Abstract
This work reports the application of Posidonia oceanica for the elimination of heavy metals M(II) (M = Zn, Cd, Ni, Cu and Pb) by biosorption in batch system. The effect of the contact time, initial M concentration, pH and temperature was considered. The kinetic and equilibrium models for the M-biosorption were tested namely the pseudo-first-order, pseudo-second-order and Elovich kinetic models. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms models have also been used to fit equilibrium adsorption data. The adsorption kinetics follow a pseudo-second-order model for all studied systems, and the equilibrium data are suitably fitted by the above models. The amount of adsorbed metals by biosorption is 48.33, 43.9, 41.02, 37.90 and 30.22 mg/g for Pb(II), Cu(II), Ni(II), Zn(II) and Cd(II), respectively. The thermodynamic parameters suggested that the metal biosorption is spontaneous with an endothermic nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The environmental pollution by heavy metals is a serious threat and a complex problem that has been and is still a focus of attention throughout the world since they are non-biodegradable and exist in all the food chain. Many organic, inorganic and biological substances have been reported as water contaminants (Gupta et al. 2002; Saravanan et al. 2013), whose toxicity has been observed to exceed the permissible limit (Gupta and Saleh 2013). The metals are among the major pollutants in surface and underground waters, and both industrial and municipal wastewaters contain metal ions which are hazardous to the human health (Krishnani et al. 2008; Gupta et al. 2013). Waste streams containing toxic metals are often encountered in the industry and can be the source of metals in the aquatic environment. Copper is widely used in various fields such as plating, mining and electroplating, refining of oil, etc.… (Demirbas et al. 2009). Cadmium is employed in batteries, paints and plastics (Low et al. 2000), whereas zinc is used in acid galvanizing plants (Tunali and Akar 2006). The industry also uses nickel in various products (detergents, metal-based jewelry, steel and many other metal products) (Venkateswarlu et al. 2015). All these metals are not biodegradable and have negative effects on the flora and fauna (Rozaini et al. 2010). They cause chronic bronchitis and reduced lung function (cancer of the lungs) (Ahmaruzzaman and Gupta 2011).
The Environmental Protection Agency (EPA) has included these metals on the list of priority pollutants (Volesky 2001). However, their removal from water is a very difficult task due to the high cost of treatment methods. Conventional techniques of removing metals from wastewater are high cost with some limitations. They include chemical precipitation, mainly hydroxides and sulfides, membrane filtration (reverse osmosis, nano-filtration), electrolytic reduction, solvent extraction, electrocoagulation, ions exchange and adsorption (Huang and Wu 1977; Calugaru et al. 2016; Juang and Shiau 2000; Gupta and Ali 2004; Heidmann and Calmano 2008; Qiu and Zheng 2009; Bessbousse et al. 2008).
The elimination of metals by biosorption remains an attractive alternative and is promising because of its potential application in the environmental protection. The biosorption uses biological materials such as raw rice husk (Mohan and Sreelakshmi 2008), olive pomace (Pagnanelli et al. 2005), wood bark (Palma et al. 2003), peat (Ma and Tobin 2003), lignin (Wu et al. 2008) and agro-industrial wastes (Johnson et al. 2002; Nguyen et al. 2013). The main advantage of the biosorption is the low cost, high yield, decrease in both chemical and biological sludge, lack of nutrient requirements, biosorbent regeneration and uptake of metal recovery.
Marine biomass including chitosan, chitin, crab shell and algae represents an important resource for the biosorption; the biomass considered in this study is Posidonia oceanica. The endemic herbarium P. oceanica occupies a central position in the ecology of the Mediterranean Sea, its importance lies in its extension, high productivity and stability, and its grasslands are functional as a spawning area, hunting area or permanent habitat for different plants and animals. In addition, it possesses chelating properties toward heavy metals which makes it promising in biosorption. This ability to uptake toxic metals is attributed to the existence of alginate and to the porosity of the cell wall which allows small ionic species (Boudouresque et al. 2012).
