Abstract
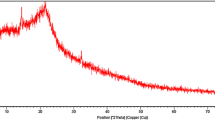
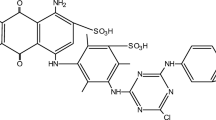
The organo-attapulgite was prepared using hexadecyltrimethylammonium bromide (HTMAB) with equation equivalent ratio of HTMAB to CEC of attapulgite added and then used as adsorbent for the removal of Congo red (CR) anionic dye from aqueous solution. Adsorbent characterizations were investigated using infrared spectroscopy and X-ray diffraction. The effects of contact time, temperature, pH and initial dye concentration on organo-attapulgite adsorption for CR were investigated. The results show that the amount adsorbed of CR on the organo-attapulgite increase with increasing dye concentration, temperature, and by decreasing pH. The adsorption kinetics was studied with the pseudo-first-order, pseudo-second-order and intraparticle diffusion models, and the rate constants were evaluated. It was found that the adsorption mechanisms in the dye/organo-attapulgite system follow pseudo-second-order kinetics with a significant contribution of film diffusion. Equilibrium data fitted perfectly with Langmuir isotherm model compared to Freundlich isotherm model, and the maximum adsorption capacity was 189.39 mg g−1 for the adsorbent. Kinetic and desorption studies both suggest that chemisorption should be the major mode of CR removal by the organo-attapulgite. The results indicate that HTMAB-modified attapulgite could be employed as low-cost material for the removal of Congo red anionic dye from wastewater.
Similar content being viewed by others
References
Aksu, Z.: Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of lead(II) ions onto Chlorella vulgaris. Process Biochem. 38(1), 89–99 (2002)
Al-Degs, Y., Khraisheh, M.A.M., Allen, S.J., : Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res. 34(3), 927–935 (2000)
Al-Futaisi, A., Jamrah, A., Al-Rawas, A., : Adsorption capacity and mineralogical and physico-chemical characteristics of Shuwaymiyah palygorskite (Oman). Environ. Geol. 51(8), 1317–1327 (2007)
Alkan, M., Demirbaş, Ö., Çelikçapa, S., Doğan, M.: Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 116(1–2), 135–145 (2004)
Alkan, M., Çelikçapa, S., Demirbaş, Ö.: Removal of reactive blue 221 and acid blue 62 anionic dyes from aqueous solutions by sepiolite. Dyes Pigments 65(3), 251–259 (2005)
Armağan, B., Turan, M., Çelik, M.S.: Equilibrium studies on the adsorption of reactive azodyes into zeolite. Desalination 170(1), 33–39 (2004)
Bache, B.W.: The measurement of cation exchange capacity of soils. J. Sci. Food Agric. 27, 273–280 (1976)
Baskaralingam, P., Pulikesi, M., Elango, D., Ramamurthi, V., Sivanesan, S.: Adsorption of acid dye onto organobentonite. J. Hazard. Mater. 128(2–3), 138–144 (2006)
Boyd, G.E., Adamson, A.W., Meyers, L.S.: The exchange adsorption of ions from aqueous solution by organic zeolites. II: Kinetics. J. Am. Chem. Soc. 69, 2836–2848 (1947)
Chen, H., Wang, A.Q.: Kinetic and isothermal studies of lead ions adsorption onto palygorskite clay. J. Colloid Interface Sci. 307(2), 309–316 (2007)
Chen, H., Zhao, Y.G., Wang, A.Q.: Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 149(2), 346–354 (2007)
Chiou, M.S., Ho, P., Ho, Y., Li, H.Y.: Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 60(1), 69–84 (2004)
Czímerová, A., Bujdák, J., Gáplovský, A.: The aggregation of thionine and methylene blue dye in smectite dispersion. Colloids Surf. A: Physicochem. Eng. Aspects 243(1–3), 89–96 (2004)
Freundlich, H.M.F.: Über die adsorption in lösungen. Z. Phys. Chem. 57, 385–470 (1906)
Frost, R.L., Locos, O.B., Ruan, H., Kloprogge, J.T.: Near-infrared and mid-infrared spectroscopic study of sepiolites and palygorskites. Vib. Spectrosc. 27(1), 1–13 (2001)
Galan, E.: Properties and applications of palygorskite-sepiolite clays. Clay Miner. 31(4), 443–453 (1996)
Giles, C.H., MacEwan, S.N., Nakhwa, S.N., Smith, D.: Studies in adsorption. Part XI. A system of classification of solution adsorption isotherm, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface area in solids. J. Chem. Soc. 3, 3973–3993 (1960)
Gong, R., Ding, Y., Li, M., Yang, C., Liu, H., Sun, Y.: Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pigments 64(3), 187–192 (2005)
Gupta, G.S., Prasad, G., Singh, V.N.: Removal of chrome dye from aqueous solutions by mixed adsorbents: Fly ash and coal. Water Res. 24(1), 45–50 (1990)
Halliday, P.J., Beszedits, S.: Color removal from textile mill wastewaters. Can. Text. J. 103, 78–84 (1986)
Harris, R.G., Wells, J.D., Johnson, B.B.: Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces. Colloids Surf. A: Physicochem. Eng. Aspects 180(1–2), 131–140 (2001)
