Abstract
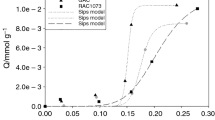
Sorption isotherms for trifluoromethane (R-23) in activated carbon have been measured at ca. 298 and 323 K using a gravimetric microbalance. High-resolution TEM images of the activated carbon show a very uniform microstructure with no evidence of any contaminants. The adsorption in the activated carbon reaches about 22.8 mol kg−1 at 2.0 MPa and 298 K or 17.6 mol kg−1 at 2.0 MPa and 323 K. Three different adsorption models (Langmuir, multi-site Langmuir, and BET equations) have been used to analyze the activated carbon sorption data, with a particular interest in the heat of adsorption (−ΔH). The heat of adsorption for R-23 in the activated carbon was about 29.78 ± 0.04 kJ mol−1 based on the multi-site Langmuir model and is within the range of typical physical adsorption. According to the IUPAC classification, the activated carbon exhibits Type I adsorption behavior and was completely reversible. Compared with our previous work for the sorption of R-23 in zeolites (5A (Ca,Na-A), 13X (Na-X), Na,K-LSX, Na-Y, K,H-Y, Rb,Na-Y) and ionic liquids ([omim][TFES] and [emim][Tf2N]) the activated carbon had the highest adsorption capacity. The adsorption process in the activated carbon also took less time than in the zeolites or the ionic liquids to reach thermodynamic equilibrium.









Similar content being viewed by others
References
Bhatia, S.K., Ding, L.P.: Vacancy solution theory of adsorption revisited. AIChE J. 47, 2136–2138 (2001)
Breck, D.W.: Zeolite Molecular Sieves. Wiley, New York (1974)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004)
Corbin, D.R., Fernandez, R.E., Mahler, B.A.: Purification of Hexafluoroethane Products, US Patent 5,523,499, 4 June 1996
Kaneko, K., Ishii, C., Ruike, M., Kuwabara, H.: Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 30, 1075–1088 (1992)
Moore, W.J.: Physical Chemistry, 4th edn. Prentice-Hall, New Jersey (1972)
Nitta, T., Shigetomi, T., Kuro-oka, M., Katayama, T.: An adsorption isotherm of multi-site occupancy model for homogeneous surface. J. Chem. Eng. Jpn. 17, 39–45 (1984)
Shiflett, M.B., Corbin, D.R., Elliott, B.A., Yokozeki, A.: Sorption of trifluoromethane in zeolites and ionic liquid. J. Chem. Thermodyn. 64, 40–49 (2013a)
Shiflett, M.B., Corbin, D.R., Yokozeki, A.: Comparison of the sorption of trifluoromethane (R-23) on zeolites and in an ionic liquid. J. Ads. Sci. Technol. 31, 59–84 (2013b)
Shiflett, M.B., Elliott, B.A., Lustig, S.R., Sabesan, S., Kelkar, M.S., Yokozeki, A.: Chemical absorption of carbon dioxide in room-temperature ionic liquid 1-ethyl-3-ethylimidazolium acetate [eeim][Ac]. Chem. Phys. Chem. 13, 1806–1817 (2012a)
Shiflett, M.B., Junk, C.P., Harmer, M.A., Yokozeki, A.: Solubility and diffusivity of 1,1,1,2-tetrafluoroethane in room-temperature ionic liquids. Fluid Phase Equilib. 242, 220–232 (2006a)
Shiflett, M.B., Junk, C.P., Harmer, M.A., Yokozeki, A.: Solubility and diffusivity of difluoromethane in room-temperature ionic liquids. J. Chem. Eng. Data 51, 483–495 (2006b)
Shiflett, M.B., Niehaus, A.M.S., Elliott, B.A., Yokozeki, A.: Phase behavior of N2O and CO2 in room-temperature ionic liquids [bmim][Tf2N], [bmim][BF4], [bmim][N(CN)2], [bmim][Ac], [eam][NO3] and [bmim][SCN]. Int. J. Thermophys. 33, 412–436 (2012b)