The aim of this research is the evaluation of the performance and efficiency of P. oceanica as a new biosorbent for the M(II) elimination (M = Ni, Cd, Cu, Pb and Zn). The influence of physical parameters affecting the biosorption such as pH, contact time, M(II) concentration, temperature and biosorbent dose was studied. The adsorption is studied in terms of pseudo-first- and pseudo-second-order kinetics, and different isotherm models are applied to experimental data to obtain information on the interaction metal/P. oceanica.
Materials and methods
Adsorbent preparation and characterization
Posidonia oceanic was collected along the shoreline of the western region of Algiers (Tipaza). This harvest site was chosen because of the high abundance of submarine herbariums of this phanerogam which is therefore conducive to the formation of balls resulting from the fraying of dead leaves. The balls were washed and dried at 40 °C for 48 h. This temperature allows desiccation of the material without affecting its structural and functional characteristics. Dried waste was reduced to a fine powder in a centrifugal ball mill for 45 min (400 rpm) and sieved to get a size fraction less than 160 μm.
The BET specific surface area was determined by adsorption–desorption isotherms of N2 at 77 K using a Micromeritics ASAP 2010 apparatus. The FTIR spectroscopy was used to give a qualitative analysis of the main chemical groups present on the biomass and responsible of the adsorption. The translucent samples disks were prepared by mixing 0.1 g of biomass/g of KBr; the spectra were recorded with a PerkinElmer Spectrum Tow. The point of zero charge (pzc) was determined by simple electrolyte addition method; 0.1 g of P. oceanica fibers was immersed into 50 mL of KNO3 (0.05 M) at different pH solutions and shaken at constant speed for 24 h. After decantation, the difference between the initial and final pHs was plotted against the initial pH (Mahmood et al. 2011). The same suspension was used to determine the zeta potential, measured with Mastesizer 3600, Malvern. Scanning electron microscopy (SEM, Jeol-JSM-6360 LV) provides a view of the morphological structure of the biosorbent.
Adsorbate
Stock M(II) solutions (1000 mg/L) with which the experiments were conducted were prepared by dissolving nitrate salts: Ni(NO3)2, Zn(NO3)2, Pb(NO3)2, Cd(NO3)2 and Cu(NO3)2 in distilled water; other concentrations (30–250 mg/L) were prepared by dilution. HCl and NaOH solutions (0.1 M) were used for the pH adjustment.
Biosorption experiment
The experimental tests were conducted in batch mode by varying pH from 2 to 8, temperatures (20, 30 and 40 °C), P. oceanica dose (0.05–0.5 mg) and M(II) concentrations (30–250 mg/L). For each experiment, an accurate quantity of P. oceanica was added to 50 mL of M(II) solutions in conical flasks (100 mL), shaken in a thermostatic shaker (300 rpm). The suspensions were filtered with Whatman 41 filter paper. The M(II) quantities before and after equilibrium were analyzed by atomic adsorption spectrometry (PerkinElmer 3030), and the adsorbed amount (qe) was calculated from the formula.
where C0 and Ce are the initial and equilibrium concentrations (mg/L), m the amount of adsorbent (g), and V the volume of solution (L). The removal efficiency percentage of metal (E%) was calculated as follows:
Biosorption kinetics and equilibrium
Kinetics
The kinetics were studied in conical flasks; 0.1 mg of biosorbent was contacted with 50 mL of M(II) solution (100 mg/L); the concentration was determined at regular time intervals. The adsorption kinetic data were fitted by the pseudo-first-order, pseudo-second-order and Elovich models.
The pseudo-first order is given by Belhamdi et al. (2016):
where qe and qt (mg g−1) are the amounts of M(II) adsorbed at equilibrium and time t(min), respectively and k1 the equilibrium rate constant (min−1). qe and k1 are calculated from the slope and intercept of the plot \( {\text{ln}}\left( {q_{\text{e}} - q_{\text{t}} } \right) \) versus t.
The linear form of pseudo-second-order equation is expressed by the relation (Belhamdi et al. 2016; Ghaedi et al. 2015):
where k2 (g mg−1 min−1) is the equilibrium rate constant. qe and k2 are calculated from the plot \( \frac{t}{{q_{t} }} \) versus t.
