Abstract
In the present study, adsorption of Cr(VI) ions from aqueous solutions was investigated and compared under various conditions using two different nano-structured adsorbents, i.e., carbon nanotubes (CNTs) and low-cost activated carbons (AC). Walnut shell, an agricultural solid waste, was used as a raw material for the preparation of ACs. Multi-walled CNTs (MWCNTs) with average diameter of 20 nm and length of about 2 mm were also used for comparison purposes. Adsorption studies were carried out by varying the parameters such as: treatment time, metal ion concentration, adsorbent amount and pH. The adsorption capacities of AC and MWCNT for Cr(VI) ions were measured as 35 and 24 mg g−1, respectively. The efficiency was observed fairly high at pH = 2–3 for AC and pH = 5–6 for MWCNTs. The adsorption was significantly enhanced by increasing the adsorbent dose up to 0.4 and 0.3 g for AC and MWCNTs, respectively. It was also determined that Cr(VI) adsorption behavior follows both Langmuir and Freundlich isotherms. The content of functional groups, which was obtained by applying the Boehm’s method, revealed that phenolic groups are mostly present on the surface of MWCNTs, while basic groups are predominant on the walnut shell AC structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals are often present in the aquatic streams through different industrial wastewaters. Due to their toxicological importance in the ecosystem, agriculture and human health, pollution by heavy metals has received wide spread attention [1, 2]. Chromium, one of the extremely toxic heavy metals affecting the environment, is present in the waste water as a result of its industrial application such as tanning, metallurgy, plating and metal finishing. Once chromium is introduced into the environment, it exists in two stable oxidation states, i.e., Cr(III) and Cr(VI) [3]. The trivalent form is relatively innocuous, but the hexavalent chromium is very toxic, highly mobile in soil and aquatic system, and also is a strong oxidant capable of being adsorbed by the skin [4]. Therefore, the tolerance limit of Cr(VI) for discharge into the inland surface water is set to 0.05 mg L−1 [5].
Various methods were employed by researchers for the removal of heavy metals from aqueous solutions such as: chemical precipitation [6], reverse osmosis [7], ion exchange [8], coagulation [9], and adsorption [10–12]. Biosorption method is also a property of certain types of inactive, dead, microbind biomass to bind and concentrate heavy metals from very dilute aqueous solutions such as green algae, bagass fly ash and some other adsorbents [13–25].
Most commonly, the recommended adsorbents for Cr(VI) ion removal are alumina, silica and activated carbons. Several researches have been also performed some studies on the development of low-cost activated carbons from cheaper and readily available materials for the removal of heavy metals from waters [26–29]. Walnut shell is one of the agricultural waste products, which is mainly used as a fuel. On the other hand, carbon nanotubes (CNTs) are increasingly attracting interest in the field of metal adsorption [30–34]. Their small sizes, large surface areas, high mechanical strength and remarkable electrical conductivities have indicated their tremendous potential for several engineering applications.
Although there are many studies on the removal of chromium using ACs and to some extend by CNTs, but there is no comparison between the adsorption activities of these two different types of carbon-based materials. In the present study, the adsorptions of Cr(VI) ions from aqueous solutions are investigated and compared under various conditions using two different types of adsorbents; an ordinary low-cost sorbent, i.e., walnut shell-activated carbon and multi-walled carbon nanotubes.
Materials and methods
Materials
Potassium dichromate was obtained from Merck Company in analytical grade. Stock solutions were prepared by dissolving potassium dichromate in de-ionized water. Activated carbon (AC) was prepared by physical activation using carbon dioxide as an activating agent according to the method described elsewhere [35]. Briefly, walnut shell (WS) was washed with distilled water to remove all soluble impurities. Sample was then dried in an electrical oven for 24 h at 110 °C. Dried sample was carbonized in nitrogen atmosphere and then activated for 12 h at 800 °C by CO2. The activated carbon was ground and sieved to obtain particle sizes in the range of 1–2 mm. The iodine number and BET surface area (measured by Quantachrome, ASAP-2010) of AC products were measured as 830 mg g−1 and 900 m2 g−1, respectively.
