Abstract
The ability of articular cartilage to withstand significant mechanical stresses during activities, such as walking or running, relies on its distinctive structure. Integrating detailed tissue properties into subject-specific biomechanical models is challenging due to the complexity of analyzing these characteristics. This limitation compromises the accuracy of models in replicating cartilage function and impacts predictive capabilities. To address this, methods revealing cartilage function at the constituent-specific level are essential. In this study, we demonstrated that computational modeling derived individual constituent-specific biomechanical properties could be predicted by a novel nanoparticle contrast-enhanced computer tomography (CECT) method. We imaged articular cartilage samples collected from the equine stifle joint (n = 60) using contrast-enhanced micro-computed tomography (µCECT) to determine contrast agents’ intake within the samples, and compared those to cartilage functional properties, derived from a fibril-reinforced poroelastic finite element model. Two distinct imaging techniques were investigated: conventional energy-integrating µCECT employing a cationic tantalum oxide nanoparticle (Ta2O5-cNP) contrast agent and novel photon-counting µCECT utilizing a dual-contrast agent, comprising Ta2O5-cNP and neutral iodixanol. The results demonstrate the capacity to evaluate fibrillar and non-fibrillar functionality of cartilage, along with permeability-affected fluid flow in cartilage. This finding indicates the feasibility of incorporating these specific functional properties into biomechanical computational models, holding potential for personalized approaches to cartilage diagnostics and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Articular cartilage has the very challenging task of withstanding significant mechanical stresses during daily activities such as walking or running. Consequently, cartilage possesses a distinctive composition and structure that endow it with the necessary biomechanical properties. Articular cartilage is primarily composed of proteoglycans (PGs), collagens, and fluid, which all play a crucial role in its biomechanical behavior [32]. The collagen network and fluid pressure allow cartilage to withstand impacts and cyclic loading, whereas PGs with their capability to bind water define the equilibrium stiffness of the tissue when the free fluid has flown out during a prolonged loading [9,10,11, 21].
The biomechanics of cartilage are critical for joint function and, therefore, profound understanding of it is essential. To deepen our understanding, computational models are used to simulate scenarios inside and outside of the experimental range, enabling investigations into the individual constituents’ (collagen and proteoglycan) impact on cartilage mechanical behavior, stresses, strains, and fluid flow [10, 52]. Computational models have increasingly gained popularity since the early 2000s, and consistently, they have been demonstrated to be effective in mimicking cartilage behavior [31, 35, 61]. One such advanced model is the fibril-reinforced poroelastic (FRPE) material model, which has been applied and validated several times, showing its ability to capture the complex behavior and distinguish between normal and osteoarthritic cartilage [9,10,11, 26, 27, 31, 41, 52]. The model enables us to analyze cartilage function at a constituent-specific level, meaning that we can extract functional properties that describe proteoglycan-defined behavior (non-fibrillar matrix modulus Enf), collagen-defined behavior (both initial Ef0 and strain-dependent fibril network modulus Efε), and fluid flow (permeability k0 and permeability strain-dependency factor M). The non-fibrillar matrix modulus Enf determines tissue equilibrium stiffness and resilience, with higher values indicating greater resistance to prolonged deformation (e.g., standing). The initial fibril network modulus Ef0 represents collagen stiffness at the onset of deformation (e.g., jumping), while the strain-dependent modulus Efε captures collagen’s nonlinear behavior and stiffening under strain. Permeability k0 and its strain-dependency M regulate fluid flow, impacting load bearing, and hydration. Higher permeability, which reflects increased porosity, promotes faster tissue relaxation, while lower permeability results in higher fluid pressurization. These aspects are challenging to explore through conventional analytical biomechanical measurements. This is particularly significant because fluid flow, which is determined by the collective of solid constituents, is recognized as a key contributor to cartilage health and function [39, 40].
For the knee joint model generation and simulation, various medical imaging methods, e.g., magnetic resonance imaging, standard radiography, and computed tomography (CT), are utilized [15, 46, 49, 55, 63]. However, providing data on individual cartilage constituent-specific biomechanical properties (material properties) is exceedingly difficult [56]. Instead, these imaging techniques provide cartilage volume and topography information but not data on the constituents which is critical to gaining a comprehensive understanding of the intricacies of cartilage mechanics [17, 56].
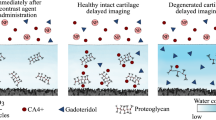
To develop more accurate computational models and to assess the condition of the cartilage, the use of advanced imaging techniques such as contrast-enhanced computed tomography (CECT) is highly advantageous. Contrast agents can be designed to selectively target specific cartilage constituents, such as PGs and collagen, or reflect porosity [1, 4, 13, 58]. Furthermore, CECT capability improves when combined with photon-counting detectors (PCD-CT) [54]. Compared to conventional energy-integrating detectors (EIDs), PCDs detect individual photons and classify them by their energy, enabling spectral imaging and the separation of multiple contrast agents with a single scan. Importantly, this is achieved without increasing radiation doses or the need for cumbersome co-registering of multiple image sets. To harness this feature, we have reported a quantitative dual-energy CT technique [3,4,5, 18,19,20, 54, 58] that combines an ‘active’ cationic contrast agent and a ‘passive’ neutral contrast agent. The cationic contrast agent binds to anionic PGs [33, 45, 57, 58], while neutral contrast agent reflects free water content and steric hindrance created by the solid constituents of the cartilage [54, 58]. Moreover, the dual-contrast method can be further refined by converting measured concentrations to relative partitions (i.e., dividing the measured concentration by the original concentration), and unifying them (i.e., dividing the active contrast agent partition by the partition of the neutral agent) to form combined partition. This approach merges free water, proteoglycans, and steric hindrance effects, thereby enhancing the sensitivity of CECT imaging [3, 5, 19, 58]. Numerous prior studies [1, 2, 30, 33, 45, 50, 57, 58] have already showcased the capacity of CECT to capture cartilage constituents, structures, and biomechanics defined from analytical experiments. However, an unexplored aspect is the integration of contrast agents' diffusion data directly into the material parameters of the finite element (FE) cartilage models. Such an approach offers a fresh and intricate perspective on the relationship between contrast agent diffusion and cartilage function, potentially offering refinement for subject-specific models. By providing biomechanical parameters of the target tissue, such as permeability, this methodology could significantly enhance the precision of the advanced cartilage models [47, 48, 53] and ultimately lead to more accurate simulation outcomes.