Ho, Y.S., McKay, G.: Pseudo-second order model for sorption processes. Process Biochem. 34(5), 451–465 (1999)
Hu, Q.H., Qiao, S.Z., Haghseresht, F., Wilson, M.A., Lu, G.Q.: Adsorption study for removal of basic red dye using bentonite. Ind. Eng. Chem. Res. 45(2), 733–738 (2006)
Huang, J.H., Liu, Y.F., Jin, Q.Z., : Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J. Hazard. Mater. 143(1–2), 541–548 (2007)
Kannan, N., Sundaram, M.M.: Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pigments 51(1), 25–40 (2003)
Lagergren, S.: About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetensk. Handl. 24(4), 1–39 (1898)
Langmuir, I.: The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 40(9), 1361–1403 (1918)
Lorenc-Grabowska, E., Gryglewicz, G.: Adsorption characteristics of Congo red on coal-based mesoporous activated carbon. Dyes Pigments 74(1), 34–40 (2007)
Mall, I.D., Srivastava, V.C., Kumar, G.V.A., Mishra, I.M.: Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf. A: Physicochem. Eng. Aspects 278(1–3), 175–187 (2006)
Mckay, G., Otterburn, M.S., Aga, J.A.: Fuller’s earth and fired clay as adsorbents for dyestuffs. Water Air Soil Pollut. 24(3), 307–322 (1985)
Neamtu, M., Yediler, A., Siminiceanu, I., Macoveanu, M., Kellrup, A.: Decolorization of disperse red 354 azo dye in water by several oxidation processes-a comparative study. Dyes Pigments 60(1), 61–68 (2004)
Nir, S., Undabeytia, T., Yaron-Marcovich, D., El-Nahhal, Y., : Optimization of adsorption of hydrophobic herbicides on montmorillonite preadsorbed by monovalent organic cations: interaction between phenyl rings. Environ. Sci. Technol. 34(7), 1269–1274 (2000)
Ogawa, M., Kawai, R., Kuroda, K.: Adsorption and aggregation of a cationic cyanine dye on smectites. J. Phys. Chem. 100(40), 16218–16221 (1996)
Özcan, A.S., Erdem, B., Özcan, A.: Adsorption of Acid Blue 193 from aqueous solutions onto BTMA-bentonite. Colloids Surf. A: Physicochem. Eng. Aspects 266(1–3), 73–81 (2005)
Özcan, A.S., Tetik, S., Özcan, A.: Adsorption of acid dyes from aqueous solutions onto sepiolite. Sep. Sci. Technol. 39(2), 301–320 (2004)
Özcan, A., Öncü, E.M., Özcan, A.S.: Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A: Physicochem. Eng. Aspects 277(1–3), 90–97 (2006)
Özcan, A.S., Özcan, A.: Adsorption of acid dyes from aqueous solutions onto acid-activated bentonite. J. Colloid Interface Sci. 276(1), 39–46 (2004)
de Paiva, L.B., Morales, A.R., Valenzuela Díaz, F.R.: Organoclays: Properties, preparation and applications. Appl. Clay Sci. 42(1–2), 8–24 (2008)
Patil, S.S., Shinde, V.M.: Biodegradation studies of aniline and nitrobenzene in aniline plant wastewater by gas chromatography. Environ. Sci. Technol. 22(10), 1160–1165 (1988)
Pelekani, C., Snoeyink, V.L.: A kinetic and equilibrium study of competitive adsorption between atrazine and Congo red dye on activated carbon: the importance of pore size distribution. Carbon 39(1), 25–37 (2001)
Rao, K.C.L.N., Ashutosh, K.K.: Color removal from a dyestuff industry effluent using activated carbon. Indian J. Chem. Technol. 1, 13–19 (1994)
Rao, V.V.B., Rao, S.R.M.: Adsorption studies on treatment of textile dyeing industrial effluent by flyash. Chem. Eng. J. 116(1), 77–84 (2006)
Reichenberg, D.: Properties of ion exchange resins in relation to their structure. III. Kinetics of exchange. J. Am. Chem. Soc. 75(3), 589–597 (1953)
Seshadri, S., Bishop, P.L., Agha, A.M.: Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag. 15(2), 127–137 (1994)
Tahir, S.S., Rauf, N.: Removal of a cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63(11), 1842–1848 (2006)
Wahi, R.K., Yu, W.W., Liu, Y.P., : Photodegradation of Congo Red catalyzed by nanosized TiO2. J. Mol. Catal. A: Chem. 242(1–2), 48–56 (2005)
Wang, C.C., Juang, L.C., Hsu, T.C., Lee, C.K., Lee, J.F., Huang, F.C.: Adsorption of basic dyes onto montmorillonite. J. Colloid Interface Sci. 273(1), 80–86 (2004)
Wang, S., Boyjoo, Y., Choueib, A.: A comparative study of dye removal using fly ash treated by different methods. Chemosphere 60(10), 1401–1407 (2005)
Wang, S., Li, H., Xu, L.: Application of zeolite MCM-22 for basic dye removal from wastewater. J. Colloid Interface Sci. 295(1), 71–78 (2006)
Weber, W.J. Jr., Morriss, J.C.: Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 89, 31–60 (1963)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 15, 381–389 (2009). https://doi.org/10.1007/s10450-009-9155-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-009-9155-z