The MWCNTs with an average diameter of 20 nm, length of about 2 mm, and purity >95 % were supplied by Iran research institute of petroleum industry (RIPI). The nanotubes had been produced by the CVD method using methane gas.
Boehm’s test
The Boehm’s test was performed to measure main functional groups on the adsorbents surfaces. The procedure can be described as follows: 0.5 g sorbent was placed in a series of flasks, each containing 50 mL of 0.05 N sodium bicarbonate, sodium carbonate, sodium hydroxide and hydrochloric acid, respectively. Flasks were sealed and shaken for 24 h, after which the solutions were filtered. Then 10 mL of each solution was titrated with 0.05 N sodium hydroxide and/or hydrochloric acid, depending on the original solution used. The amount of acidic groups on the sorbent was calculated under the assumption that NaOH neutralizes carboxylic, lactonic, and phenolic groups; Na2CO3 neutralizes carboxylic and lactonic groups and NaHCO3 only neutralizes carboxylic group. The number of surface basic sites was calculated from the amount of HCl reacted with the sorbent. The reaction between reagents and acidic oxygenated functional groups on the surface is based on the difference in acid/base strength. The strength of acidic and basic groups is in the order of: Carboxylic > Lactonic > Phenolic [36].
Batch adsorption
Adsorption of Cr(VI) ion from solutions was studied by the batch technique. The general method used is described as follows: the specified doses of adsorbent were mixed with 50 mL of 100 ppm Cr(VI) solution in the 250 mL conical flasks and kept for predetermined time intervals on a mechanical shaker at 30 °C. The reactor was a jar cell with a double cylindrical jacket in which the temperature was controlled by circulating water through the jacket and stirring speed was kept 720 rpm to keep the sorbent particles in suspension [37].
The pH was adjusted using sulfuric acid. Solutions were then filtered using filter papers and filtrate was analyzed for residual Cr(VI) ion using an atomic adsorption spectrophotometer (Varian, spectra-110-220/880) equipped with a Zeeman atomizer. The uptake of metal ion in the solution was calculated by the difference in their initial and final concentrations. Each experiment was repeated three times and the results reported are the average values. These data were used to calculate equilibrium Cr(VI) uptake capacity according to the following equation:
where qe (mg g−1) is the amount of metal in the adsorbate phase at equilibrium, C0 and Ce are the initial and equilibrium concentrations of metal ion (mg L−1) in the aqueous solution, V is the volume of the solution (L), and m is the weight of sorbent (g) in the mixture.
The effect of different parameters, including adsorbent dosage (0–0.8 g for WS AC and 0–0.4 g for MWCNT), pH of solution (2–11), initial concentration (20–110 ppm), temperature (30–50 °C) and contact time (0–40 min), was evaluated.
Results and discussion
Characterization of adsorbents
Chemical and thermal treatment processing could affect the adsorption capability of adsorbents for the removal of chromium ions as these treatments result in some functional groups on the solid surface [38]. To study the effect of functional groups on the Cr(VI) adsorption, these groups were measured quantitatively for both adsorbents using the Boehm’s titration method. The results obtained from the Boehm’s method are presented in Table 1. As shown in the table, the amounts of acidic and basic groups on both adsorbents are in the following order:
Walnut shell AC: Basic > Lactonic > Phenolic > Carboxylic.
MWCNTs: Phenolic > Lactonic > Carboxylic > Basic.
Effect of temperature and contact time
Figure 1a shows the effect of operating time on the adsorption of Cr(VI) at different temperatures for a fixed dose of 0.3 g AC. The initial concentrations were kept constant at 110 mg L−1 for all different cases. The experimental data show that adsorption of Cr(VI) is enhanced by increasing temperature (30–50 °C). It confirms that adsorption process has an exothermic nature; therefore, the tendency of chromium molecules for adsorption on the solid surface would decrease with increasing temperature.