The primary aim of this study is to quantify cartilage function through contrast-enhanced micro-computed tomography (µCECT) imaging utilizing computational modeling. To achieve this, FRPE FE modeling of cartilage function under experimental compression is conducted to determine the constituent-specific material properties. Contrast agent diffusion experiments are performed using conventional EID-µCT and experimental PCD-µCT setups to follow the uptake of cationic and neutral contrast agents. We hypothesize that cartilage permeability, extracted from FE modeling, serves as a highly sensitive biomarker for µCECT imaging parameters to assess cartilage function. This hypothesis builds upon previous studies that have demonstrated the ability of cationic contrast agents to target negatively charged PGs [33, 45, 57, 58] and the neutral contrast agent’s dependence on porous structure [54, 58], which together define the permeability. The secondary aim is to evaluate the efficacy of the dual-contrast agent and the PCD-µCT method in assessing FE model derived cartilage function. To accomplish this, we will use both single- and dual-contrast agents and employ EID- and PCD-based µCT setups, respectively.
Materials and Methods
Samples
The samples used in this study were previously utilized in a prior publication [16]. Thirty equine stifle joints were carefully chosen (joints with macroscopic abnormalities were excluded), and cylindrical osteochondral plugs of 10 mm depth and 8.5 mm diameter were harvested using a hollow drill. Samples were harvested from two different sites within the joint to account for variations in biomechanics and structures: the distal intertrochlear groove (n = 30) and the medial femoral condyle (n = 30). Separate single-contrast and dual-contrast experiments were performed on batches of fifteen samples from each group (n = 30). Cartilage thickness measurements were obtained using µCT (Nikon XT H 225, Nikon Metrology Europe, Leuven, Belgium) by measuring the samples in the air without any presence of contrast agents. Samples were inside a sealed container during the imaging to prevent drying.
Biomechanics
The experimental tests were conducted in indentation geometry (Fig. 1a). The measuring geometry consisted of a displacement actuator with a resolution of 0.1 µm (PM1A11939, Newport, Irvine, CA, USA) and a load cell of 0.25 kg (Model 31, Honeywell International Inc., Charlotte, NC, USA). A goniometer was applied to ensure perpendicularity between the cartilage surface and the plane-ended cylindrical indenter. The indenter diameter was 0.55 mm. The samples were fixed with three screws through the subchondral bone. The sample holder was then filled with phosphate-buffered saline (PBS, 310 mOsmol/L).
The study workflow: a Indentation testing (n = 60) using a stress-relaxation protocol was followed by modeling samples as Fibril-Reinforced Poroelastic (FRPE) materials, optimizing five material parameters. b Half of the samples (n = 30) immersed in a Ta2O5-cNP bath were imaged using EID-µCT, while the other half (n = 30) immersed in a Ta2O5-cNP and iodixanol mixture were imaged using PCD-µCT. Contrast agent partitions within samples were calculated from the images based on attenuation. Material parameters and contrast agent partitions were then statistically compared using Spearman correlation.
Full contact on surfaces between the indenter and the cartilage was ensured with an equilibrium pre-stress of 40 kPa, resulting in minor deformation (from 8 to 60 µm). Cartilage recovery time was set to 600 s after preliminary tests, ensuring equilibrium before each indentation test. The testing protocol included four steps of compression, which corresponded to 4% of the remaining cartilage thickness. The ramp rate was 100% per second relative to cartilage thickness, and each compression step was followed by 600 s of relaxation time.
Computational Modeling
A sample-specific axisymmetric FRPE FE model was created to replicate experimental stress-relaxation tests (Fig. 1a) using Abaqus (v6.12-3, Dassault Systèmes, Vèlizy-Villacoublay, France). The total stress tensor is defined as the sum of stress tensors caused by the fibrillar and non-fibrillar matrices, excluding (pore) fluid pressure [9, 10]:
where \( {\sigma }_{\text {nf}}\) and \({\sigma }_{\text {f}}\) are the stress tensors of the non-fibrillar and fibrillar matrices, \(p\) is the fluid pressure, and \(I\) is the unit tensor. The stress tensor of the Neo-Hookean hyperelastic non-fibrillar matrix is [10, 52]:
where \({\sigma }_{\text {nf}}\) is the stress tensor of the non-fibrillar matrix, \({K}_{\text {nf}}\) and \({G}_{\text {nf}}\) are the bulk and shear moduli of the non-fibrillar matrix, respectively, \(F\) is the deformation gradient tensor, \(J\) is the determinant of the \(F\) and \(I\) is the unit tensor. The bulk and shear moduli of the non-fibrillar matrix can be expressed as a function of the elastic modulus (Enf) and Poisson’s ratio (\({v}_{\text {nf}}\) = 0.42 [24, 25, 31]) of the non-fibrillar matrix [10, 40]:
Darcy’s law is employed to describe the fluid flow inside the porous matrix as follows:
where \(q\) is the rate of the fluid flow, \(k\) is the permeability of the material, and \(\nabla p\) is the pressure gradient. The deformation in the porous material causes a change in the void ratio and, hence, the permeability changes, which is then described as follows [60]:
where \(k\) and \({k}_{\text {0}}\) are the current and initial values of the permeability, \(e\) and \({e}_{\text {0}}\) are the current and the initial values for the void ratio, respectively, and M is a constant describing the deformation dependency of permeability. The value for the initial void ratio was set to 3.5 [25, 35, 36], yielding a 78% fluid volume fraction, which corresponds to the average of earlier reported fluid volume fraction throughout the cartilage depth [35, 38]. The collagen fibril network is modeled as a nonlinear elastic fibrillar matrix [10, 52]. The stress in tension of an individual collagen fibril was modeled as follows [10, 37, 52]:
where \({\sigma }_{\text {f}, i}\) and \({\varepsilon }_{\text {f}, i}\) are stress and strain of the i:th fibril, Ef0 is the initial fibril network modulus, and Efε is the strain-dependent fibril network modulus. The collagen network was created using primary and secondary fibrils, and their ratio was defined using a relative density. The relative density describes how much more of the stress is applied to the primary fibrils [61]:
and
where \({\sigma }_{\text {f,p}}\) is primary fibril stress, \({\sigma }_{\text {f,s}}\) is secondary fibril stress, \(C\) is the relative density and \({\rho }_{\text {z}}\) is a factor accounting for fibril density as a function of depth, which is equal to 1 in our model. The collagen network consisted of four organized primary fibrils and thirteen randomly oriented secondary fibrils, with a relative density of 12.16 between the primary and secondary fibrils [7, 26, 40, 61, 62]. The primary fibrils’ direction and number were determined in a way that they form a mesh-like structure parallel to the surface. It was achieved with four fibrils at every node. The secondary fibrils were employed in the x-, y-, and z-directions, and in all directions at 45° angles to the x-, y-, and z-axes, totaling 13 secondary fibrils as originally suggested by Wilson et al. [61].