The Elovich model was applied satisfactorily to chemisorption processes (Hannachi 2012):
where a (mg g−1 min−1) is the initial biosorption rate and b (g mg−1) the desorption constant related to the extent of surface coverage and activation energy; they are obtained from the linear plot of qt versus ln t.
Equilibrium
Equilibrium studies were carried out at 20, 30 and 40 °C. The biomass (0.1 mg) was contacted with M(II) solutions in the concentrations range (30–250 mg/L) at pH 6, for 80 min.
The Freundlich, Langmuir, Temkin and Dubinin–Radushkevich (D–R) models were used for describing the solid–liquid adsorption system.
The Freundlich model assumes a heterogeneous surface with adsorption sites of different energies (Freundlich 1907; Mittal et al. 2010):
where KF is a constant related to the bonding energy, 1/n the heterogeneity factor and n (g L−1) measures the deviation from linearity of adsorption. The equilibrium constants are determined from the plot log qe versus log Ce. The regression coefficient (R2) measures the fit goodness of the experimental data to the isotherm models.
The Langmuir adsorption model considers specific homogeneous sites on the surface, when a site is occupied by a molecule; no further adsorption can take place and is expressed by Ahmad et al. (2018) and Mohammadi et al. (2011):
where KL is a constant related to the energy of adsorption and qmax the maximum adsorption capacity (mg g−1). They are calculated from the linear plot of Ce/qe versus Ce.
The Temkin model assumes that the adsorption energy decreases linearly with the surface coverage due to adsorbent–adsorbate interactions (Dada et al. 2012); its linear form is given by:
where br is a constant related to heat of sorption (J mol−1) and KT the isotherm constant (L g−1); these constants were obtained from the plot of qe versus ln Ce.
The D–R model is semiempirical, and the adsorption follows a pore-filling mechanism. It assumes that the adsorption has a multilayer character and involves Van Der Waals forces; it is applicable for physical adsorption, and the linear form is expressed by Ahmad et al. (2018):
where qm is the D–R constant (mg g−1), β the constant related to free energy and \( \varepsilon \) the Polanyi potential which is defined as:
Results and discussion
Characterization of biosorbent
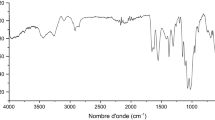
The FTIR spectra of P. oceanica (data not shown) show a peak at 3333 cm−1 due to (–OH) or amine groups (–NH), while the band at 2903 cm−1 represents the stretching of –CH groups. The bands 1407 and 772 cm−1 are attributed to –CH bending, and those observed at 1720 and 1672 cm−1 are assigned to C=O stretching groups. The peak at 1600 cm−1 could be attributed to N–H bending, while those at 1407 and 1027 cm−1 are assigned to –CN stretching. Similar results are reported on the copper removal by P. oceanica (Hannachi et al. 2014).
A relationship exists between the zeta potential and biosorption capacity; the latter varies with pH and helps to understand the behavior of suspended biomaterials in solution (Feng et al. 2009).
The zeta potential was determined under different pHs (Fig. 1), and the potential of the biomass decreases from − 5.9 to − 18.9 mV with increasing pH (6–11). The negative charges on the biosorbent surface could be explained by the alkalization of the solution. Indeed, according to Kam and Gregory (2001), basic pH induces the hydrolysis and loss of cationic groups and reduces the protonation of surface groups of the algae. The (–OH) groups deprotonate at high pH, it convert to O−, and such transformation generates negative charges on the surface (Park and Regalbuto 1995).
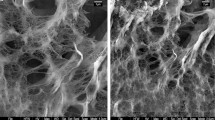
The P. oceanica fibers, characterized by SEM analysis (Fig. 2), have lignocellulosic fibrous structure with cylindrical fibers shape. Furrows are observed on the outer surface of the biomass, due to the dehydration of the cell wall. This morphology plays an important role in the adsorption and precipitation of M(II) on the external surface; other physicochemical are gathered in Table 1.