The adsorption of Cr(VI) is increased by increasing the contact time, due to higher contact between the chromium ions and adsorbent particle surfaces. The amounts of Cr(VI) adsorbed are increased during the first 10 min of adsorption and at the temperature of 30 °C as an example it reached up to about 75 % of its removal efficiency. Subsequently, the adsorption is enhanced gradually and reached nearly equilibrium after about 30 min. The results also indicate that there is not much differences among the amounts of Cr(VI) adsorbed at various temperatures. This is a reason for great adsorption potential between Cr(VI) and WS activated carbon.
Figure 1b shows the effect of operating time on the adsorption of Cr(VI) with a initial concentration of 100 mg L−1 at 30 °C for 0.15 g MWCNTs. The experimental results show enhancement of Cr(VI) ion adsorption by time. The rate of Cr(VI) removal is fast in the first 2 h reaching about 50 % of its final value and then gradually reduced. The equilibrium adsorption time is seen to be much shorter in the case of WS AC compared to that of MWCNTs. The shorter time needed to attain equilibrium could be attributed to high adsorption efficiency of WS AC and readily available basic sites on its surface.
Effect of Cr(VI) initial concentration
The effect of Cr(VI) initial concentration at different levels of 21, 61, 87 and 110 mg L−1 and constant AC dose of 0.3 g are presented in Fig. 2a. The results show that the Cr(VI) ions removal is concentration dependent and removal efficiency is increased by increasing Cr(VI) initial concentration (from about 50 to 73 %). The limited size of pore openings and the electrostatic repulsion between negative charges of adsorbate ions result in the reduction of adsorption percentage. Interestingly, the equilibrium times are found to be the same for all the concentration levels studied.
The effect of Cr(VI) initial concentration at 20, 60 and 100 mg L−1 levels and constant dose of 0.15 g with contact time of 30 min are seen in Fig. 2b for MWCNTs. Different behavior is seen for this adsorbent, i.e., the percentage of Cr(VI) removal is increased with decreasing the initial concentration from about 100 to 53 %. It should be noted that the time scale for adsorption in the case of MWNT is very high compared to that of AC. At long time and higher initial concentrations, the ratio of available adsorption sites of MWCNTs to chromium ions is less and the binding sites being saturated.
Effect of sorbent dose
The effect of sorbent dose was investigated by changing the amount of AC from 0.2 to 0.8 g at 30 min contact time, keeping the initial Cr(VI) concentration and temperature constant at 100 mg L−1 and 30 °C, respectively (Fig. 3). Our observations show that the remaining chromium ions in the solution (unadsorbed ions) decreased by enhancing the sorbent dose for a fixed initial concentration due to greater surface area available for adsorption and also more effective contact between the adsorbent and Cr(VI) ions. It is also found that there is an optimum value of sorbent for each initial concentration which gives maximum removal.
Similar results can be found in Fig. 3b for MWCNTs. The amounts of MWCNTs were 0.1, 0.15, 0.2, 0.3, and 0.4 g for the same initial concentration and temperature at 5 h contact time. Adsorption was enhanced by increasing the adsorbent dose until it reached to a constant value due to equilibrium.
Effect of pH
pH is one of the most important parameters controlling the metal ion adsorption. Figure 4 shows the effect of pH on adsorption of Cr(VI) with initial concentration of 100 mg L−1 at 30 °C for both adsorbents. For all different cases, the adsorbent amounts and contact times were kept constant at 0.3 g and 30 min for AC and 0.15 g and 6 h for MWCNTs. This figure shows that WS AC is more active at the lower pH range. The maximum adsorption of Cr(VI) is found in the pH range of 2–3 for AC and the removal percentage is almost negligible at pH values higher than 11. But in the case of MWCNTs, the best performance is seen in the pH range of 5–6.
