Abstract
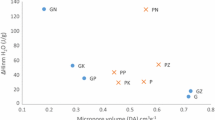
This work conducted an experimental study on the sorption characteristics of seven activated carbons from agroindustrial origin. Activated carbons derived from spent-coffee ground, orange peel, sugarcane bagasse, cedar sawdust, agave bagasse, coconut shell, and citric residue have been prepared with phosphoric acid activation and subsequent carbonization. The obtained materials were characterized using FTIR, SEM, EDS, Boehm potentiometric titration, and PZC measurements. Once the samples were characterized, the sorption of anionic and cationic model molecules was studied using kinetic models and compared to density functional theory calculations. The understanding of the surface characteristics of activated carbons allowed to describe and correlate the adsorption behavior of anionic and cationic model molecules. According to FTIR spectroscopy and Boehm titration results, the surface of the activated carbon substrates has acidic and basic oxygenated functional groups. Adsorption experiments were carried out to compare the sorption of two model molecules: methylene blue and methyl orange as cationic and anionic species, respectively, under near neutral pH, very acidic for methyl orange, and very basic for methylene blue, based on the corresponding pKa. Molecular modeling using DFT was also used to calculate adsorption Gibbs’ free energies for sorption reactions between the model molecules and the oxygenated functional groups. Almost all the free energies were negative which means that adsorption interactions are thermodynamically favorable. As expected for adsorption processes, in all cases, the enthalpic contribution dominated the entropic one. In addition, it is shown that the predominating interactions are H-bonding and π-π interactions. Methylene blue uptakes range from 0.026 mmol/g for graphite to 0.121 mmol/g for coconut shell carbon that corresponds to 24.8% and 100% removal, respectively. Increasing the methylene blue’s pH from 6.6 to 12 raised the cationic molecule uptake by ~ 30%. Furthermore, the coconut shell also removes the higher amount of methyl orange, near 99% at neutral and low pH. Citric residue does not adsorb methyl orange at neutral pH whereas at acidic conditions (pH = 3), it adsorbs 0.011 mmol/g (11% removal); in general, methyl orange adsorption was slightly lower than that of methylene blue.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study has been included in this manuscript and in its supplementary material file.
References
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sustain Energy Rev 46:218–235. https://doi.org/10.1016/j.rser.2015.02.051
Hagemann N, Spokas K, Schmidt HP et al (2018) Activated carbon, biochar and charcoal: linkages and synergies across pyrogenic carbon’s ABCs. Water (Switzerland) 10:1–19. https://doi.org/10.3390/w10020182
Cagnon B, Py X, Guillot A et al (2009) Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour Technol 100:292–298. https://doi.org/10.1016/j.biortech.2008.06.009
Li L, Quinlivan PA, Knappe DRU (2002) Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon N Y 40:2085–2100. https://doi.org/10.1016/S0008-6223(02)00069-6
Heidarinejad Z, Dehghani MH, Heidari M et al (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18:393–415. https://doi.org/10.1007/s10311-019-00955-0
Gao Y, Yue Q, Gao B, Li A (2020) Insight into activated carbon from different kinds of chemical activating agents: a review. Sci Total Environ 746https://doi.org/10.1016/j.scitotenv.2020.141094
Gao Y, Yue Q, Xu S, Gao B (2015) Activated carbons with well-developed mesoporosity prepared by activation with different alkali salts. Mater Lett 146:34–36. https://doi.org/10.1016/j.matlet.2015.01.161
González-García P (2018) Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew Sustain Energy Rev 82:1393–1414. https://doi.org/10.1016/j.rser.2017.04.117