Shiflett, M.B., Yokozeki, A.: Binary vapor-liquid and vapor-liquid-liquid equilibria of hydrofluorocarbons (HFC-125 and HFC-143a) and hydrofluoroethers (HFE-125 and HFE-143a) with ionic liquid [emim][Tf2N]. J. Chem. Eng. Data 53, 492–497 (2008)
Shiflett, M.B., Yokozeki, A.: Chemical absorption of sulfur dioxide in room-temperature ionic liquids. Ind. Eng. Chem. Res. 49, 1370–1377 (2010)
Shiflett, M.B., Yokozeki, A.: Gaseous absorption of fluoromethane, fluoroethane, and 1,1,2,2-tetrafluoroethane in 1-butyl-3-methylimidazolium hexafluorophosphate. Ind. Chem. Eng. Res. 45, 6375–6382 (2006a)
Shiflett, M.B., Yokozeki, A.: Hydrogen substitution effect on the solubility of perhalogenated compounds in ionic liquid [bmim][PF6]. Fluid Phase Equilib. 259, 210–217 (2007a)
Shiflett, M.B., Yokozeki, A.: Phase behavior of carbon dioxide in ionic liquids: [emim][acetate], [emim][trifluoroacetate], and [emim][acetate] + [emim][trifluoroacetate] mixtures. J. Chem. Eng. Data 54, 108–114 (2009a)
Shiflett, M.B., Yokozeki, A.: Solubilities and diffusivities of carbon dioxide in ionic liquids: [bmim][PF6] and [bmim][BF4]. Ind. Eng. Chem. Res. 44, 4453–4464 (2005)
Shiflett, M.B., Yokozeki, A.: Solubility and diffusivity of hydrofluorocarbons in room-temperature ionic liquids. AIChE J. 52, 1205–1219 (2006b)
Shiflett, M.B., Yokozeki, A.: Solubility differences of halocarbon isomers in ionic liquid [emim][Tf2N]. J. Chem. Eng. Data 52, 2007–2015 (2007b)
Shiflett, M.B., Yokozeki, A.: Solubility of CO2 in room-temperature ionic liquid [hmim][Tf2N]. J. Phys. Chem. B 111, 2070–2074 (2007c)
Shiflett, M.B., Yokozeki, A.: Solubility of fluorocarbons in room temperature ionic liquids. In: Plechkova, N.V., Rogers, R.D., Seddon, K.R. (eds.) Ionic Liquids: From Knowledge to Applications, ACS Symposium Series 1030, pp. 21–42. American Chemical Society, Washington DC (2009b)
Shiflett, M.B., Yokozeki, A.: Solubility of gases in ionic liquids. In: Plechkova, N.V., Seddon, K.R. (eds.) Ionic Liquids, pp. 349–386. Wiley, UnCOILed (2013)
Shiflett, M.B., Yokozeki, A.: Solubility of N2O and CO2 in olive oil. J. Fluid Phase Equilib. 305, 127–131 (2011)
Surya Prakash, G.K., Jog, P.V., Batamack, P.T.D., Olah, G.A.: Taming of fluoroform: direct nucleophilic trifluoromethylation of Si, B, S, and C Centers. Science 338, 1324–1327 (2012)
Yang, R.T.: Adsorbents: Fundamentals and Applications, pp. 79–123. Wiley, New Jersey (2003)
Acknowledgments
The authors thank Dr. Lloyd Abrams (retired), Dr. Alan M. Allgeier and Mr. Brian L. Wells from the DuPont Experimental Station for their assistance with obtaining the surface area and pore volume of the activated carbon and the R-23 gravimetric solubility measurements. The present work was supported by DuPont Central Research and Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shiflett, M.B., Corbin, D.R., Elliott, B.A. et al. Sorption of trifluoromethane in activated carbon. Adsorption 20, 565–575 (2014). https://doi.org/10.1007/s10450-014-9601-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-014-9601-4