FE mesh convergence was ensured and the meshes consisted of 350 linear axisymmetric pore pressure continuum elements. To replicate the experimental tests, five sample-specific model parameters were optimized: strain-dependent fibril network modulus (Efε), non-fibrillar matrix modulus (Enf), initial permeability (k0), and permeability strain-dependency factor (M). Each optimized model parameter contributes uniquely to the biomechanical output, meaning that different combinations of moduli and permeability cannot produce identical behavior, particularly when considering multiple strain-dependent stress-relaxation steps [8, 52]. The optimization was done by minimizing the mean-squared relative error between the measured and simulated reaction forces in the 2nd, 3rd, and 4th stress-relaxation steps [9, 40, 52]. The axisymmetric boundary conditions and limitations to displacements were applied similarly like in previous studies [9, 40, 52]. The structure, composition, and fluid fraction/void ratio of each sample were assumed to be homogenous in order to obtain mechanical properties independent from the composition and structure of the tissue, as done previously [10, 31, 41, 52].
µCECT Imaging
Before µCECT experiments, samples were cut into quarters. To allow the contrast agent diffusion only through the articulating surface, the sides of the quarter samples utilized in µCECT experiments were sealed with glue (ethyl cyanoacrylate) before immersing them in a contrast agent bath. µCECT experiments were conducted using both single- and dual-contrast techniques (Fig. 1b). In single-contrast experiments, one contrast agent was utilized, and the samples were imaged with an EID-µCT device. In turn, dual-contrast experiments were conducted using the mix of two contrast agents, and the samples were imaged with a PCD-µCT setup.
Single-Contrast Experiments (Utilizing EID-µCT)
A quarter of each sample was immersed in a bath containing cationic tantalum oxide nanoparticle contrast agent (Ta2O5-cNP, hydrodynamic diameter = 2.55 ± 0.96 nm, concentration 30 mg(Ta2O5)/mL [14, 33]) for 96 h and imaged in air with EID-µCT, i.e., a conventional µCT device (Nikon XT H 225, voxel size 40 × 40 × 40 µm). The X-ray spectrum was generated with 150 kVp voltage, 0.17 mA current, and filtered with a 0.5 mm copper filter [23]. Flat-field calibration was utilized to calibrate the detector.
Dual-Contrast Experiments (Utilizing PCD-µCT)
A quarter from each sample was utilized for dual-contrast experiments by immersing it for 96 hours in a bath containing a mix of cationic Ta2O5-cNP (hydrodynamic radius 2.55 ± 0.96 nm) and neutral iodixanol (approximated spherical size ~ 1.5 nm). The concentrations of the contrast agents in the bath were 20 mg(Ta2O5)/mL and 40 mg(I)/mL for Ta2O5-cNP and iodixanol, respectively. A custom-built PCD-µCT setup (detector: XCounter XC-Flite FX15, Danderyd, Sweden, voxel size 68 × 68 × 68 µm) was utilized, and the X-ray source (VJX IXS1203 Mini-Focus, Bohemia, NY, USA) was configured with a voltage of 120 kVp, a current of 0.25 mA, and filtered with 3.0 mm aluminum and 0.5 mm copper filters [54]. To enable spectral imaging, the lower and upper thresholds were set to 10 keV and 80 keV, respectively, giving us three distinctive energy bins: low (10–80 keV), high (80–120 keV), and total (10–120 keV) energy bins. For the dual-contrast analysis, only low and total energy bins were used. Signal-to-thickness calibration was utilized to calibrate the detector [28, 29, 54].
Image Analysis
The uptake of contrast agent was analyzed with a custom-made MATLAB (R2020b, MathWorks, Natick, MA, USA) script. The script was used to determine contrast agent partitions within the samples by subtracting bulk cartilage attenuation (Hounsfield unit) at the 0-h timepoint from the attenuation at the 96-h timepoint and dividing the result by the initial contrast agent bath attenuation [18, 54].
For dual-contrast experiments, a calibration-based material decomposition was utilized to accurately separate the two contrast agent concentrations, as previously described [3, 18, 54]. Similarly, the material decomposition was validated using multiple mixtures containing various ratios of both contrast agents. The estimated contrast agent concentrations were converted to partitions correspondingly. Combined tantalum-iodine partition was calculated by dividing Ta2O5-cNP partition by the iodixanol partition.