Effect of pH
The pH has a great influence on the M(II) removal by adsorption, because it directly affects the surface charge and the nature of ionic species of the adsorbates. In this context, the pH effect was studied in the range (2–8). The removal capacity of M(II) increases with increasing pH (Fig. 3); the minimal adsorption at pH 2 is due to the high concentration of H+ ions which compete with the M(II) adsorption and are preferentially fixed by carboxyl, hydroxyl and amine groups present on the P. oceanica surface (Pérez-Marín et al. 2007). When the pH increases, the biomass surface becomes negatively charged, leading to increased M(II) uptake and reaches equilibrium at pH 6. The retention capacity of M(II) decreases above pH 6 because of the apparition of insoluble M(OH)2, and all hydroxides have a solubility product smaller than 10−15. Adsorption and precipitation are the two mechanisms responsible for the M(II) elimination (Wang and Qin 2005).
Effect of biosorbent dose
The effect of biosorbent dose was carried out over the range (50–500 mg); these quantities were added to 50 mL of M(II) solution (100 mg/L), and the M(II) concentration was determined at equilibrium. The M(II) uptakes were found to increase linearly with increasing the dose (Fig. 4); they reach 97, 98, 88, 85 and 70% for Pb(II), Cu(II), Ni(II), Cd(II) and Zn(II), respectively, for a biomass dose of 250 mg. The availability of biosorption sites depends upon the biosorbent dose. The stability of M(II) uptake with increases in the biosorbent dose is due to the split in the flux between the M(II) concentration in the solution and the sorbent surface (Wang and Qin 2005).
Effect of the contact time
In order to control the effect of the contact time on the metal biosorption and to estimate the time sufficient to achieve equilibrium, the experiments were carried out at pH ~ 6, a biomass dose of 0.1 g, an initial M(II) concentration of 100 mg/L and a temperature of 25 °C. The concentration of M(II) in solution was determined at regular time intervals (Fig. 5). The biosorption increases over time and reaches equilibrium after 80 min. The removal efficiency of the biosorbent increases rapidly in the initial stage (0–30 min) because of the abundant active binding sites on the biomass (Madala et al. 2017) and becomes less efficient during the second stage (30–80 min) to reach a saturation; this can be explained by the interaction with the functional groups located on the biosorbent surface and intercellular accumulation (Liu et al. 2006). Based on these results, a shaking time of 80 min was suitable for further biosorption experiments.
Effect of the initial concentration of heavy metals
The effect of the M(II) initial concentration on the biosorption by P. oceanica is investigated by varying the initial M(II) concentration in solution from 30 to 250 mg/L, maintaining the other parameters constant. The results show that the retention capacity of metal by the biomass increases with raising the M(II) concentration in solution (Fig. 6). This due to the interaction M(II)/biosorbent which provides the driving force to overcome the resistance to the mass transfer of M(II) ions between the solution and solid phase. In addition, the enhanced biosorption with the M(II) concentration is due to an increase in the electrostatic interactions (physical adsorption versus covalent interactions) (Arshadi et al. 2014). The M(II) absorption can be attributed to various mechanisms of ion exchange and adsorption processes (Bektaş and Kara 2004; Buasri et al. 2007).
Kinetics studies
The biosorption kinetics of M(II) on P. oceanica were undertaken to establish the adsorption equilibrium time (Fig. 7) and kinetic parameters (Table 2). The validity of each model was verified by the fitness of the correlation coefficient (R2). The qe(cal) values calculated from the pseudo-first-order model differ from the experimental ones. By contrast, in the pseudo-second-order model, the calculated qe(cal) values are very close to qe(exp), and the R2 values converge to 1, indicating the validity of the pseudo-second order (Ncibi et al. 2009). For the Elovich model, the plots are not linear with bad coefficients R2, suggesting that the biosorption follows rather the pseudo-second-order kinetic model based on the assumption that the rate-determining step is a chemisorption, involving valence forces through sharing or exchange of electrons between adsorbent/adsorbate (Ghodbane and Hamdaoui 2008).
Isotherm studies
The isotherms data provide information on the capacity of the adsorbent or the amount required to remove a unit mass of pollutant under the operating conditions. In this study, the equilibrium data of M(II) adsorbed onto P. oceanica were fitted by the Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherms, and the corresponding parameters are given in Table 3. On the basis of correlation coefficients R2, it is concluded that the M(II) biosorption is better fitted to the Langmuir and Temkin models. This suggests that the biosorption of M(II) on the surface of P. oceanica occurs on homogeneous binding sites with equivalent adsorption energies and monolayer coverage (Ncibi et al. 2009).