Yunus ZM, Al-Gheethi GYA et al (2020) Advanced methods for activated carbon from agriculture wastes; a comprehensive review. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1717477
Ukanwa KS, Patchigolla K, Sakrabani R, et al (2019) A review of chemicals to produce activated carbon from agricultural waste biomass. Sustain 11 https://doi.org/10.3390/su11226204
Danish M, Ahmad T (2018) A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew Sustain Energy Rev 87:1–21. https://doi.org/10.1016/j.rser.2018.02.003
Martínez De Yuso A, Rubio B, Izquierdo MT (2014) Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process Technol 119:74–80. https://doi.org/10.1016/j.fuproc.2013.10.024
Kaouah F, Boumaza S, Berrama T et al (2013) Preparation and characterization of activated carbon from wild olive cores (oleaster) by H3PO4 for the removal of Basic Red 46. J Clean Prod 54:296–306. https://doi.org/10.1016/j.jclepro.2013.04.038
Meza CL, del Rosario M, Sun Kou NRA (2012) Astillas De Eucalipto Chromium ( Vi ) Adsorption with activated carbons prepared from wood chips of eucaliytus by chemical activation. Rev Soc Quim Peru 78:14–26
Lim WC, Srinivasakannan C, Balasubramanian N (2010) Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J Anal Appl Pyrolysis 88:181–186. https://doi.org/10.1016/j.jaap.2010.04.004
Güzel F, Koyuncu F (2021) Conversion of citrus industrial processing solid residues to well-developed mesoporous powder-activated carbon and its some water pollutant removal performance. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01726-0
Suárez-García F, Martínez-Alonso A, Tascón JMD (2002) Pyrolysis of apple pulp: chemical activation with phosphoric acid. J Anal Appl Pyrolysis 63:283–301. https://doi.org/10.1016/S0165-2370(01)00160-7
Zhang ZB, Liu XY, Li DW et al (2018) Effects of the ultrasound-assisted H3PO4 impregnation of sawdust on the properties of activated carbons produced from it. Xinxing Tan Cailiao/New Carbon Mater 33:409–416. https://doi.org/10.1016/S1872-5805(18)60349-X
Mahmood T, Ali R, Naeem A et al (2017) Potential of used Camellia sinensis leaves as precursor for activated carbon preparation by chemical activation with H3PO4; optimization using response surface methodology. Process Saf Environ Prot 109:548–563. https://doi.org/10.1016/J.PSEP.2017.04.024
Negara DNKP, Nindhia TGT, Surata IW et al (2020) Textural characteristics of activated carbons derived from tabah bamboo manufactured by using H3PO4 chemical activation. Mater Today Proc 22:148–155. https://doi.org/10.1016/j.matpr.2019.08.030
Puziy AM, Poddubnaya OI, Socha RP et al (2008) XPS and NMR studies of phosphoric acid activated carbons. Carbon N Y 46:2113–2123. https://doi.org/10.1016/j.carbon.2008.09.010
Giraldo S, Robles I, Ramirez A et al (2020) Mercury removal from wastewater using agroindustrial waste adsorbents. SN Appl Sci 2:1029. https://doi.org/10.1007/s42452-020-2736-x
Martínez RJ, Farrell J (2017) Understanding nitrilotris(methylenephosphonic acid) reactions with ferric hydroxide. Chemosphere 175:490–496. https://doi.org/10.1016/j.chemosphere.2017.02.015
Abdelbassit MS, Popoola SA, Saleh TA et al (2020) DFT and kinetic evaluation of chloromethane removal using cost-effective activated carbon. Arab J Sci Eng 45:4705–4716. https://doi.org/10.1007/s13369-020-04458-x
Vieira de Souza TN, Leão de Carvalho SM, Adeodato Vieira MG et al (2018) Adsorption of basic dyes onto activated carbon: experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl Surf Sci 448:662–670. https://doi.org/10.1016/j.apsusc.2018.04.087
Robles I, Espejel-Ayala F, Velasco G, et al (2020) A statistical approach to study the valorization process of spent coffee ground. Biomass Convers Biorefinery 1–13 https://doi.org/10.1007/s13399-020-00854-3