Statistical Analysis
Linear correlation analysis (Spearman) was used to determine the relationships between the optimized model parameters and contrast agent partitions. The Mann–Whitney U-test was used to compare the differences in contrast agents’ partitions and material model parameters between methods and the two locations. The threshold for statistical significance was set at p < 0.05. Statistical analysis was conducted using MATLAB.
Results
The FE models accurately replicated the experimental stress-relaxation data (R2 = 0.95 ± 0.05, Fig. 1a). The mean values and 95% confidence intervals (CIs) of the optimized FE model parameters are shown in Table 1. A statistically significant difference in material parameters was found only in the non-fibrillar matrix modulus Enf and initial permeability k0 between locations. The non-fibrillar matrix modulus Enf was higher in the medial femoral condyle compared to the distal intertrochlear groove (p < 0.001).
For the µCECT measurements, the mean bulk partitions and 95% CIs are shown in Table 2. The Ta2O5-cNP diffusion was elevated without the presence of iodixanol (p < 0.001). Furthermore, there was a notably higher Ta2O5-cNP partition in the medial femoral condyle compared to the distal intertrochlear groove (p < 0.001). Conversely, the partition of iodixanol was increased in the distal intertrochlear groove compared to the medial femoral condyle (p < 0.01).
The single-contrast Ta2O5-cNP partition correlated positively and negatively with the non-fibrillar matrix modulus Enf and the initial permeability k0, respectively (R = 0.84 and R = − 0.47, respectively, Fig. 2A, B). The dual-contrast combined tantalum-iodine partition correlated positively and negatively with the fibrillar matrix modulus Enf and the initial permeability k0, respectively (R = 0.74 and R = − 0.42, respectively, Fig. 3C, D). The iodixanol partition negatively correlated with the non-fibrillar matrix modulus Enf and the strain-dependent fibril network modulus Efε, and positively correlated with the initial permeability k0: R = − 0.44, R = − 0.74, R = 0.45 (Fig. 4A–C). All correlations between contrast agents’ partitions and model parameters are shown in the figures, and no additional statistically significant correlations (p < 0.05) were observed.
Discussion
In this study, we investigated the ability of CT contrast agents to reveal FE model derived cartilage function, by using single- and dual-contrast methods. For the single-contrast measurements, we used Ta2O5-cNPs, while in dual-contrast measurements, we used a mixture of Ta2O5-cNPs and iodixanol. The two contrast agents are strikingly different in composition, size, and charge, and as such their diffusion patterns within cartilage vary [30, 33, 34, 45, 57, 58]. The diffusion of the larger and positively charged Ta2O5-cNPs depends on the negatively charged PGs [22, 33, 45, 57, 58], while the diffusion of the smaller and neutral iodixanol molecule reflects the amount of free water and porosity [54, 58]. Based on the results, Ta2O5-cNPs and iodixanol allow revealing specific functional properties of cartilage, including permeability as we hypothesized, the non-fibrillar matrix modulus Enf, and the strain-dependent fibril network modulus Efε.
When examining data collected from the single-contrast experiments, the strongest correlation was between Ta2O5-cNP partition and non-fibrillar matrix modulus Enf (R = 0.84, Fig. 2A). This correlation can be attributed to the presence of negatively charged PGs, to which the cationic nanoparticle is attracted [22, 33, 45, 57, 58]. PGs serve as a primary constituent influencing the non-fibrillar matrix modulus Enf, as highlighted in prior research [10, 11, 31, 41]. Thus, the increased Ta2O5-cNP diffusion correlates with the higher stiffness of the non-fibrillar structure of the target tissue.
The observation of a negative correlation between Ta2O5-cNP partition and initial permeability k0 was in contrast with that typically seen for conventional small molecule contrast agents where a positive correlation exists (Fig. 2B) [6, 43, 44, 51]. This unexpected finding (particularly in the context of larger charged nanoparticles like Ta2O5-cNP), where the influence of the charge supersedes the size-related effects, challenges the conventional understanding [6, 44, 51]. Typically, a higher proteoglycan (PG) content associates with lower permeability, indicative of better overall tissue health [59]. However, our results introduced a new and more nuanced perspective, suggesting that electrostatic attraction overrides the anticipated limiting effect of steric hindrance on diffusion. This conclusion is supported by the robust positive correlation observed between Ta2O5-cNP and the non-fibrillar matrix modulus Enf, coupled with its simultaneous negative correlation with permeability. Thus, the observed complex interplay among charge, matrix properties, and permeability aligns with a higher PG content contributing to healthier tissue, leading to lower permeability. These findings provide critical insights into the determination of overall cartilage health, particularly when assessed with a single-contrast agent.
Previous research has shown that the combined use of cationic and neutral contrast agents enhanced correlations with cartilage constituent properties and allowed the correlations to be reached at earlier time points [3, 5, 19, 58]. In our dual-contrast experiments, we observed higher correlation coefficients with the combined tantalum-iodine partition (Fig. 3A, B) compared to the Ta2O5-cNP partition alone in dual-contrast experiments (Fig. 3C, D). It is noteworthy that the results obtained with Ta2O5-cNP partition in single-contrast experiments, conducted with a separate set of samples, were consistent with the results obtained with combined partition in dual-contrast experiments (Figs. 2, 3). The combined tantalum-iodine partition correlated positively with the non-fibril matrix modulus Enf and negatively with initial permeability k0. In addition, it is worth noting that the Ta2O5-cNP partition inside the samples decreased in dual-contrast experiments compared to single-contrast experiments (Table 2). This reduction in Ta2O5-cNP intake was most likely because the smaller iodixanol diffused more rapidly into the cartilage, simultaneously reducing the diffusion of Ta2O5-cNP. Importantly, this dynamic did not compromise the correlations, as the inclusion of iodixanol is advantageous for enhancing the characterization of cartilage.
