, 104–110 (2013)
Krysztafkiewicz, A., Binkowski, S., Jesionowski, T.: Adsorption of dyes on a silica surface. Appl. Surf. Sci. 199, 31–39 (2002)
Lin, Y.-R., Teng, H.: Mesoporous carbons from waste tire char and their application in wastewater discoloration. Microporous Mesoporous Mater. 54, 167–174 (2002)
Maroto-Valer, M.M., Tang, Z., Zhang, Y.: CO2 capture by activated and impregnated anthracites. Fuel Process. Technol. 86, 1487–1502 (2005)
Nakagawa, K., Mukai, S.R., Suzuki, T., Tamon, H.: Gas adsorption on activated carbons from PET mixtures with a metal salt. Carbon 41, 823–831 (2003)
Nowicki, P., Pietrzak, R.: Effect of ammoxidation of activated carbons obtained from sub-bituminous coal on their NO2 sorption capacity under dry conditions. Chem. Eng. J. 166, 1039–1043 (2011)
Nowicki, P., Skibiszewska, P., Pietrzak, R.: NO2 removal on adsorbents prepared from coffee industry waste materials. Adsorption 19, 521–528 (2013)
Nowicki, P., Skibiszewska, P., Pietrzak, R.: Hydrogen sulphide removal on carbonaceous adsorbents prepared from coffee industry waste materials. Chem. Eng. J. 248, 208–215 (2014)
Nowicki, P., Kazmierczak, J., Sawicka, K., Pietrzak, R.: Nitrogen-enriched activated carbons prepared by the activation of coniferous tree sawdust and their application in the removal of nitrogen dioxide. J. Environ. Sci Technol. 12, 2233–2244 (2015a)
Nowicki, P., Kazmierczak, J., Pietrzak, R.: Comparison of physicochemical and sorption properties of activated carbons prepared by physical and chemical activation of cherry stones. Powder Technol. 269, 312–319 (2015b)
Pietrzak, R., Wachowska, H., Nowicki, P.: Preparation of nitrogen-enriched activated carbons from brown coal. Energy Fuels 20, 1275–1280 (2006)
Pietrzak, R., Bandosz, T.J.: Reactive adsorption of NO2 at dry conditions on sewage sludge-derived materials. Environ. Sci. Technol. 41, 7516–7522 (2007)
Pietrzak, R., Nowicki, P., Wachowska, H.: Comparison of physicochemical properties of nitrogen-enriched activated carbons prepared by physical and chemical activation of brown coal. Energy Fuels 22, 4133–4138 (2008)
Pokonova, Y.V.: Production of carbon adsorbents from brown coal. Carbon 34(3), 411–415 (1996)
Qian, Q., Gong, C., Zhang, Z., Yuan, G.: Removal of VOCs by activated carbon microspheres derived from polymer: a comparative study. Adsorption 21(4), 333–341 (2015)
Sharma, Y.C., Upadhyay, S.N.: Removal of a cationic dye from wastewaters by adsorption on activated carbon developed from coconut coir. Energy Fuels 23(6), 2983–2988 (2009)
Soleimani, M., Kaghazchi, T.: Adsorption of gold ions from industrial wastewater using activated carbon derived from hard shell of apricot stones—an agricultural waste. Bioresour. Technol. 99, 5374–5383 (2008)
Starck, J., Burg, P., Cagniant, D., Tascon, J.M.D., Martinez-Alonso, A.: The effect of demineralisation on a lignite surface properties. Fuel 83, 845–850 (2004)
Teng, H., Yeh, T.-S., Hsu, L.-Y.: Preparation of activated carbon from bituminous coal with phosphoric acid activation. Carbon 36(9), 1387–1395 (1998)
Thomas, A.G., Syres, K.L.: Adsorption of organic molecules on rutile TiO2 and anatase TiO2 single crystal surfaces. Chem. Soc. Rev. 41, 4207–4217 (2012)
Toles, C., Rimmer, S., Hower, J.C.: Production of activated carbons from a Washington lignite using phosphoric acid activation. Carbon 34(11), 1419–1426 (1996)
Wang, T., Tan, S., Liang, C.: Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon 47, 1880–1883 (2009)
Wiśniewska, M., Chibowski, S., Urban, T.: The temperature influence on the adsorption and electrokinetical properties in the nonionic polymer/controlled porosity glass (CPG) system. Mater. Chem. Phys. 103, 216–221 (2007)
Wiśniewska, M.: Studies of temperature influence on adsorption behaviour of nonionic polymers at the zirconia—solution interface. J. Therm. Anal. Calorim. 101, 743–751 (2010)
Wiśniewska, M.: The temperature effect on the adsorption mechanism of polyacrylamide on the silica surface and its stability. Appl. Surf. Sci. 258, 3094–3101 (2012)
Wiśniewska, M., Nosal-Wiercińska, A., Dąbrowska, I., Szewczuk-Karpisz, K.: Effect of the solid pore size on the structure of polymer film at the metal oxide/polyacrylic acid solution interface—temperature impast. Microporous Mesoporous Mater. 175, 92–98 (2013)
Wiśniewska, M., Urban, T., Nosal-Wiercińska, A., Zarko, V., Gunko, V.: Comparison of stability properties of poly(acrylic acid) adsorbed on the surface of silica, alumina and mixed silica-alumina nanoparticles—application of turbidimetry method. Cent. Eur. J. Chem. 12(4), 476–479 (2014)
Yuan, W., Bandosz, T.J.: Removal of hydrogen sulfide from biogas on sludge-derived adsorbents. Fuel 86, 2736–2746 (2007)
Acknowledgments
Financial support received from the Polish Ministry of Higher Education and Science (Project Iuventus Plus No. IP2012 004072) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nowicki, P. The effect of mineral matter on the physicochemical and sorption properties of brown coal-based activated carbons. Adsorption 22, 561–569 (2016). https://doi.org/10.1007/s10450-015-9729-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9729-x