Thermodynamic studies
To study the effect of thermal on the M(II) biosorption by P. oceanica, the temperature was varied from 20 to 40 °C using constant parameters at equilibrium. The test was performed below 40 °C to avoid evaporation of the solution. The M(II) biosorption at different temperatures (Figs. 8 and 9) shows that the uptake capacity increases with augmenting temperature and confirms the endothermic nature of retention of M(II) by Posidonia oceanic (Ghodbane and Hamdaoui 2008). The metal uptake is favored at higher temperature, since it activates the meal ions for enhancing the biosorption at the coordinating sites of the mineral (Rajamohan et al. 2014). The amounts of M(II) adsorbed versus the final concentration for different temperatures show that the biosorption creases with raising temperatures, confirming the endothermic nature and could be explained by availability of more active sites (Gupta and Rastogi 2008).
The thermodynamic parameters (ΔG°, ΔH° and ΔS°) were determined from the following equation:
where R is the universal gas constant. The thermodynamic equilibrium constant KC is determined by:
where CAe and Ce are the equilibrium concentrations of M(II) (mg/L) on adsorbent and in solution, respectively (Krika et al. 2016). The thermal effect on thermodynamic constant is determined by Eq. (13)
where ΔH° and ΔS° are calculated from the slope and intercept of the linear plot, of ln K versus 1/T. The free energy is given by Eq. (14):
The free energy (ΔG°) is negative and increases with temperature (Table 4), indicating that the best biosorption is obtained at higher temperature while the positive enthalpy ΔH° suggests an endothermic adsorption. A similar behavior was reported by Ahmaruzzaman and Gupta (2011) for the adsorption of heavy metals by rice husk ash, and the positive entropy ΔS° confirms the increased randomness at the solid–liquid interface. The necessity of heat to remove the M(II) ions from the solution makes the biosorption process endothermic (Al-Sou’od 2012).
Comparison with literature
The maximum biosorption capacity of P. oceanica for the removal of Pb(II), Cu(II), Ni(II), Zn(II) and Cd(II) was compared with that of other biosorbents, and the maximum biosorption capacities (qmax) are gathered in Table 5. It can be noteworthy that the P. oceanica biomass has a considerable potential for the removal of metals compared to those of the literature. The reduction of the above metals on semiconducting oxides upon solar light is our next objective; preliminary results were satisfactory and will be reported very soon.
Conclusion
The present work showed that P. oceanica fibers, a low cost biosorbent available abundantly at Algerian coasts, has an important capacity to remove the metals Zn(II), Ni(II), Cd(II), Cu(II) and Pb(II) from the aqueous medium. Based on the experimental results, it was concluded that:
- 1.
The retention rate increases with increasing the contact time until reaching equilibrium after 80 min;
- 2.
The maximum adsorption was determined in the pH range (6–8), close to the natural medium. The amount of adsorbed metals follows the sequence: 48.33, 43.9, 41.02, 37.90 and 30.22 mg/g, for Pb(II), Cu(II), Ni(II), Zn(II) and Cd(II), respectively;
- 3.
The elimination capacity of M(II) is influenced by the biosorbent dose which peaks at 2 g/L, allowing the elimination of 97, 98, 88, 85 and 70% of Pb(II), Cu(II), Ni(II), Cd(II) and Zn(II), respectively;
- 4.
The equilibrium data fit well with the Langmuir, Freundlich, Temkin and Dubinin–Radushkevich models;
- 5.
From thermodynamic studies, the free energy (ΔG°) is negative and increases with temperature. This indicated that better biosorption is obtained at higher temperature, thus reflecting the feasibility and spontaneous nature of the process. The positive enthalpy ΔH° suggested an endothermic biosorption.