The diffusion of neutral contrast agents, like iodixanol, is mainly influenced by free water and steric hindrance [54, 58]. Consequently, iodixanol partition positively correlated with initial permeability k0 in the present study (Fig. 4B), as increased permeability can be considered to boost iodixanol intake. The inverse correlation with the non-fibrillar matrix modulus Enf can be attributed to a secondary correlation, as PGs influence tissue porosity [6, 12, 44], affirming that iodixanol diffusion is not dependent on the electrostatic attraction of PGs. The diffusion of iodixanol also negatively correlated with the strain-dependent fibril network modulus Efε. This parameter characterizes the strain-dependent behavior of the collagen fibril network, and it is known to depend more on the organization or the architecture of the collagen network, than on collagen quantity [11]. Thus, this result indicates that the tightly organized collagen structure prevents iodixanol from entering the cartilage, which further suggests the potential utility of iodixanol as a reflector of the organized structure of collagen. Previous literature supports this finding, by showing the influence of collagen on both strain-dependent fibril network modulus Efε [10] and the diffusion of neutral nanoparticles [4].
In summary, dual-contrast enables the characterization of more functional parameters, compared to a single-contrast agent (Figs. 2, 3, 4). The contrasting correlations observed between Ta2O5-cNP and iodixanol with cartilage functional properties (Figs. 2A, B & 4A, B) underscore the intricate interplay between contrast agent behavior and cartilage composition and structure. These findings emphasize the significance of selecting the appropriate contrast agent when assessing cartilage function through µCECT methods. The choice of contrast agent yields valuable insights into specific facets of cartilage function. Furthermore, PCD technology provides a platform for spectral imaging, which is essential for multi-contrast methods, and removes the need for image co-registration making the analysis less laborious and faster. In the light of these findings and regarding the secondary aim of this study to evaluate the effectiveness of dual-contrast in conjunction with PCD-µCT, it is evident that this method demonstrates effectiveness in cartilage functional characterization.
In terms of limitations, this study employed equine samples. Although the characteristics of equine articular cartilage are known to be very close to those of human cartilage [42], this still could pose a limitation if we aim to extend our research findings to humans. The behavior of contrast agents in human cartilage may not necessarily mirror that in equine cartilage. Additionally, it is important to note that this study was conducted in an ex vivo setting using osteochondral plugs, and the transferability of these findings to whole joints, especially in vivo, requires further investigation. Emphasizing that in vivo studies face challenges, and obtaining permits for the use of contrast agents under development is a complex and time-consuming process. Alternatively, considering the promising results obtained with the contrast agents used in this study, future investigations may explore the in vivo applicability of the methodology using just the clinically approved iodixanol. Finally, four samples were excluded from the study because we could not replicate their mechanical response with our model. Nonetheless, the model proved suitable for all other samples, justifying its utilization. Also, one sample was excluded due to cracks in the articulating surface originating from sample drilling. Considering the total number of samples (n = 60) in the study, excluding those five samples had minimal impact on our results.
In conclusion, by utilizing FE modeling, which has not been done before in this context, we gain a more detailed evaluation of cartilage function compared to analytically derived equilibrium and instantaneous moduli [1, 3, 12, 33]. Computational modeling allows for the separation of function between specific structural components, whereas parameters determined solely through analytical methods may be more influenced by interactions among multiple structural components (e.g., Ebrahimi et al. [10]). Furthermore, these models enable a more nuanced estimation of cartilage properties arising from the porosity and water flow, which are otherwise challenging to define experimentally. Given the significant impact of early-stage osteoarthritis on cartilage composition and properties, particularly permeability [40], exploring methods to image functional properties presents an exciting avenue for advancing our understanding of cartilage biomechanics. These techniques hold potential for the development of personalized computational models based on imaging data.
Our study demonstrates that single- and dual-contrast µCECT are capable of assessing the intricate functional properties of cartilage, derived from FE modeling. Specifically, the dual-contrast approach enables a thorough assessment of the functional condition of the cartilage at a constituent-specific level, the distinctive attributes of Ta2O5-cNP serving as an excellent reflector of the non-fibrillar matrix, and neutral iodixanol, unveiling permeability with sensitivity to fibrillar stiffness. The ability to independently monitor the partition of contrast agents, or combine their partition, further expands our results, providing a more nuanced understanding of cartilage dynamics. This comprehensive insight holds promise for integrating such detailed information into future cartilage models, for the accurate prediction of the onset of osteoarthritis, and for assessing the precise effects of treatment.
References
Bansal, P. N., N. S. Joshi, V. Entezari, M. W. Grinstaff, and B. D. Snyder. Contrast enhanced computed tomography can predict the glycosaminoglycan content and biomechanical properties of articular cartilage. Osteoarthr. Cartil. 18:184–191, 2010.
Bansal, P. N., N. S. Joshi, V. Entezari, B. C. Malone, R. C. Stewart, B. D. Snyder, and M. W. Grinstaff. Cationic contrast agents improve quantification of glycosaminoglycan (GAG) content by contrast enhanced CT imaging of cartilage. J. Orthop. Res. 29:704–709, 2011.
Bhattarai, A., J. T. J. Honkanen, K. A. H. Myller, M. Prakash, M. Korhonen, A. E. A. Saukko, T. N. Viré, A. Joukainen, A. N. Patwa, H. Kro, M. W. Grinstaff, and J. S. Jurvelin. Quantitative dual contrast CT technique for evaluation of articular cartilage properties. Ann. Biomed. Eng. 46:1038–1046, 2018.
Bhattarai, A., J. T. A. Mäkelä, B. Pouran, H. Kröger, H. Weinans, M. W. Grinstaff, J. Töyräs, and M. J. Turunen. Effects of human articular cartilage constituents on simultaneous diffusion of cationic and nonionic contrast agents. J. Orthop. Res. 39:771–779, 2021.
