Abstract
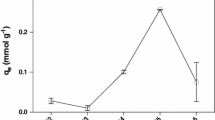
The biosorption of Cu(II) ions on Cladophora crispata was investigated as a function of the initial pH, temperature and initial Cu(II) ion concentration. Algal biomass exhibited the highest Cu(II) uptake capacity at 25∘C and at the initial pH of 4.5. Equilibrium data fitted very well to both the Langmuir and Freundlich isotherm models. The pseudo second order kinetic model was applied to describe the kinetic data and the rate constants were evaluated in the studied concentration range of Cu(II) ions at all the temperatures studied. The experimental data fitted well to the pseudo second order kinetic model with a high correlation coefficient (R 2 > 0.99), which indicates that the external mass transfer limitations in the system can be neglected and the chemical sorption is the rate-limiting step. The pseudo second order kinetic constants were also used to calculate the activation energy of Cu(II) biosorption.
Similar content being viewed by others
References
Aksu, Z., Y. Saĝ, and T. Kutsal, “The Biosorption of Copper(II) by C. vulgaris and Z. ramigera,” Environ. Technol., 13, 579–586 (1992).
Aksu, Z. and S. Tezer, “Equilibrium and Kinetic Modelling of Biosorption of Remazol Black B by R. arrhizus in a Batch System: Effect of Temperature,” Process Biochemistry, 36, 431–439 (2000).
Aksu, Z., “Equilibrium and Kinetic Modelling of Cadmium(II) Biosorption by C. vulgaris in a Batch System: Effect of Temperature,” Separation and Purification Technology, 21, 285–294 (2001).
Akthar, N., S. Sastry, and M. Mohan, “Biosorption of Silver Ions by Processed Aspergillus niger Biomass,” Biotech. Letters, 17, 551–556 (1995).
Allen, S.J. and P.A. Brown, “Isotherm Analysis for Single Component and Multi-Component Metal Sorption onto Lignite,” J. Chem. Technol. Biotechnol., 62, 17–24 (1995).
Anoop Krishnan, K. and T.S. Anirudhan, “Removal of Mercury(II) from Aqueous Solutions and Chlor-Alkali Industry Effluent by Steam Activated and Sulphurised Activated Carbons Prepared from Bagasse Pith: Kinetics and Equilibrium Studiees,” Journal of Hazardous Materials, B92, 161–183 (2002).
Asmal, M, A.H. Khan, S. Ahmad, and A. Ahmad, “Cole of Sawdust in the Removal of Copper(II) from Industrial Wastes,” Water Research, 32, 3085–3091 (1998).
Bedell, G.W. and D.W. Darnall, “Immobilization of Nonviable, Biosorbent, Algal Biomass for the Recovery of Metal Ions,” Biosorption of Heavy Metals, B. Volesky (Ed.), pp. 314–326, CRC Press, Boca Raton, 1990.
Benguella, B. and H. Benaissa, “Cadmium Removal from Aqueous Solutions by Chitin: Kinetic and Equilibrium Studies,” Water Research, 36, 2463–2474 (2002).
Chang, J.S., R. Law C.C. Chang,“Biosorption of Lead, Copper and Cadmium by Biomass of Pseudomonas aeruginosa PU21,” Water Research, 31, 1651–1658 (1997).
Chen, P. and Y.P. Ting, “Effect of Heavy Metal Uptake on the Electrokinetic Properties of Saccharomyces cerevisiae,” Biotechnology Letters, 17, 107–112 (1995).
Chiou, M.S. and H.Y. Li, “Equilibrium and Kinetic Modeling of Adsorption of Reactive Dye on Cross-Linked Chitosan Beads,” J of Hazardous Materials, B93, 233–248 (2002).
Crist, R.H., K. Oberholser, N. Shank, and M. Nguyen, “Nature of Bonding Between Metallic Ionc and Algal Cell Walls,” Environ. Sci. Technol., 15, 1212–1217 (1981).
Cruz, C.C.V., A.C. A.da Costa, and A.S. Henriques Cai Luna, “Kinetic Modeling and Equilibrium Studies During Cadmium Biosorption by Dead Sargassum sp. Biomass,” Bioresource Technology, 91, 249–257 (2004).
Darnall D.W., B. Greene, M. Hosea, R.A. McPherson, M. Henzl, and M.D. Alexander, “Recovery of Heavy Metals by Immobilized Algae: In Trace Metal Removal from Aqueous Solutions,” in Industrial Division of the Royal Society of Chemistry Annual Chemical Congress, R. Thomson (Ed.), pp. 1–24, UK, 1986.
Elliott, H.A. and C.P. Huang, “Adsorption Characteristic of Some Cu(II) Complexes on Alumino Silicates,” Water Research, 15, 849–855 (1981).
Esposito, A., F. Pagnelli, and F. Veglio, “pH-Related Equilibria Models for Biosorption in Single Metal Systems,” Chemical Engineering Science, 57, 307–313 (2002).