The pH of system controls the adsorption capacity due to its influence on the surface properties of the adsorbent as well as ionic forms of the chromium solutions. The chromic acid predominates at pH values less than about 1.0, HCrO4− at pH range of 1.0–6.0, and CrO42− and Cr2O72– at pH values above 6.0. In the case of AC, more adsorption at acidic pH indicates that lower pH results in an increase in H+ ions on the adsorbent surface that results in significantly strong electrostatic attraction between positively charged adsorbent surface and chromium ions. Lesser adsorption of Cr(VI) at pH values greater than 6.0 may be due to the dual competition of two anions (CrO42– and OH–) to be adsorbed on the surface of the adsorbent, of which OH– predominates. This is in accordance with earlier studies on the removal of Cr(VI) by different adsorbents [39]. It has also been suggested that, under acidic conditions, Cr(VI) could be reduced to Cr(III) in the presence of AC adsorbent.
The result of Boehm’s test for WS AC shows that basic groups on the carbon surface are more than others. Therefore, by decreasing pH (or increasing H+) of the solution, basic groups could adsorb chromium ions. In other words, by decreasing the negative charge density on the adsorbent surface, the electrostatic force of adsorption between Cr(VI) ions and surface increases.
The Boehm’s test indicates that most of the functional groups on MWCNTs surface are phenolic. In the aqueous environment (especially at low pH), phenolic groups transform into C x O. On the other hand, at low pH, both HCrO4− and Cr2O72− ions coexist in the solution. In the presence of a reducing substrate (C x OH), the Cr(IV) species are quickly reduced into Cr(III) ions as indicated by the following equations [40].
where Cx is the carbon. Cr(III) ions are not adsorbed or poorly adsorbed at these low pH values. Therefore, at pH < 3.0 the adsorption capacity is very low. Since the concentration of H+ decreases at pH >3.0, the chemical reactions of (2) and (3) will not proceed. The adsorbent surface may become negative and adsorption operation does not happen anymore. One of the reasons for slow adsorption operation with MWCNTs is hydrolysis of phenolic group which is a slow reaction.
Adsorption isotherms
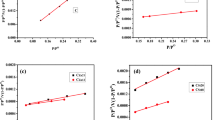
Langmuir and Freundlich equations are commonly used for describing adsorption equilibrium of water and wastewater treatment applications. The linear form of Langmuir isotherm is given by the following equation [41]:
where qe is the adsorbed amount at equilibrium (mg g−1), Ce is the equilibrium concentration (mg L−1), b is a constant related to the energy or net enthalpy of adsorption (L mg−1), and qm is the Langmuir constant related to maximum adsorption capacity. When 1/qe is plotted against 1/Ce, the straight line with slope of 1/bqm is obtained, which shows that the adsorption of chromium on WS AC follows Langmuir isotherm. Langmuir constants, b and qm, were then calculated and these values are given in Table 2.
The adsorption data are also analyzed by Freundlich model. The logarithmic form of Freundlich model is given by the following equation:
where Kf and n are the Freundlich constants related to adsorption capacity and adsorption intensity, respectively. The Freundlich parameters are also given in Table 2. The adsorption isotherms were determined at fixed temperature of 30 °C for Cr(VI) concentration range of 100, 150, 200, and 250 mg L−1. All solutions contained fixed dose of 0.3 g adsorbent.
The good correlation coefficients showed that both Langmuir and Freundlich models are suitable for adsorption equilibrium of chromium ion.
RL value indicates the type of isotherm. RL values between 0 and 1 indicate favorable adsorption.
where b is the Langmuir constant and C0 is the initial metal ion concentration (mg L−1). The results are presented in Table 3. RL values for chromium were found to be between 0 and 1 for all concentrations at 30 °C, indicating favorable adsorption.
For comparison purposes, adsorption capacities of other adsorbents are collected from the literature and reported in Table 4. It is clear from the Table that the adsorption capacities of WS activated carbon for Cr(VI) ion are comparable with most other adsorbents.
Conclusion
Based on the present investigation, it can be concluded that the adsorption capacity of low-cost adsorbent (AC) from an agricultural based material is comparable with that of MWCNTs for chromium ion removal. In the batch adsorption technique studied, the ion removal was dependent on the adsorption time, metal ion concentration, adsorbent amount and pH of solution.