Bhattarai, A., B. Pouran, J. T. A. Mäkelä, R. Shaikh, M. K. M. Honkanen, M. Prakash, H. Kröger, M. W. Grinstaff, H. Weinans, J. S. Jurvelin, and J. Töyräs. Dual contrast in computed tomography allows earlier characterization of articular cartilage over single contrast. J. Orthop. Res. 38:2230–2238, 2020.
Didomenico, C. D., M. Lintz, and L. J. Bonassar. Molecular transport in articular cartilage—what have we learned from the past 50 years? Nat. Rev. Rheumatol. 14:393–403, 2018.
DiSilvestro, M. R., and J. K. F. Suh. A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. J. Biomech. 34:519–525, 2001.
Ebrahimi, M. Structure, Composition and Function of Human Tibiofemoral Joint Cartilage. Publications of the University of Eastern Finland Dissertations in Forestry and Natural Sciences No: 486, 2022, 13–64 pp.
Ebrahimi, M., M. A. J. Finnilä, A. Turkiewicz, M. Enhlund, S. Saarakkala, R. K. Korhonen, and P. Tanska. Elastic, dynamic viscoelastic and model-derived fibril-reinforced poroelastic mechanical properties of normal and osteoarthritic human femoral condyle cartilage. Ann. Biomed. Eng. 49:2622–2634, 2021.
Ebrahimi, M., S. Ojanen, A. Mohammadi, M. A. Finnilä, A. Joukainen, H. Kröger, S. Saarakkala, R. K. Korhonen, and P. Tanska. Elastic, viscoelastic and fibril-reinforced poroelastic material properties of healthy and osteoarthritic human tibial cartilage. Ann. Biomed. Eng. 47:953–966, 2019.
Ebrahimi, M., M. J. Turunen, M. A. Finnilä, A. Joukainen, H. Kröger, S. Saarakkala, R. K. Korhonen, and P. Tanska. Structure-function relationships of healthy and osteoarthritic human tibial cartilage: experimental and numerical investigation. Ann. Biomed. Eng. 48:2887–2900, 2020.
Evans, R. C., and T. M. Quinn. Solute diffusivity correlates with mechanical properties and matrix density of compressed articular cartilage. Arch. Biochem. Biophys. 442:1–10, 2005.
Fowkes, M. M., P. D. N. Borges, F. Cacho-Nerin, P. E. Brennan, T. L. Vincent, and N. H. Lim. Imaging articular cartilage in osteoarthritis using targeted peptide radiocontrast agents. PLoS ONE. 17(5):e0268223, 2022.
Freedman, J. D., H. Lusic, B. D. Snyder, and M. W. Grinstaff. Tantalum oxide nanoparticles for the imaging of articular cartilage using X-ray computed tomography: visualization of ex vivo/in vivo murine tibia and ex vivo human index finger cartilage. Angew. Chem. Int. Ed. 53:8406–8410, 2014.
Fripp, J., S. Crozier, S. K. Warfield, and S. Ourselin. Automatic segmentation of articular cartilage in magnetic resonance images of the knee. In: Medical Image Computing and Computer-Assisted Intervention, 2007, pp. 186–194.
Fugazzola, M., M. T. Nissinen, J. Jäntti, J. Tuppurainen, S. Plomp, N. Te Moller, J. T. A. Mäkelä, and R. van Weeren. Composition, architecture and biomechanical properties of articular cartilage in differently loaded areas of the equine stifle. Equine Vet. J. 56(3):573–585, 2023.
Halonen, K. S., M. E. Mononen, J. S. Jurvelin, J. Töyräs, and R. K. Korhonen. Importance of depth-wise distribution of collagen and proteoglycans in articular cartilage—a 3D finite element study of stresses and strains in human knee joint. J. Biomech. 46:1184–1192, 2013.
Honkanen, M. K. M., H. Matikka, J. T. J. Honkanen, A. Bhattarai, M. W. Grinstaff, A. Joukainen, H. Kröger, J. S. Jurvelin, and J. Töyräs. Imaging of proteoglycan and water contents in human articular cartilage with full-body CT using dual contrast technique. J. Orthop. Res. 37:1059–1070, 2019.
Honkanen, M. K. M., A. E. A. Saukko, M. J. Turunen, R. Shaikh, M. Prakash, G. Lovric, A. Joukainen, H. Kröger, M. W. Grinstaff, and J. Töyräs. Synchrotron MicroCT reveals the potential of the dual contrast technique for quantitative assessment of human articular cartilage composition. J. Orthop. Res. 38:563–573, 2020.
Honkanen, M. K. M., A. E. A. Saukko, M. J. Turunen, W. Xu, G. Lovric, J. T. J. Honkanen, M. W. Grinstaff, V. P. Lehto, and J. Töyräs. Triple contrast CT method enables simultaneous evaluation of articular cartilage composition and segmentation. Ann. Biomed. Eng. 48:556–567, 2020.
Huttu, M. R. J., J. Puhakka, J. T. A. Mäkelä, Y. Takakubo, V. Tiitu, S. Saarakkala, Y. T. Konttinen, I. Kiviranta, and R. K. Korhonen. Cell-tissue interactions in osteoarthritic human hip joint articular cartilage. Connect. Tissue Res. 55:282–291, 2014.
Jäntti, J., A. Joenathan, M. Fugazzola, J. Tuppurainen, J. T. J. Honkanen, J. Töyräs, R. van Weeren, B. D. Snyder, M. W. Grinstaff, H. Matikka, and J. T. A. Mäkelä. Cationic tantalum oxide nanoparticle contrast agent for micro computed tomography reveals articular cartilage proteoglycan distribution and collagen architecture alterations. Osteoarthr. Cartil. 32:299–309, 2024.
