Abstract
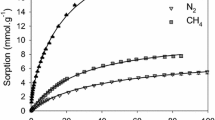
Upgrading raw biogas and landfill gas to methane purity >98 % is a vital prerequisite for the utilization of biogas for compressed natural gas pipeline applications. Pressure swing adsorption (PSA) is an industrial separation technology widely used for the separation and purification of methane rich streams from raw biogas and landfill gas. Current PSA technologies make use of differential adsorption of gases on traditional adsorbents, such as zeolites, activated alumina and molecular sieves. In order to evaluate the potential of new adsorbent materials, such as metal–organic frameworks (MOFs) for PSA applications, and for PSA process development, thermodynamic equilibrium adsorption isotherms data of the pure components and mixtures and their adsorption isosteric heats are required. In this work we use extended Langmuir (ELM) model and Doong–Yang multi-component (DYM) adsorption model to predict the isotherms of biogas compositions containing binary and ternary mixtures of CO2, CH4 and N2 on activated carbon Maxsorb and metal–organic framework Cu-BTC. The model parameters required for predicting the mixture adsorption isotherms using the ELM and DYM are obtained, respectively from the single-site Langmuir and Dubinin–Astakhov non-linear regression of pure gas isotherms experimentally measured at 298 K and over a pressure range of 0–5 MPa. Predicted data are compared with the experimental binary and ternary mixture adsorption isotherms on Norit R1 extra and Cu-BTC available in the literature. Selectivity and thermodynamic delta-loading of equimolar mixtures of CH4 and CO2 on Cu-BTC and Maxsorb are determined from the predicted mixture isotherms and are compared with that of a traditional PSA adsorbent, zeolite 13X.










Similar content being viewed by others
References
Amankwah, K.A.G., Schwarz, J.A.: A modified approach for estimating pseudo-vapor pressures in the application of the Dubinin-Astakhov equation. Carbon 33(9), 1313–1319 (1995)
Bae, J.-S., Bhatia, S.K.: High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 20(6), 2599–2607 (2006)
Bae, Y.-S., Mulfort, K.L., Frost, H., Ryan, P., Punnathanam, S., Broadbelt, L.J., Hupp, J.T., Snurr, R.Q.: Separation of CO2 from CH4 using mixed-ligand metal–organic frameworks. Langmuir 24(16), 8592–8598 (2008)
Bai, R., Yang, R.T.: A modification of the doong–yang model for gas mixture adsorption using the Lewis Relationship. Langmuir 21(18), 8326–8332 (2005)
Bárcia, P.S., Nicolau, M.P.M., Gallegos, J.M., Chen, B., Rodrigues, A.E., Silva, J.A.C.: Modeling adsorption equilibria of xylene isomers in a microporous metal–organic framework. Microporous Mesoporous Mater. 155, 220–226 (2012)
Casas, N., Schell, J., Pini, R., Mazzotti, M.: Fixed bed adsorption of CO2/H2 mixtures on activated carbon: experiments and modeling. Adsorption 18(2), 143–161 (2012)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49(4), 1095–1101 (2004)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Separation of mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 61(12), 3893–3906 (2006)
Cavenati, S., Grande, C.A., Rodrigues, A.E., Kiener, C., Müller, U.: Metal organic framework adsorbent for biogas upgrading. Ind. Eng. Chem. Res. 47(16), 6333–6335 (2008)
Chowdhury, P., Mekala, S., Dreisbach, F., Gumma, S.: Adsorption of CO, CO2 and CH4 on Cu-BTC and MIL-101 metal organic frameworks: effect of open metal sites and adsorbate polarity. Microporous Mesoporous Mater. 152, 246–252 (2012)
Doong, S.J., Yang, R.T.: A simple potential-theory model for predicting mixed-gas adsorption. Ind. Eng. Chem. Res. 27(4), 630–635 (1988)
Dreisbach, F., Staudt, R., Keller, J.U.: High pressure adsorption data of methane, nitrogen, carbon dioxide and their binary and ternary mixtures on activated carbon. Adsorption 5(3), 215–227 (1999)
Dubinin, A.M.M.: A study of the porous structure of active carbons using a variety of methods. Q Rev Chem Soc 9(2), 101–114 (1955)
Dubinin, M.M., Astakhov, V.A.: Development of the concepts of volume filling of micropores in the adsorption of gases and vapors by microporous adsorbents. Russ. Chem. Bull. 20(1), 3–7 (1971)
Dundar, E., Zacharia, R., Chahine, R., Bénard, P.: Performance comparison of adsorption isotherm models for supercritical hydrogen sorption on MOFs. Fluid Phase Equilib. 363, 74–85 (2014a)
Dundar, E., Zacharia, R., Chahine, R., Bénard, P.: Potential theory for prediction of high-pressure gas mixture adsorption on activated carbon and MOFs. Sep. Purif. Technol. 135, 229–242 (2014b)