The experimental results indicated optimum operating conditions for each adsorbent. Chromium removal was found to be pH dependent and the efficiency was observed fairly high at pH = 2–3 for AC, in comparison with the pH = 5–6 for MWCNTs. Adsorption was increased by increasing the adsorbent dose and time at initial stages and then it became somewhat constant due to the attainment of equilibrium. The percentage of Cr(VI) removal was increased by enhancing the adsorbate concentration for WS AC and the opposite trend for MWCNTs (but at longer time).
The adsorption process can be modeled by Langmuir and Freundlich adsorption isotherms. The adsorptive capacity of WS activated carbon is comparable with other sorbents for chromium ion. This low-cost adsorbent with its rapid adsorptive ability would offer a promising technique for industrial wastewaters cleanup.
References
Mittal, A., Mittal, J., Malviya, A., Gupta, V.K.: Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J. Colloid Interface Sci. 344, 497–507 (2010)
Gupta, V.K., Rastogi, A., Nayak, A.: Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J. Colloid Interface Sci. 342, 533–539 (2010)
Selvi, K., Pattabhi, S., Kadirvalu, K.: Removal of Cr from aqueous solutions by adsorption on to activated carbon. Bioresour. Technol. 80, 87–89 (2001)
Hu, Z., Lei, L., Li, Y., Ni, Y.: Chromium adsorption on high performance activated carbon from aqueous solutions. Sep. Technol. 31, 13–18 (2003)
EPA (Environmental Protection Agency), Environmental Pollution Control Alternatives. EPA/625/5-90/25, EPA/625/4-89/023. Cincinaati OH, USA (1990)
Mauchauffée, S., Meux, E.: Use of sodium decanoate for selective precipitation of metals contained in industrial wastewater. Chemosphere 69, 763–768 (2007)
Malamis, S., Katsou, E., Takopoulos, K., Demetriou, P., Loizidou, M.: Assessment of heavy metal removal, biomass activity and RO concentrate treatment in an MBR-RO system. J. Hazard. Mater. 209–210, 1–8 (2012)
Verma, V.K., Tewari, S., Rai, J.P.N.: Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 99, 1932–1938 (2008)
Samrani, A.G.E., Lartiges, B.S., Villiéras, F.: Chemical coagulation of combined sewer overflow: heavy metal removal and treatment optimization. Water Res. 42, 951–960 (2008)
Zabihi, M., Asl, A.H., Ahmadpour, A.: Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 174, 251–256 (2010)
Ahmadpour, A., Zabihi, M., Tahmasbi, M., Bastami, T.R.: Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solutions. J. Hazar. Mater. 182, 552–556 (2011)
Gupta, V.K., Sharma, S.: Removal of Zinc from Aqueous Solutions Using Bagasse Fly Ash − a Low Cost Adsorbent. Ind. Eng. Chem. Res. 42, 6619–6624 (2003)
Mittal, A., Mittal, J., Malviya, A., Kaur, D., Gupta, V.K.: Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 343, 463–473 (2010)
Gupta, V.K., Jain, R., Mittal, A., Mathur, M., Sikarwar, S.: Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J. Colloid Interface Sci. 309, 464–469 (2007)
Gupta, V.K., Mittal, A., Malviya, A., Mittal, J.: Adsorption of carmoisine A from wastewater using waste materials-Bottom ash and deoiled soya. J. Colloid Interface Sci. 335, 24–33 (2009)
Gupta, V.K., Mittal, A., Kurup, L., Mittal, J.: Adsorption of a hazardous dye, erythrosine, over hen feathers. J. Colloid Interface Sci. 304, 52–57 (2006)
Mittal, A., Mittal, J., Malviya, A., Gupta, V.K.: Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 340, 16–26 (2009)