Jäntti, J., A. Joenathan, M. Fugazzola, R. van Weeren, B. D. Synder, M. W. Grinstaff, J. Töyräs, H. Matikka, and J. T. Mäkelä. Tantalum oxide nanoparticles for contrast enhanced computed tomography imaging of cartilage. Osteoarthr. Cartil. 30:S277, 2022.
Julkunen, P., T. Harjula, J. Iivarinen, J. Marjanen, K. Seppänen, T. Närhi, J. Arokoski, M. J. Lammi, P. A. Brama, J. S. Jurvelin, and H. J. Helminen. Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthr. Cartil. 17:1628–1638, 2009.
Julkunen, P., T. Harjula, J. Marjanen, H. J. Helminen, and J. S. Jurvelin. Comparison of single-phase isotropic elastic and fibril-reinforced poroelastic models for indentation of rabbit articular cartilage. J. Biomech. 42:652–656, 2009.
Julkunen, P., P. Kiviranta, W. Wilson, J. S. Jurvelin, and R. K. Korhonen. Characterization of articular cartilage by combining microscopic analysis with a fibril-reinforced finite-element model. J. Biomech. 40:1862–1870, 2007.
Julkunen, P., W. Wilson, H. Isaksson, J. S. Jurvelin, W. Herzog, and R. K. Korhonen. A review of the combination of experimental measurements and fibril-reinforced modeling for investigation of articular cartilage and chondrocyte response to loading. Comput. Math. Methods Med.2013:326150, 2013.
Juntunen, M. A. K. Technical and algorithmic approaches for medical photon counting computed tomography in the example of coronary artery calcium quantification. Acta Univ. Oul. D 1592, 2020.
Juntunen, M. A. K., S. I. Inkinen, J. H. Ketola, A. Kotiaho, M. Kauppinen, A. Winkler, and M. T. Nieminen. Framework for photon counting quantitative material decomposition. IEEE Trans. Med. Imaging. 39:35–47, 2020.
Kokkonen, H. T., J. Mäkelä, K. A. M. Kulmala, L. Rieppo, J. S. Jurvelin, V. Tiitu, H. M. Karjalainen, R. K. Korhonen, V. Kovanen, and J. Töyräs. Computed tomography detects changes in contrast agent diffusion after collagen cross-linking typical to natural aging of articular cartilage. Osteoarthr. Cartil. 19:1190–1198, 2011.
Korhonen, R. K., M. S. Laasanen, J. Töyräs, R. Lappalainen, H. J. Helminen, and J. S. Jurvelin. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J. Biomech. 36:1373–1379, 2003.
Laasanen, M. S., J. Töyräs, R. K. Korhonen, J. Rieppo, S. Saarakkala, M. T. Nieminen, J. Hirvonen, and J. S. Jurvelin. Biomechanical properties of knee articular cartilage. Biorheology. 40:133–140, 2003.
Lawson, T., A. Joenathan, A. Patwa, B. D. Snyder, and M. W. Grinstaff. Tantalum oxide nanoparticles for the quantitative contrast-enhanced computed tomography of ex vivo human cartilage: assessment of biochemical composition and biomechanics. ACS Nano. 15:19175–19184, 2021.
Leddy, H. A., and F. Guilak. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31:753–760, 2003.
Li, L. P., M. D. Buschmann, and A. Shirazi-Adl. A fibril reinforced nonhomogeneous poroelastic model for articular cartilage: inhomogeneous response in unconfined compression. J. Biomech. 33:1533–1541, 2000.
Li, L. P., and W. Herzog. Arthroscopic evaluation of cartilage degeneration using indentation testing—influence of indenter geometry. Clin. Biomech. 21:420–426, 2006.
Li, L. P., J. Soulhat, M. D. Buschmann, and A. Shirazi-Adl. Nonlinear analysis of cartilage in unconfined ramp compression using a fibril reinforced poroelastic model. Clin. Biomech. 14:673–682, 1999.
Lipshitz, H., R. Etheredge 3rd., and M. J. Glimcher. Changes in the hexosamine content and swelling ratio of articular cartilage as functions of depth from the surface. J. Bone Jt. Surg. Am. 58:1149–1153, 1976.
Mäkelä, J. Dissertations in forestry and natural sciences: structural and functional alterrations of articular cartilage in osteoarthritis. 2016.
Mäkelä, J. T. A., S.-K. Han, W. Herzog, and R. K. Korhonen. Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J. Biomech. 48:3369–3376, 2015.
Mäkelä, J. T. A., M. R. J. Huttu, and R. K. Korhonen. Structure–function relationships in osteoarthritic human hip joint articular cartilage. Osteoarthr. Cartil. 20:1268–1277, 2012.
Malda, J., K. E. M. Benders, T. J. Klein, J. C. de Grauw, M. J. L. Kik, D. W. Hutmacher, D. B. F. Saris, P. R. van Weeren, and W. J. A. Dhert. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 20:1147–1151, 2012.
Maroudas, A. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10:365–379, 1970.
Maroudas, A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 12:233–248, 1975.
Meng, H., Q. Quan, X. Yuan, Y. Zheng, J. Peng, Q. Guo, A. Wang, and S. Lu. Diffusion of neutral solutes within human osteoarthritic cartilage: effect of loading patterns. J. Orthop. Transl. 22:58–66, 2020.
Mohammadi, A., K. A. H. Myller, P. Tanska, J. Hirvasniemi, S. Saarakkala, J. Töyräs, R. K. Korhonen, and M. E. Mononen. Rapid CT-based estimation of articular cartilage biomechanics in the knee joint without cartilage segmentation. Ann. Biomed. Eng. 48:2965–2975, 2020.
Mononen, M. E., M. K. Liukkonen, and R. K. Korhonen. Utilizing atlas-based modeling to predict knee joint cartilage degeneration: data from the osteoarthritis initiative. Ann. Biomed. Eng. 47:813–825, 2019.