References
Abu Al-Rub FA (2006) Biosorption of zinc on palm tree leaves: equilibrium, kinetics, and thermodynamics studies. Sep Sci Technol 41:3499–3515
Ahmad ZU, Yao L, Wang J, Gang DD, Islam F, Lian Q, Zappi ME (2018) Neodymium embedded ordered mesoporous carbon (OMC) for enhanced adsorption of sunset yellow: characterizations, adsorption study and adsorption mechanism. Chem Eng J 359:814–826
Ahmaruzzaman M, Gupta VK (2011) Rice husk and its ash as low-cost adsorbents in water and wastewater treatment. Ind Eng Chem Res 50:13589–13613
Al-Sou’od K (2012) Adsorption isotherm studies of chromium (VI) from aqueous solutions using Jordanian pottery materials. APCBEE Proc 1:116–125
Amarasinghe B, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132:299–309
Anayurt RA, Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd (II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem Eng J 151:255–261
Arshadi M, Amiri M, Mousavi S (2014) Kinetic, equilibrium and thermodynamic investigations of Ni (II), Cd (II), Cu (II) and Co (II) adsorption on barley straw ash. Water Resour Ind 6:1–17
Bektaş N, Kara S (2004) Removal of lead from aqueous solutions by natural clinoptilolite: equilibrium and kinetic studies. Sep Purif Technol 39:189–200
Belhamdi B, Merzougui Z, Trari M, Addoun A (2016) A kinetic, equilibrium and thermodynamic study of l-phenylalanine adsorption using activated carbon based on agricultural waste (date stones). J Appl Res Technol 14:354–366
Bessbousse H, Rhlalou T, Verchère J-F, Lebrun L (2008) Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly (ethyleneimine) in a poly (vinyl alcohol) matrix. J Membr Sci 307:249–259
Boudouresque C-F, Bernard G, Bonhomme P, Charbonnel E, Diviacco G, Meinesz A, Pergent G, Pergent-Martini C, Ruitton S, Tunesi L (2012) Protection and conservation of Posidonia oceanica meadows, RAMOGE and RAC/SPA
Buasri A, Chaiyut N, Ponpatcharasakul N, Artsalee P, Potisook S (2007) Factors affecting the removal of copper (II) and zinc (II) from aqueous solutions with clinoptilolite. J Res Eng Technol 4:1–17
Calugaru IL, Neculita CM, Genty T, Bussière B, Potvin R (2016) Performance of thermally activated dolomite for the treatment of Ni and Zn in contaminated neutral drainage. J Hazard Mater 310:48–55
Dada A, Olalekan A, Olatunya A, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2 + unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45
Demirbas E, Dizge N, Sulak M, Kobya M (2009) Adsorption kinetics and equilibrium of copper from aqueous solutions using hazelnut shell activated carbon. Chem Eng J 148:480–487
Deng L, Zhu X, Wang X, Su Y, Su H (2007) Biosorption of copper (II) from aqueous solutions by green alga Cladophora fascicularis. Biodegradation 18:393–402
Dursun AY (2006) A comparative study on determination of the equilibrium, kinetic and thermodynamic parameters of biosorption of copper (II) and lead (II) ions onto pretreated Aspergillus niger. Biochem Eng J 28:187–195
Fan T, Liu Y, Feng B, Zeng G, Yang C, Zhou M, Zhou H, Tan Z, Wang X (2008) Biosorption of cadmium (II), zinc (II) and lead (II) by Penicillium simplicissimum: isotherms, kinetics and thermodynamics. J Hazard Mater 160:655–661
Feng Q, Sugita S, Gong F (2004) Removal of lead ion from aqueous solution using rice husk ash. In: Tong Z, Kim SH (eds) Frontiers on separation science and technology, pp 807–813
Feng N, Guo X, Liang S (2009) Adsorption study of copper (II) by chemically modified orange peel. J Hazard Mater 164:1286–1292
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Ghaedi M, Hajjati S, Mahmudi Z, Tyagi I, Agarwal S, Maity A, Gupta VK (2015) Modeling of competitive ultrasonic assisted removal of the dyes—methylene blue and Safranin-O using Fe3O4 nanoparticles. Chem Eng J 268:28–37