Esteves, I.A.A.C., Lopes, M.S.S., Nunes, P.M.C., Mota, J.P.B.: Adsorption of natural gas and biogas components on activated carbon. Sep. Purif. Technol. 62(2), 281–296 (2008)
Follivi.: Caractérisation des matériaux adsorbants pour le stockage de l’hydrogène. Université du québec à Trois-Rivières. (2015)
Grande, C.A., Blom, R., Möller, A., Möllmer, J.: High-pressure separation of CH4/CO2 using activated carbon. Chem. Eng. Sci. 89, 10–20 (2013)
Halliburton.: Coalbed Methane: Principles and Practices. http://www.halliburton.com/public/pe/contents/Books_and_Catalogs/web/CBM/H06263_Chap_03.pdf (2007). Accessed 10 June 2015
Hamon, L., Jolimaître, E., Pirngruber, G.D.: CO2 and CH4 separation by adsorption using Cu-BTC metal–organic framework. Ind. Eng. Chem. Res. 49(16), 7497–7503 (2010)
Hamon, L., Llewellyn, P.L., Devic, T., Ghoufi, A., Clet, G., Guillerm, V., Pirngruber, G.D., Maurin, G., Serre, C., Driver, G., Beek, W.V., Jolimaître, E., Vimont, A., Daturi, M., Férey, G.: Co-adsorption and separation of CO2–CH4 mixtures in the highly flexible MIL-53(Cr) MOF. J. Am. Chem. Soc. 131(47), 17490–17499 (2009)
Harpalani, S., Pariti, U.M.: Study of coal sorption isotherms using a multicomponent gas mixture. In: Proceedings of the 1993 International Coalbed Methane Symposium, pp. 151–160 (1993)
Himeno, S., Komatsu, T., Fujita, S.: High-pressure adsorption equilibria of methane and carbon dioxide on several activated carbons. J. Chem. Eng. Data 50(2), 369–376 (2005)
Hirscher, M., Panella, B., Schmitz, B.: Metal-organic frameworks for hydrogen storage. Microporous Mesoporous Mater. 129(3), 335–339 (2010)
Lemmon, E.W., Huber, M.L., McLinden, M.O.: NIST reference fluid thermodynamic and transport properties–REFPROP, Version (2013)
Liang, Z., Marshall, M., Chaffee, A.L.: CO2 adsorption-based separation by metal organic framework (Cu-BTC) versus zeolite (13X). Energy Fuels 23(5), 2785–2789 (2009)
Mosher, K., He, J., Liu, Y., Rupp, E., Wilcox, J.: Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 109–110, 36–44 (2013)
Myers, A.L., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11(1), 121–127 (1965)
Otowa, T., Tanibata, R., Itoh, M.: Production and adsorption characteristics of MAXSORB: high-surface-area active carbon. Gas Sep. Purif. 7(4), 241–245 (1993)
Rasi, S., Veijanen, A., Rintala, J.: Trace compounds of biogas from different biogas production plants. Energy 32(8), 1375–1380 (2007)
Rege, S.U., Yang, R.T., Qian, K., Buzanowski, M.A.: Air-prepurification by pressure swing adsorption using single/layered beds. Chem. Eng. Sci. 56(8), 2745–2759 (2001)
Reich, R., Ziegler, W.T., Rogers, K.A.: Adsorption of methane, ethane, and ethylene gases and their binary and ternary mixtures and carbon-dioxide on activated carbon at 212-301 K and pressures to 35 atmospheres. Ind. Eng. Chem. Process Des. Dev. 19(3), 336–344 (1980)
Richard, M.A., Bénard, P., Chahine, R.: Gas adsorption process in activated carbon over a wide temperature range above the critical point. Part 1: modified Dubinin-Astakhov model. Adsorption 15(1), 43–51 (2009)
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Schell, J., Casas, N., Pini, R., Mazzotti, M.: Pure and binary adsorption of CO2, H2, and N2 on activated carbon. Adsorption 18(1), 49–65 (2012)
Severn Wye Energy Agency.: Biomethane regions. Introduction to the Production of Biomethane from Biogas - A Guide for ENGLAND and WALES (UK). http://www.bio-methaneregions.eu/ (2012). Accessed on Mar 2015
Sheikh, M.A., Hassan, M.M., Loughlin, K.F.: Adsorption equilibria and rate parameters for nitrogen and methane on Maxsorb activated carbon. Gas Sep. Purif. 10(3), 161–168 (1996)
Thu, K., Kim, Y.-D., Ismil, A.B., Saha, B.B., Ng, K.C.: Adsorption characteristics of methane on Maxsorb III by gravimetric method. Appl. Therm. Eng. 72(2), 200–205 (2014)
Weigang, Z., Vanessa, F., Aylon, E., Izquierdo, M.T., Alain, C.: High-performances carbonaceous adsorbents for hydrogen storage. J. Phys. 416(1), 012024 (2013)
Yang, R.T.: Gas Separation by Adsorption Processes. Imperial College Press, London (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomez, L.F., Zacharia, R., Bénard, P. et al. Multicomponent adsorption of biogas compositions containing CO2, CH4 and N2 on Maxsorb and Cu-BTC using extended Langmuir and Doong–Yang models. Adsorption 21, 433–443 (2015). https://doi.org/10.1007/s10450-015-9684-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9684-6