Tovar AK, Godínez LA, Espejel F et al (2019) Optimization of the integral valorization process for orange peel waste using a design of experiments approach: production of high-quality pectin and activated carbon. Waste Manag 85:202–213. https://doi.org/10.1016/j.wasman.2018.12.029
Ramirez AP, Giraldo S, Ulloa M, et al (2017) Production and characterization of activated carbon from wood wastes. In: Journal of Physics: Conference Series. Institute of Physics Publishing
Nieto-Delgado C, Terrones M, Rangel-Mendez JR (2011) Development of highly microporous activated carbon from the alcoholic beverage industry organic by-products. Biomass Bioenerg 35:103–112. https://doi.org/10.1016/j.biombioe.2010.08.025
Sánchez Martínez BS (2020) Elaboración de Carbonos Activados a Partir de Residuos Agroindustriales. Tecnológico de estudios superiores de san Felipe del progreso
Oickle AM, Tarasuk AC, Goertzen SL et al (2009) Standardization of the Boehm titration. Part I. CO 2 expulsion and endpoint determination. Carbon N Y 48:9. https://doi.org/10.1016/j.carbon.2009.11.050
Fulazzaky MA (2019) Study of the dispersion and specific interactions affected by chemical functions of the granular activated carbons. Environ Nanotechnology, Monit Manag 12https://doi.org/10.1016/j.enmm.2019.100230
Di L, Kerns EH (2016) pKa Methods. In: Di L, Kerns EH (eds) Drug-Like Properties, 2nd ed. Elsevier, pp 307–312. https://doi.org/10.1016/B978-0-12-801076-1.00024-1
Adan-Mas A, Alcaraz L, Arévalo-Cid P et al (2021) Coffee-derived activated carbon from second biowaste for supercapacitor applications. Waste Manag 120:280–289. https://doi.org/10.1016/j.wasman.2020.11.043
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026. https://doi.org/10.1016/j.procbio.2004.04.008
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3:773–790. https://doi.org/10.1007/s13201-013-0117-y
Largitte L, Pasquier R (2016) A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des 109:495–504. https://doi.org/10.1016/j.cherd.2016.02.006
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Revision A.03. Gaussian Inc., Wallingford, CT
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98:1372–1377. https://doi.org/10.1063/1.464304
Frisch MJ, Pople JA, Binkley JS (1998) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265. https://doi.org/10.1063/1.447079
de Souza TNV, de Carvalho SML, Vieira MGA et al (2018) Adsorption of basic dyes onto activated carbon: experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl Surf Sci 448:662–670. https://doi.org/10.1016/j.apsusc.2018.04.087
Martínez RJ, Farrell J (2019) Water splitting activity of oxygen-containing groups in graphene oxide catalyst in bipolar membranes. Comput Theor Chem 1164 https://doi.org/10.1016/j.comptc.2019.112556
Giraldo S, Robles I, Godínez LA, et al (2021) Experimental and theoretical insights on methylene blue removal from wastewater using an adsorbent obtained from the residues of the orange industry. Sometido Mol
Ramirez A, Ocampo R, Giraldo S, et al (2020) Removal of Cr (VI) from an aqueous solution using an activated carbon obtained from teakwood sawdust: Kinetics, equilibrium, and density functional theory calculations. J Environ Chem Eng 8 https://doi.org/10.1016/j.jece.2020.103702
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396. https://doi.org/10.1021/jp810292n
Ochterski JW (2000) Thermochemistry in Gaussian. In: Gaussian, Inc. https://gaussian.com/thermo/. Accessed 22 Dec 2021
Orth ES, Ferreira JGL, Fonsaca JES et al (2016) pKa determination of graphene-like materials: validating chemical functionalization. J Colloid Interface Sci 467:239–244. https://doi.org/10.1016/J.JCIS.2016.01.013
Weinhold F, Landis CR (2012) Discovering chemistry with natural bond orbitals. John Wiley & Sons Inc, Hoboken, New Jersey
Foster JP, Weinhold F (1980) Natural hybrid orbitals. J Am Chem Soc 102:7211–7218. https://doi.org/10.1021/JA00544A007