Gupta, M., Satpathy, I., Roy, A., Pratibha, R.: Nanoparticle induced director distortion and disorder in liquid crystal-nanoparticle dispersions. J. Colloid Interface Sci. 352, 292–298 (2010)
Gupta, V.K., Rastogi, A.: Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 163, 396–402 (2009)
Mittal, A., Kurup, L., Gupta, V.K.: Use of waste materials-Bottom Ash and De-Oiled Soya, as potential adsorbents for the removal of Amaranth from aqueous solutions. J. Hazard. Mater. 117, 171–178 (2005)
Gupta, V.K., Jain, R., Varshney, S.: Removal of Reactofix golden yellow 3 RFN from aqueous solution using wheat husk-An agricultural waste. J. Hazard. Mater. 142, 443–448 (2007)
Gupta, V.K., Gupta, B., Rastogi, A., Agarwal, S., Nayak, A.: A comparative investigation on adsorption performances of mesoporous activated carbon prepared from waste rubber tire and activated carbon for a hazardous azo dye-Acid Blue 113. J. Hazard. Mater. 186, 891–901 (2011)
Mittal, A., Gupta, V.K., Malviya, A., Mittal, J.: Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (Metanil Yellow) by adsorption over waste materials (Bottom Ash and De-Oiled Soya). J. Hazard. Mater. 151, 821–832 (2008)
Jain, A.K., Gupta, V.K., Bhatnagar, A., Suhas, A.: A Comparative Study of Adsorbents Prepared from Industrial Wastes for Removal of Dyes. Sep. Sci. Technol. 38, 463–481 (2003)
Karthikeyan, S., Gupta, V.K., Boopathy, R., Titus, A., Sekaran, G.: A new approach for the degradation of high concentration of aromatic amine by heterocatalytic Fenton oxidation: kinetic and spectroscopic studies. J. Mol. Liquids 173, 153–163 (2012)
Cronje, K.J., Chetty, K., Carsky, M., Sahu, J.N., Meikap, B.C.: Optimization of chromium(VI) sorption potential using developed activated carbon from sugarcane bagasse with chemical activation by zinc chloride. Desalination 275, 276–284 (2011)
Bhatnagar, A., Hogland, W., Marques, M., Sillanpää, M.: An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 219, 499–511 (2013)
Gupta, V.K., Ali, I., Saini, V.K.: Defluoridation of wastewaters using waste carbon slurry. Water Res. 41, 3307–3316 (2007)
Gupta, V.K., Srivastava, S.K., Mohan, D., Sharma, S.: Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste for the removal of some heavy metal ions. Waste Manage. 17, 517–522 (1997)
Ren, X., Chen, C., Nagatsu, M., Wang, X.: Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem. Eng. J. 170, 395–410 (2011)
Gupta, V.K., Agarwal, S., Saleh, T.A.: Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 185, 17–23 (2011)
Gupta, V.K., Ali, I., Saleh, T.A., Nayak, A., Agarwal, S.: Chemical treatment technologies for waste-water recycling-an overview. RSC Adv. 2, 6380–6388 (2012)
Saleh, T.A., Gupta, V.K.: Column with CNT/magnesium oxide composite for lead(II) removal from water. Environ. Sci. Pollut. Res. 19, 1224–1228 (2012)
Tofighy, M.A., Mohammadi, T.: Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 185, 140–147 (2011)
Nguyen, C., Ahmadpour, A., Do, D.D.: Effect of Gasifying Agents on the Characterization of Nutshell-derived Activated Carbon. Adsorp. Sci. Tech. 12, 247–258 (1995)
Wibowo, N., Setiyadhi, L., Wibowo, D., Setiawan, J., Ismadji, S.: Adsorption of benzene and toluene from aqueous solutions onto activated carbon and its acid and heat treated forms: influence of surface chemistry on adsorption. J. Hazard. Mater. 146, 237–242 (2007)
Ahmadpour, A., Tahmasbi, M., Rohani, T.: Rapid removal of cobalt ion from aqueous solution by almond green hull. J. Hazard. Mater. 166, 925–930 (2009)
Ngah, W.S.W., Hanafiah, M.A.K.M.: Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour. Tech. 99, 3935–3948 (2008)