Friis, N. and P. Myers-Keith, “Biosorption of Uranium and Lead by Streptomyces longwoodensis,” Biotech. and Bioeng., 28, 21–28 (1989).
Holan, Z.R. and B. Volesky, “Biosorption of Lead and Nickel by Biomass of Marine Algae,” Biotech. and Bioeng, 43, 1001–1009 (1994).
Ho, Y.S. and G. McKay, “Pseudo-Second Order Model for Sorption Processes,” Process Biochemistry, 34, 451–465 (1999a).
Ho, Y.S. and G. McKay, “The Sorption of Lead(II) Ions on Peat,” Water Research, 33, 578–584 (1999b).
Ho, Y.S. and G. McKay, “Competitive Sorption of Copper and Nickel Ions from Aqueous Solution Using Peat,” Adsorption, 5, 409–417 (1999c).
Ho, Y.S. and G. McKay, “The Kinetics Sorption of Divalent Metal Ions Onto Sphagnum Moss Peat,” Water Research, 34, 735–742 (2000).
Huang, J.P., C.P. Huang, and A.L, Morehart, “Removal of Heavy Metals by Fungal (Aspergillus oryzae) Adsorption,” Heavy Metals in the Environment, J.P. Vernet (Ed.), pp. 329–349, Elsevier, London, 1991.
Kaewsarn, P., “Biosorption of Copper(II) from Aqueous Solutions by Pre-Treated Biomass of Marine Algae Padina sp.,” Chemosphere, 47, 1081–1085 (2002).
Kratochvil D., E. Fourest and B. Volesky, “Biosorption of Copper by Sargassum fluitans Biomass in a Fixed Bed Column,” Biotechnology Letters, 17, 777–782 (1995).
Manohar, D.M., K. Anoop Krishnan, and T.S. Anirudhan, “Removal of Mercury(II) from Aqueous Solutions and Chlor-Alkali Industry Wastewater Using 2-mercaptobenzimidazole-Clay,” Water Research, 36, 1609–1619 (2002).
Mathialagon, T. and T. Viraraghavan. “Adsorption of Cadmium from Aqueous Solutions by Perlite,” J. Hazardous Material, B94, 291–303 (2002).
Özer, A. and D. Özer, “Modelling of Copper(II) Adsorption by Using Saccharomyces cerevisiae in Batch Stirred Reactors in Series,” Chimica Acta Turcica, 26, 75–80 (1998).
Özer, A., D. Özer, and H.İ. Ekiz, “Application of Freundlich and Langmuir Models to Multistage Purification Process to Remove Heavy Metal Ions by Using Schizomeris leibleinii,” Process Biochemistry, 34, 919–927 (1999).
Palmieri, M.C., O. Jr. Garcia, and P. Melnikov, “Neodymium Biosorption from Acidic Solutions in Batch System,” Process Biochemistry, 36, 441–444 (2000).
Saĝ, Y. and T. Kutsal, “Determination of the Biosorption Heats of Heavy Metal Ions on Zooglea ramigera and Rhizopus arrhizus,” Biochem. Eng. J., 6, 145–151 (2000).
Say, R., A. Denizli, M.Y. Aríca,“Biosorption of Cadmium(II), Lead(II) and Copper(II) with the Filamentous Fungus P. Chrysosporium,” Bioresource Technology, 76, 67–70 (2001).
Scott, J.A. and A.M. Karanjkar, “Adsorption Isotherms and Diffusion Coefficients for Metals Biosorbed by Biofilm Coated Granular Activated Carbon,” Biotechnology Letters, 17, 1267–1270 (1995).
Smith, J.M., Chemical Engineering Kinetics, pp. 314–320, McGraw-Hill, Chemical Engineering Series, Singapore, 1981.
Ting, Y.P., F. Lawson, and I.G. Prince, “Uptake of Cadmium and Zinc by the Alga Chlorella vulgaris Part I: Individual Ion Species,” Biotech. and Bioeng., 34, 990–999 (1989).
Tobin, J.M., D.G. Copper, and R.J. Neufeld, “Uptake of Metal Ions by Rhizopus arrhizus Biomass,” Appl. Environ. Microbiol., 47, 821–824 (1984).
Treybal, R.E., Mass-Transfer Operations, pp. 566–575, McGraw-Hill, Singapore, 1980.
Tsezos, M., S.H. Noh, and M.A. Baird, “Batch Reactor Mass Transfer Kinetic Model for Immobilized Biomass Biosorption,” Biotech. and Bioeng., 32, 545–553 (1988).
Tsezos, M. and B. Volesky, “Biosorption of Uranium and Thorium,” Biotech. and Bioeng., 25, 583–604 (1981).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ÖZER, A., Özer, D. & İbrahim Ekİz, H. The Equilibrium and Kinetic Modelling of the Biosorption of Copper(II) Ions on Cladophora crispata . Adsorption 10, 317–326 (2005). https://doi.org/10.1007/s10450-005-4817-y
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10450-005-4817-y