Wllkins DJ (1975) Compression buckling tests of laminated graphite-epoxy curved panels. AIAA J 13:465–470. https://doi.org/10.2514/3.49732
Paul E, Vannke MA (1991) A drifts study of the formation of surface groups on carbon by oxidation. Carbon N Y 31:721–730
Ortiz-Martínez AK, Godínez LA, Martínez-Sánchez C et al (2021) Preparation of modified carbon paste electrodes from orange peel and used coffee ground. New materials for the treatment of dye-contaminated solutions using electro-Fenton processes. Electrochim Acta 390:138861. https://doi.org/10.1016/j.electacta.2021.138861
Chia CH, Gong B, Joseph SD et al (2012) Imaging of mineral-enriched biochar by FTIR, Raman and SEM-EDX. Vib Spectrosc 62:248–257. https://doi.org/10.1016/j.vibspec.2012.06.006
Otero V, Sanches D, Montagner C et al (2014) Characterisation of metal carboxylates by Raman and infrared spectroscopy in works of art. J Raman Spectrosc 45:1197–1206. https://doi.org/10.1002/JRS.4520
Zeleňák V, Vargová Z, Györyová K (2007) Correlation of infrared spectra of zinc(II) carboxylates with their structures. Spectrochim Acta Part A Mol Biomol Spectrosc 66:262–272. https://doi.org/10.1016/J.SAA.2006.02.050
Eluyemi MS, Eleruja MA, Adedeji AV et al (2016) Synthesis and characterization of graphene oxide and reduced graphene oxide thin films deposited by spray pyrolysis method. Graphene 5:143–154. https://doi.org/10.4236/GRAPHENE.2016.53012
De Araujo RE, Gomes ASL, De Araújo CB (2000) Measurements of pKa of organic molecules using third-order nonlinear optics. Chem Phys Lett 330:347–353. https://doi.org/10.1016/S0009-2614(00)01108-8
Disanto AR, Wagner JG (1972) Pharmacokinetics of highly ionized drugs II: methylene blue—absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci 61:1086–1090. https://doi.org/10.1002/jps.2600610710
Fuente E, Menéndez JA, Suárez D, Montes-Morán MA (2003) Basic surface oxides on carbon materials: a global view. Langmuir 19:3505–3511. https://doi.org/10.1021/LA026778A
Schönherr J, Buchheim JR, Scholz P, Adelhelm P (2018) Boehm titration revisited (Part I): practical aspects for achieving a high precision in quantifying oxygen-containing surface groups on carbon materials. Carbon Res 1–13https://doi.org/10.3390/c4020021
Rufford TE, Hulicova-Jurcakova D, Zhu ZJ (2014) Green carbon materials - advances and applications, 1st Editio. CRC Press Taylor & Francis Group
Montes-Morán MA, Menéndez JA, Fuente E, Suárez D (1998) Contribution of the basal planes to carbon basicity: an ab initio study of the H3O+−π interaction in cluster models. J Phys Chem B 102:5595–5601. https://doi.org/10.1021/JP972656T
Figueiredo JL, Pereira MFR, Freitas MMA, Órfão JJM (1999) Modification of the surface chemistry of activated carbons. Carbon N Y 37:1379–1389. https://doi.org/10.1016/S0008-6223(98)00333-9
Ania CO, Parra JB, Pis JJ (2002) Influence of oxygen-containing functional groups on active carbon adsorption of selected organic compounds. Fuel Process Technol 79:265–271. https://doi.org/10.1016/S0378-3820(02)00184-4
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem - A Eur J 15:4195–4203. https://doi.org/10.1002/chem.200802097
Domingo-García M, López-Garzón FJ, Pérez-Mendoza M (2000) Effect of some oxidation treatments on the textural characteristics and surface chemical nature of an activated carbon. J Colloid Interface Sci 222:233–240. https://doi.org/10.1006/jcis.1999.6619
Puziy AM, Poddubnaya OI, Martínez-Alonso A et al (2002) Synthetic carbons activated with phosphoric - acid I. Surface chemistry and ion binding properties. Carbon N Y 40:1493–1505. https://doi.org/10.1016/S0008-6223(01)00317-7
Puziy AM, Poddubnaya OI, Ziatdinov AM (2006) On the chemical structure of phosphorus compounds in phosphoric acid-activated carbon. Appl Surf Sci 252:8036–8038. https://doi.org/10.1016/j.apsusc.2005.10.044
OriginPro 8.5 SR1 (2010) OriginLab Corporation, Northampton, MA