Ghodbane I, Hamdaoui O (2008) Removal of mercury (II) from aqueous media using eucalyptus bark: kinetic and equilibrium studies. J Hazard Mater 160:301–309
Guler UA, Sarioglu M (2014) Mono and binary component biosorption of Cu (II), Ni (II), and Methylene Blue onto raw and pretreated S. cerevisiae: equilibrium and kinetics. Desalination Water Treat 52:4871–4888
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271:321–328
Gupta V, Rastogi A (2008) Sorption and desorption studies of chromium (VI) from nonviable cyanobacterium Nostoc muscorum biomass. J Hazard Mater 154:347–354
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environ Sci Pollut Res 20:2828–2843
Gupta VK, Jain CK, Ali I, Chandra S, Agarwal S (2002) Removal of lindane and malathion from wastewater using bagasse fly ash—a sugar industry waste. Water Res 36:2483–2490
Gupta VK, Ali I, Saleh TA, Siddiqui M, Agarwal S (2013) Chromium removal from water by activated carbon developed from waste rubber tires. Environ Sci Pollut Res 20:1261–1268
Hannachi Y (2012) Characterization of the biosorption of lead and cadmium with the red alga (Ceramium virgatum). Holist Approach Environ 2:93–109
Hannachi Y, Rezgui A, Boubaker T (2014) Biosorption potential of the mediterranean plant (Posidonia oceanica) for the removal of Cu2 + ions from aqueous media: equilibrium, kinetic, thermodynamic and mechanism analysis. Korean J Chem Eng 31:1211–1218
Heidmann I, Calmano W (2008) Removal of Zn (II), Cu (II), Ni (II), Ag (I) and Cr (VI) present in aqueous solutions by aluminium electrocoagulation. J Hazard Mater 152:934–941
Ho Y-S, Ofomaja AE (2006) Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent. Biochem Eng J 30:117–123
Huang C, Wu M (1977) The removal of chromium (VI) from dilute aqueous solution by activated carbon. Water Res 11:673–679
Johnson P, Watson M, Brown J, Jefcoat I (2002) Peanut hull pellets as a single use sorbent for the capture of Cu (II) from wastewater. Waste Manag 22:471–480
Juang R-S, Shiau R-C (2000) Metal removal from aqueous solutions using chitosan-enhanced membrane filtration. J Membr Sci 165:159–167
Kam S-K, Gregory J (2001) The interaction of humic substances with cationic polyelectrolytes. Water Res 35:3557–3566
Krika F, Azzouz N, Ncibi MC (2016) Adsorptive removal of cadmium from aqueous solution by cork biomass: equilibrium, dynamic and thermodynamic studies. Arab J Chem 9:S1077–S1083
Krishnani KK, Meng X, Christodoulatos C, Boddu VM (2008) Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J Hazard Mater 153:1222–1234
Liu Y-G, Fan T, Zeng G-M, Li X, Tong Q, Ye F, Zhou M, Xu W-H, Huang Y-E (2006) Removal of cadmium and zinc ions from aqueous solution by living Aspergillus niger. Trans Nonferrous Met Soc China 16:681–686
Low K, Lee C, Liew S (2000) Sorption of cadmium and lead from aqueous solutions by spent grain. Process Biochem 36:59–64
Lu S, Gibb SW (2008) Copper removal from wastewater using spent-grain as biosorbent. Bioresour Technol 99:1509–1517
Ma W, Tobin J (2003) Development of multimetal binding model and application to binary metal biosorption onto peat biomass. Water Res 37:3967–3977
Madala S, Nadavala SK, Vudagandla S, Boddu VM, Abburi K (2017) Equilibrium, kinetics and thermodynamics of Cadmium (II) biosorption on to composite chitosan biosorbent. Arab J Chem 10:S1883–S1893
Mahmood T, Saddique MT, Naeem A, Westerhoff P, Mustafa S, Alum A (2011) Comparison of different methods for the point of zero charge determination of NiO. Ind Eng Chem Res 50:10017–10023
Malkoc E (2006) Ni (II) removal from aqueous solutions using cone biomass of Thuja orientalis. J Hazard Mater 137:899–908
Mittal A, Mittal J, Malviya A, Gupta VK (2010) Removal and recovery of Chrysoidine Y from aqueous solutions by waste materials. J Colloid Interface Sci 344:497–507
