Abstract
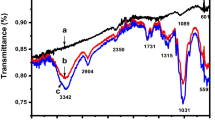
Activated carbons from watermelon shell (GACW) and walnut shell (GACN) were synthesized through chemical activation with phosphoric acid 40 % w/w, as an alternative low-cost adsorbent for the removal of lead(II) and zinc(II) ions from aqueous solutions. The yield of production was 85 and 80 % for GACW and GACN respectively. To compare the differences and similarities between the two activated carbons the following tests were performed: surface and pore width with SEM, nitrogen adsorption isotherms at −196 °C (77 K), IR spectroscopy, TGA, point of zero charge (PZC) and Boehm titration. The GACN has 10 % more surface area (789 m2 g−1 for GACN and 710 m2 g−1 for GACW) and 13 % more pore volume than GACW. Also, GACN has a better resistance to high temperatures than GACW (the loss of mass at 900 °C was 20 % for GACN, while for GACW was 31 %). The effect of the initial concentration of lead(II) and zinc(II) ions on the adsorption process was studied in a batch process mode. To quantify the adsorption of lead and zinc adsorption isotherms of both metals in aqueous solution were performed for each carbon using analytic technique of atomic absorption. The adsorption isotherm data were better fitted by Langmuir model. Experimental results suggests that one gram of GACW adsorbs more milligrams of lead(II) and zinc(II) than one gram of GACN; it is suggest that the pore distribution is a significant variable in the adsorption process because GACW present mesopores and micropores, while GACN has only micropores. Also, the surface chemistry is an important variable in the adsorption process because GACW presents a lower pHPZC than GACN (3.05 for GACW and 4.5 for GACN) and the solution’s pH of each metal was adjusted in 4.5, for that it could be suggested that the electrostatic interactions were increased between the ion and the carbon surface.








Similar content being viewed by others
References
Ahmdnea, M., Marshall, W.E., Rao, R.M.: Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bio. Tech. 71, 113–123 (1999)
Ash, B., Satapathy, D., Mukherjee, P.S., Nanda, B., Gumaste, J.L., Mishra, B.K.: Characterization and application of activated carbon prepared from waste coir pith. J. Sci. Ind. Res. 65, 1008–1012 (2006)
Bellamy, L.J.: The Infra-Red Spectra of Complex Molecules. Wiley, New York (1954)
Boehm, H.: Surface oxides on carbon and their analysis: a critical. Carbon. 40, 145–149 (2001)
Chilton, Ng, Losso, Jack, Marshall, Wayne, Rao, Ramu: Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bio. Tech. 85, 131–135 (2002)
Corbridge: Infra-red analysis of phosphorus compounds. J. Appl. Chem. 6, 456–465 (1956)
Depci, T., Kul, A.R., Onal, Y.: Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: study in single- and multi-solute systems. Chem. Eng. J. 200–202, 224–236 (2012)
Elizalde-Gonzalez, M.P., Hernandez-Montoya, V.: Characterization of mango pit as raw material in the preparation of activated carbon for wastewater treatment. Biochem. Eng. J. 36, 230–238 (2007)
El-Sayed, Y., Bandosz, T.J.: Adsorption of valeric acid from aqueous solution onto activated carbons: role of surface basic sites. J. Colloid Interface Sci. 273, 64–72 (2004)
Giraldo, L., Ladino, Y., Moreno-Piraján, J., Rodríguez, M.P.: Synthesis and characterization of activated carbon fibers from Kevlar. Eclét. Quím. 32, 55–62 (2007)
Giraldo, L., Moreno, J.C.: The relation between immersion enthalpy and adsorption parameters for an activated carbon in aqueous Pb2+ solutions. Rev. Colomb. Quím. 35, 41–49 (2006)
Gratuito, M.K.B., Panyathanmaporn, T., Chumnanklang, R.-A., Sirinuntawittaya, N., Dutta, A.: Production of activated carbon from coconut shell: optimization using response surface methodology. Bioresour. Technol. 99, 4887–4895 (2007)
Hawari, A., Rawajfih, Z., Nsour, N.: Equilibrium and thermodynamic analysis of zinc ions adsorption by olive oil mill solid residues. J. Hazard Mater. 168, 1284–1289 (2009)
IUPAC: Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Int. Union Pure Appl. Chem. 57, 603–619 (1985)
Jaramillo, J., Álvarez, P.M., Gómez-Serrano, V.: Preparation an ozone-surface modification of activated carbon. Thermal stability of oxygen surface groups. App. Surf. Sci. 256, 5232–5236 (2010)
Kalmykova, Y., Stromvall, A.M., Steenari, B.M.: Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. J. Hazard Mater. 152, 885–891 (2008)
Kaneko, K.: Determination of pore size and pore size distribution: adsorbents and catalysts. J. Membr. Sci. 96, 59–89 (1994)
Karagoz, S., Tray, T., Ucar, S., Erdem, M.: Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 99, 6214–6222 (2008)