Conde O, Teixeira J (1983) Hydrogen bond dynamics in water studied by depolarized Rayleigh scattering. J Phys 44:525–529. https://doi.org/10.1051/jphys:01983004404052500ï
Esteban-Arranz A, Compte-Tordesillas D, Muñoz-Andrés V et al (2018) Effect of surface, structural and textural properties of graphenic materials over cooperative and synergetic adsorptions of two chloroaromatic compounds from aqueous solution. Catal Today 301:104–111. https://doi.org/10.1016/j.cattod.2017.03.048
Carrales-Alvarado DH, Rodríguez-Ramos I, Leyva-Ramos R et al (2020) Effect of surface area and physical–chemical properties of graphite and graphene-based materials on their adsorption capacity towards metronidazole and trimethoprim antibiotics in aqueous solution. Chem Eng J 402:126155. https://doi.org/10.1016/j.cej.2020.126155
Mattson JA, Mark HB, Malbin MD et al (1969) Surface chemistry of active carbon: Specific adsorption of phenols. J Colloid Interface Sci 31:116–130. https://doi.org/10.1016/0021-9797(69)90089-7
Carrales-Alvarado DH, Ocampo-Pérez R, Leyva-Ramos R, Rivera-Utrilla J (2014) Removal of the antibiotic metronidazole by adsorption on various carbon materials from aqueous phase. J Colloid Interface Sci 436:276–285. https://doi.org/10.1016/j.jcis.2014.08.023
Annadurai G, Juang RS, Lee DJ (2002) Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater 92:263–274. https://doi.org/10.1016/S0304-3894(02)00017-1
Yu J, Zhang X, Wang D, Li P (2018) Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci Technol 77:1303–1312. https://doi.org/10.2166/wst.2018.003
Yang J, Pignatello JJ, Yang K et al (2021) Adsorption of Organic Compounds by Biomass Chars: Direct role of aromatic condensation (ring cluster size) revealed by experimental and theoretical studies. Environ Sci Technol. https://doi.org/10.1021/acs.est.0c04852
Yakout SM, Hassan MR, El-Zaidy ME, et al (2019) Kinetic study of methyl orange adsorption on activated carbon derived from pine (Pinus strobus) sawdust. BioResources 14:4560–4574. https://doi.org/10.15376/biores.14.2.4560-4574
Zhang B, Wu Y, Cha L (2020) Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: performance, isotherm, and kinetic studies. J Dispers Sci Technol 41:125–136. https://doi.org/10.1080/01932691.2018.1561298
Rattanapan S, Srikram J, Kongsune P (2017) Adsorption of methyl orange on coffee grounds activated carbon. Energy Procedia 138:949–954. https://doi.org/10.1016/j.egypro.2017.10.064
Franca AS, Oliveira LS, Ferreira ME (2009) Kinetics and equilibrium studies of methylene blue adsorption by spent coffee grounds. Desalination 249:267–272. https://doi.org/10.1016/j.desal.2008.11.017
Dawood S, Sen TK, Phan C (2017) Synthesis and characterization of slow pyrolysis pine cone bio-char in the removal of organic and inorganic pollutants from aqueous solution by adsorption: kinetic, equilibrium, mechanism and thermodynamic. Bioresour Technol 246:76–81. https://doi.org/10.1016/j.biortech.2017.07.019
Rosas-Castor JM, Garza-González MT, García-Reyes RB et al (2014) Methylene blue biosorption by pericarp of corn, alfalfa, and agave bagasse wastes. Environ Technol (United Kingdom) 35:1077–1090. https://doi.org/10.1080/09593330.2013.861022
Zubair M, Mu’azu ND, Jarrah N, et al (2020) Adsorption behavior and mechanism of methylene blue, crystal violet, eriochrome Black T, and methyl orange dyes onto biochar-derived date palm fronds waste produced at different pyrolysis conditions. Water Air Soil Pollut 231 https://doi.org/10.1007/s11270-020-04595-x
Ding G, Wang B, Chen L, Zhao S (2016) Simultaneous adsorption of methyl red and methylene blue onto biochar and an equilibrium modeling at high concentration. Chemosphere 163:283–289. https://doi.org/10.1016/j.chemosphere.2016.08.037
Li H, Sun Z, Zhang L et al (2016) A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloids Surfaces A Physicochem Eng Asp 489:191–199. https://doi.org/10.1016/J.COLSURFA.2015.10.041