Mononen, M. E., P. Tanska, H. Isaksson, and R. K. Korhonen. New algorithm for simulation of proteoglycan loss and collagen degeneration in the knee joint: data from the osteoarthritis initiative. J. Orthop. Res. 36:1673–1683, 2018.
Myller, K. A. H., J. T. J. Honkanen, J. S. Jurvelin, S. Saarakkala, J. Töyräs, and S. P. Väänänen. Method for segmentation of knee articular cartilages based on contrast-enhanced CT images. Ann. Biomed. Eng. 46:1756–1767, 2018.
Nieminen, H. J., T. Ylitalo, S. Karhula, J. P. Suuronen, S. Kauppinen, R. Serimaa, E. Hæggström, K. P. H. Pritzker, M. Valkealahti, P. Lehenkari, M. Finnilä, and S. Saarakkala. Determining collagen distribution in articular cartilage using contrast-enhanced micro-computed tomography. Osteoarthr. Cartil. 23:1613–1621, 2015.
Nimer, E., R. Schneiderman, and A. Maroudas. Diffusion and partition of solutes in cartilage under static load. Biophys. Chem. 106:125–146, 2003.
Nissinen, M. T., N. Hänninen, M. Prakash, J. T. A. Mäkelä, M. J. Nissi, J. Töyräs, M. T. Nieminen, R. K. Korhonen, and P. Tanska. Functional and structural properties of human patellar articular cartilage in osteoarthritis. J. Biomech.126:110634, 2021.
Orozco, G. A., A. S. A. Eskelinen, J. P. Kosonen, M. S. Tanaka, M. Yang, T. M. Link, B. Ma, X. Li, A. J. Grodzinsky, R. K. Korhonen, and P. Tanska. Shear strain and inflammation-induced fixed charge density loss in the knee joint cartilage following ACL injury and reconstruction: a computational study. J. Orthop. Res. 40:1505–1522, 2022.
Paakkari, P., S. I. Inkinen, M. K. M. Honkanen, M. Prakash, R. Shaikh, M. T. Nieminen, M. W. Grinstaff, J. T. A. Mäkelä, J. Töyräs, and J. T. J. Honkanen. Quantitative dual contrast photon-counting computed tomography for assessment of articular cartilage health. Sci. Rep. 11:5556, 2021.
Pedoia, V., X. Li, F. Su, N. Calixto, and S. Majumdar. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. J. Magn. Reson. Imaging. 43:970–980, 2016.
Räsänen, L. P., M. E. Mononen, E. Lammentausta, M. T. Nieminen, J. S. Jurvelin, and R. K. Korhonen. Three dimensional patient-specific collagen architecture modulates cartilage responses in the knee joint during gait. Comput. Methods Biomech. Biomed. Eng. 19:1225–1240, 2016.
Saukko, A. E. A., J. T. J. Honkanen, W. Xu, S. P. Väänänen, J. S. Jurvelin, V.-P. Lehto, and J. Töyräs. Dual contrast CT method enables diagnostics of cartilage injuries and degeneration using a single CT image. Ann. Biomed. Eng. 45:2857–2866, 2017.
Saukko, A. E. A., M. J. Turunen, M. K. M. Honkanen, G. Lovric, V. Tiitu, J. T. J. Honkanen, M. W. Grinstaff, J. S. Jurvelin, and J. Töyräs. Simultaneous quantitation of cationic and non-ionic contrast agents in articular cartilage using synchrotron MicroCT imaging. Sci. Rep. 9:7118, 2019.
Torzilli, P. A., J. M. Arduino, J. D. Gregory, and M. Bansal. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30:895–902, 1997.
van der Voet, A. A comparison of finite element codes for the solution of biphasic poroelastic problems. Proc. Inst. Mech. Eng. H. 211:209–211, 1997.
Wilson, W., C. C. Van Donkelaar, B. Van Rietbergen, K. Ito, and R. Huiskes. Stresses in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study. J. Biomech. 37:357–366, 2004.
Wilson, W., C. C. Van Donkelaar, B. Van Rietbergen, K. Ito, and R. Huiskes. Erratum to “Stresses in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study” [Journal of Biomechanics 37 (2004) 357–366] and “A fibril-reinforced poroviscoelastic swelling model for articular cartilage.” J. Biomech. 38:2138–2140, 2005.
Zhang, K., W. Lu, and P. Marziliano. Automatic knee cartilage segmentation from multi-contrast MR images using support vector machine classification with spatial dependencies. Magn. Reson. Imaging. 31:1731–1743, 2013.
Acknowledgments
Instrumentarium Science Foundation (190021), Orion Research Foundation sr, State Research Funding for university-level health research Kuopio University Hospital (5063579, 5041795), Research Council of Finland (324529, 348410, 357787), Regional Council of Pohjois-Savo (A74798), and Biocenter Kuopio are acknowledged for financial and infrastructural support.
Funding
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital). This work was supported by Instrumentarium Science Foundation (190021), Orion Research Foundation sr, State Research Funding for university-level health research Kuopio University Hospital (5063579, 5041795), Research Council of Finland (324529, 348410, 357787), Regional Council of Pohjois-Savo (A74798), and Biocenter Kuopio.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Juuso Tuppurainen, Petri Paakkari, Jiri Jäntti, Mikko Nissinen, Maria Fugazzola, and Anisha Joenathan. The first draft of the manuscript was written by Juuso Tuppurainen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Author Heikki Kröger is on the board of directors of an orthopedic professional society (Nordic Orthopaedic Federation) and receives no compensation as member of the board of directors.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tuppurainen, J., Paakkari, P., Jäntti, J. et al. Revealing Detailed Cartilage Function Through Nanoparticle Diffusion Imaging: A Computed Tomography & Finite Element Study. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03552-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03552-7