Leofanti, G., Padovan, M., Tozzola, G., Venturelli, B.: Surface area and pore texture of catalysts. Catal. Today. 41, 207–219 (1998)
Lopez Ramon, M.V., Stoeckli, M., Moreno Castilla, C., Carrasco-Marin, F.: On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon. 37, 1215–1221 (1999)
McBride, M.B.: Reactions controlling heavy metal solubility in soils. Adv. Soil Sci. 10, 1–56 (1989)
McBride, M.B.: Chemisorption and precipitation of inorganic ions. Environmental Chemistry of Soils. Oxford University Press, New York (1994)
Mesquita, M.E., Vieira Silva, J.M., Silva, : Preliminary study of pH effect in the application of Langmuir and Freundlich isotherms to Cu–Zn competitive adsorption. Geoderma. 106, 219–234 (2001)
Minceva, M., Fajgar, R., Markovska, L., Meshko, V.: Comparative study of Zn(II), Cd(II), Pb(II) removal from water solution using natural clinoptilolitic zeolite and commercial granulated activated carbon Equilibrium of adsorption. Sep. Sci. Tech. 43, 2117–2143 (2008)
Mohan, D., Pittman, C.U., Steele, P.H.: Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on kraft lignin-a biosorbent. J. Colloid Interface Sci. 297, 489–504 (2006)
Momèiloviæ, M., Purenović, M., Bojiæ, A., Zarubica, A., Ranđelović, M.: Removal of lead(II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination. 276, 53–59 (2011)
Moreno C, López Ramón MV, Ramos RL, Moreno Piraján JC: (2007) Adsorción de compuestos orgánicos disueltos en agua sobre carbones activados. In: Sólidos Porosos. Preparación, Caracterización y Aplicaciones, By Juan Carlos Moreno Piraján, pp. 213–241. Ediciones Uniandes
Moreno, J.C., Giraldo, L.: Propiedades termodinámicas del proceso de adsorción de Pb+2 sobre CARBÓN activado a diferentes pH. Información Tecnológica. 19, 61–72 (2008)
Onal, Y., Akmil-Basar, C., Sarici-Ozdemir, C., Erdogan, S.: Textural development of sugar beet bagasse activated with ZnCl2. J. Hazard Mater. 142, 138–143 (2007)
Paraskeva, P., Kalderis, D., Diamadopoulos, E.: Production of activated carbon from agricultural by products. J. Chem. Technol. Biotech. 83, 581–592 (2008)
Parker PM (2011) The 2011 import and export market for fresh melons, watermelons, and papayas in Colombia. Country trade reports
Puziy, A.M., Poddubnaya, O.I., Martíınez-Alonso, A., Suárez-García, F.: Synthetic carbons activated with phosphoric acid. Surface chemistry and ion binding properties. Carbon. 40, 1493–1505 (2002)
Rahman, M.M., Awang, M., Kmaruzzaman, B.Y., Wan Nik, W.B., Adman, M.C., Mohosina, S.B.: Waste palm shell converted to high efficient activated carbon by chemical activation method and its adsorption capacity tested by water filtration. Procesia APCBEE. 1, 293–298 (2012)
Saka, C.: BET, TG–DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2. J. Ana. App. Pyr. 95, 24–29 (2012)
Sellin, R., Clacens, J.-M., Coutanceau, C.: A thermogravimetric analysis/mass spectroscopy study of the thermal and chemical stability of carbon in the Pt/C catalytic system. Carbon 48, 2244–2254 (2010)
Socrates, G.: Infrared characteristic group frequencies. Wiley, New York (1994)
Stavropoulos, G., Zabaniotou, A.: Minimizing activated carbons production cost. Fuel Process. Technol. 90, 952–957 (2009)
Sun, Y., Yue, Q., Gao, B., Huang, L., Xu, X., Li, Q.: Comparative study on characterization and adsorption properties of activated carbons with H3PO4 and H4P2O7 activation employing Cyperus alternifolius as precursor. Chem. Eng. J. 181–182, 790–797 (2011)
Valente Nabais, João M., Carlos, Eduardo C., Laginhas, C., Carrott, P.J.M., Ribeiro Carrott, M.M.L.: Production of activated carbons from almond shell. Fuel Process. Technol. 92, 234–240 (2011)
Zawadki, J.: Infrared spectroscopy in surface chemistry of carbons—from chemistry and physics of carbon. Wiley, New York (1989)
Zhang, M.: Adsorption study of Pb(II), Cu(II) and Zn(II) from simulated acid mine drainage using dairy manure compost. J. Chem. Eng. 172, 361–368 (2011)
Zhang, K., Cheung, W.H., Valix, M.: Roles of physical and chemical properties of activated carbon in the adsorption of lead ions. Chemosphere. 60, 1129–1140 (2004)
Acknowledgments
The authors thank the Framework Agreement between Universidad de los Andes and Universidad Nacional de Colombia, as well as the Agreement Statement (Acta de Acuerdo) between the Departments of Chemistry of both Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreno-Barbosa, J.J., López-Velandia, C., Maldonado, A.d.P. et al. Removal of lead(II) and zinc(II) ions from aqueous solutions by adsorption onto activated carbon synthesized from watermelon shell and walnut shell. Adsorption 19, 675–685 (2013). https://doi.org/10.1007/s10450-013-9491-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-013-9491-x