Martini BK, Daniel TG, Corazza MZ, De Carvalho AE (2018) Methyl orange and tartrazine yellow adsorption on activated carbon prepared from boiler residue: kinetics, isotherms, thermodynamics studies and material characterization. J Environ Chem Eng 6:6669–6679. https://doi.org/10.1016/j.jece.2018.10.013
Aboua KN, Yobouet YA, Yao KB et al (2015) Investigation of dye adsorption onto activated carbon from the shells of Macoré fruit. J Environ Manage 156:10–14. https://doi.org/10.1016/j.jenvman.2015.03.006
Bello MO, Abdus-Salam N, Adekola FA, Pal U (2021) Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem Data Collect 31:100607. https://doi.org/10.1016/j.cdc.2020.100607
Li Z, Hanafy H, Zhang L et al (2020) Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: experiments, characterization and physical interpretations. Chem Eng J 388:124263. https://doi.org/10.1016/J.CEJ.2020.124263
Kaya N, Uzun ZY (2021) Investigation of effectiveness of pine cone biochar activated with KOH for methyl orange adsorption and CO2 capture. Biomass Convers Biorefinery 11:1067–1083. https://doi.org/10.1007/s13399-020-01063-8
Yusop MFM, Ahmad MA, Rosli NA, Manaf MEA (2021) Adsorption of cationic methylene blue dye using microwave-assisted activated carbon derived from acacia wood: optimization and batch studies. Arab J Chem 14:103122. https://doi.org/10.1016/j.arabjc.2021.103122
Tuli FJ, Hossain A, Kibria AKMF et al (2020) Removal of methylene blue from water by low-cost activated carbon prepared from tea waste: a study of adsorption isotherm and kinetics. Environ Nanotechnol Monit Manag 14:100354. https://doi.org/10.1016/J.ENMM.2020.100354
Patawat C, Silakate K, Chuan-Udom S et al (2020) Preparation of activated carbon fromDipterocarpus alatusfruit and its application for methylene blue adsorption. RSC Adv 10:21082–21091. https://doi.org/10.1039/d0ra03427d
Han Q, Wang J, Goodman BA et al (2020) High adsorption of methylene blue by activated carbon prepared from phosphoric acid treated eucalyptus residue. Powder Technol 366:239–248. https://doi.org/10.1016/j.powtec.2020.02.013
Maheshwari K, Agrawal M, Gupta AB, et al (2021) Dye pollution in water and wastewater. 1–25. https://doi.org/10.1007/978-981-16-2892-4_1
Yönten V, Sanyürek NK, Kivanç MR (2020) A thermodynamic and kinetic approach to adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Vitis vinifera L. Surfaces and Interfaces 20:1–8. https://doi.org/10.1016/j.surfin.2020.100529
Acknowledgements
The authors thank Centro de Investigación y Desarrollo Tecnológico en Electroquímica (CIDETEQ) for the facilities to conduct this research. The authors also express their gratitude to the Mexican Council for Science and Technology, CONACYT (CB-2016- 01, Project: 285309) for the financial support of this work and A. Vela also thanks CONACYT for a Ph.D. scholarship. The authors are also grateful to E.J. Aldeco-Perez for the FTIR analysis.
Funding
This study was funded by CONACYT (CB-2016- 01), Project: 285309.
Author information
Authors and Affiliations
Contributions
Alina Vela: investigation, validation, formal analysis.
Rodrigo Martínez: investigation, validation, formal analysis, supervision.
Luis A. Godínez: methodology, formal analysis, writing — reviewing and editing.
José de Jesús Pérez-Bueno: investigation, validation, formal analysis.
Fabricio Espejel: investigation, formal analysis.
Irma Robles: conceptualization, formal analysis, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vela-Carrillo, A.Z., Martínez, R.J., Godínez, L.A. et al. Study of chemical, kinetic, and theoretical sorption properties of activated carbons obtained from agroindustrial origin: comparison of anionic and cationic model molecules. Biomass Conv. Bioref. 14, 733–750 (2024). https://doi.org/10.1007/s13399-022-02367-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02367-7