Mohammadi N, Khani H, Gupta VK, Amereh E, Agarwal S (2011) Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J Colloid Interface Sci 362:457–462
Mohan S, Sreelakshmi G (2008) Fixed bed column study for heavy metal removal using phosphate treated rice husk. J Hazard Mater 153:75–82
Ncibi M, Mahjoub B, Hamissa AB, Mansour RB, Seffen M (2009) Biosorption of textile metal-complexed dye from aqueous medium using Posidonia oceanica (L.) leaf sheaths: Mathematical modelling. Desalination 243:109–121
Nguyen T, Ngo H, Guo W, Zhang J, Liang S, Yue Q, Li Q, Nguyen T (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Pagnanelli F, Mainelli S, De Angelis S, Toro L (2005) Biosorption of protons and heavy metals onto olive pomace: modelling of competition effects. Water Res 39:1639–1651
Palma G, Freer J, Baeza J (2003) Removal of metal ions by modified Pinus radiata bark and tannins from water solutions. Water Res 37:4974–4980
Park J, Regalbuto JR (1995) A simple, accurate determination of oxide PZC and the strong buffering effect of oxide surfaces at incipient wetness. J Colloid Interface Sci 175:239–252
Pérez-Marín A, Zapata VM, Ortuno J, Aguilar M, Sáez J, Lloréns M (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater 139:122–131
Qiu W, Zheng Y (2009) Removal of lead, copper, nickel, cobalt, and zinc from water by a cancrinite-type zeolite synthesized from fly ash. Chem Eng J 145:483–488
Rajamohan N, Rajasimman M, Rajeshkannan R, Saravanan V (2014) Equilibrium, kinetic and thermodynamic studies on the removal of aluminum by modified Eucalyptus camaldulensis barks. Alex Eng J 53:409–415
Rozaini C, Jain K, Oo C, Tan K, Tan L, Azraa A, Tong K (2010) Optimization of nickel and copper ions removal by modified mangrove barks. Int J Chem Eng Appl 1:84
Saravanan R, Thirumal E, Gupta VK, Narayanan V, Stephen A (2013) The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. J Mol Liq 177:394–401
Sarı A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd (II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164:1004–1011
Shinde NR, Bankar AV, Kumar AR, Zinjarde SS (2012) Removal of Ni(II) ions from aqueous solutions by biosorption onto two strains of Yarrowia lipolytica. J Environ Manage 102:115–124
Srivastava VC, Mall ID, Mishra IM (2008) Removal of cadmium (II) and zinc (II) metal ions from binary aqueous solution by rice husk ash. Colloids Surf A 312:172–184
Srivastava VC, Mall ID, Mishra IM (2009) Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash. Chem Eng Process 48:370–379
Tunali S, Akar T (2006) Zn(II) biosorption properties of Botrytis cinerea biomass. J Hazard Mater 131:137–145
Venkateswarlu S, Kumar SH, Jyothi N (2015) Rapid removal of Ni(II) from aqueous solution using 3-mercaptopropionic acid functionalized bio magnetite nanoparticles. Water Resour Ind 12:1–7
Vijayaraghavan K, Palanivelu K, Velan M (2006) Biosorption of copper (II) and cobalt (II) from aqueous solutions by crab shell particles. Bioresour Technol 97:1411–1419
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy 59:203–216
Wang X-S, Qin Y (2005) Equilibrium sorption isotherms for of Cu2+ ion rice bran. Process Biochem 40:677–680
Wu Y, Zhang S, Guo X, Huang H (2008) Adsorption of chromium (III) on lignin. Biores Technol 99:7709–7715
Acknowledgements
This work was financially supported by the Faculty of Chemistry (USTHB, Algiers).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boulaiche, W., Belhamdi, B., Hamdi, B. et al. Kinetic and equilibrium studies of biosorption of M(II) (M = Cu, Pb, Ni, Zn and Cd) onto seaweed Posidonia oceanica fibers. Appl Water Sci 9, 173 (2019). https://doi.org/10.1007/s13201-019-1062-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1062-1