Mohan, D., Pittman, C.: Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. B137, 762–811 (2006)
Di, Z.-C., Ding, J., Peng, X.-J., Li, Y.-H., Luan, Z.-K., Liang, J.: Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 62, 861–865 (2006)
Ho, Y.S.: Citation review of Lagergren Kinetic rate equation on adsorption reactions. Scient. 59, 171–177 (2004)
Rao, M., Parwate, A.V., Bhole, A.G.: Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manage. 22, 821–830 (2002)
Daneshvar, N., Salari, D., Aber, S.: Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 94, 49–61 (2002)
Oliveira, E.A., Montanher, S.F., Andrade, A.D.: N´obrega, JA, Rollemberg, MC: equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem. 40, 3485–3490 (2005)
Hu, J., Chen, G., Lo, I.M.C.: Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 39, 4528–4536 (2005)
Agarwal, G.S., Bhuptawat, H.K., Chaudhari, S.: Biosorption of aqueous chromium(VI) by Tamarindus indica seeds. Bioresour. Technol. 97, 949–956 (2006)
Dakiky, M., Khamis, M., Manassra, A.: Mer’eb, M: selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 6, 533–540 (2002)
Acar, F.N., Malkoc, E.: The removal of chromium(VI) from aqueous solutions by Fagus orientalis L. Bioresour. Technol. 94, 13–15 (2004)
Tunali, S., Kiran, I., Akar, T.: Chromium(VI) biosorption characteristics of Neurospora crassa fungal biomass. Min. Eng. 18, 681–689 (2005)
Singh, V.K., Tiwari, P.N.: Removal recovery of chromium(VI) from industrial waste water. J. Chem. Technol. Biotechnol. 69, 376–382 (1997)
Park, S.-J., Jang, Y.-S., Shim, J.-W., Ryu, S.-K.: Studies on pore structures and surface functional groups of pitch-based activated carbon fibers. J. Colloid Interface Sci. 260, 259–264 (2003)
Rivera-Utrilla, J., Sanchez-Polo, M.: Adsorption of Cr(III) on ozonised activate carbon. Importance of Cπ-cation interactions. Water Res. 37, 3335–3340 (2003)
Hamadi, N.K., Chen, X.D., Farid, M.M., Lu, M.G.Q.: Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 84, 95–105 (2001)
Lyubchik, S.I., Lyubchik, A.I., Galushko, O.L., Tikhonova, L.P., Vital, J., Onseca, I.M., Lyubchik, S.B.: Kinetics and thermodynamics of the Cr(III) adsorption on the activated carbon from co-mingledwastes. Colloids Surf. A: Physicochem. Eng. Aspects 242, 151–158 (2004)
Gonzalez-Serrano, E., Cordero, T., Rodriguez-Mirasol, J., Cotoruelo, L., Rodriguez, J.J.: Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors. Water Res. 38, 3043–3050 (2004)
Khezami, L., Capart, R.: Removal of chromium(VI) from aqueous solution by activated carbons: kinetic and equilibrium studies. J. Hazard. Mater. 123, 223–231 (2005)
Boddu, V.M., Abburi, K., Talbott, J.L., Smith, E.D.: Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 37, 4449–4456 (2003)
Aksu, Z., Acikel, U., Kabasakal, E., Tezer, S.: Equilibrium modeling of individual and simultaneous biosorption of chromium(VI) and nickel(II) onto dried activated sludge. Water Res. 36, 3063–3073 (2002)
Acknowledgments
The authors gratefully acknowledge the financial support of the project from Khorasan Razavi Regional Water organization and help of chemical engineering research lab in FUM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Ahmadpour, A., Eftekhari, N. & Ayati, A. Performance of MWCNTs and a low-cost adsorbent for Chromium(VI) ion removal. J Nanostruct Chem 4, 171–178 (2014). https://doi.org/10.1007/s40097-014-0119-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-014-0119-9