Abstract
Physics-based modeling methods have the potential to investigate the mechanical factors associated with knee osteoarthritis (OA) and predict the future radiographic condition of the joint. However, it remains unclear what level of detail is optimal in these methods to achieve accurate prediction results in cohort studies. In this work, we extended a template-based finite element (FE) method to include the lateral and medial compartments of the tibiofemoral joint and simulated the mechanical responses of 97 knees under three conditions of gait loading. Furthermore, the effects of variations in cartilage thickness and failure equation on predicted cartilage degeneration were investigated. Our results showed that using neural network-based estimations of peak knee loading provided classification performances of 0.70 (AUC, p < 0.05) in distinguishing between knees that developed severe OA or mild OA and knees that did not develop OA eight years after a healthy radiographic baseline. However, FE models incorporating subject-specific femoral and tibial cartilage thickness did not improve this classification performance, suggesting there exists an optimal point between personalized loading and geometry for discrimination purposes. In summary, we proposed a modeling framework that streamlines the rapid generation of individualized knee models achieving promising classification performance while avoiding motion capture and cartilage image segmentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) impairs the correct functioning of synovial joints in the human body, especially the knee, affecting the well-being of millions of people worldwide and causing a high economic burden on societies [29]. Due to its multifactorial nature, developing accurate clinical tools for the prediction of OA is challenging [3]. Nevertheless, computational models have served to study risk factors that contribute to the onset and progression of this disease [38], e.g., joint malalignment [45] and chronic overloading [2] and its treatments [37]. In a previous study [35], we introduced a template-based approach to rapidly create knee finite element (FE) models, simulate their biomechanics, and predict the development of OA over the medial compartment. However, this method has limitations that need to be addressed. For example, it has been validated with a small sample (N = 21). It also assumed an even distribution of joint contact forces between the lateral and medial compartments, which is not fully realistic and personalized [45]. In addition, this modeling framework assumes that the fraction of femoral to tibial cartilage thickness in the joint space is constant from the template to the new models.
These limitations open the discussion about the impacts of personalized joint loading and geometry over the predictive capabilities of physics-based methods in cohort studies of OA. In an FE study of two subjects, Wesseling et al., [53] showed that both the loading and geometry affect the acetabular contact pressure in the hip joint, with geometry having a larger influence. In a musculoskeletal and joint contact study of 37 models created from 14 subjects using principal component analysis, Clouthier et al., [9] showed that variations in the local knee geometry may affect the overall functioning of the joint, increasing the risk of developing knee OA for some phenotypes. Similarly, through sensitivity analyses in an FE and experimental study of three knees, Yao et al., [56] concluded that geometry prevails over material formulation on menisci biomechanics, highlighting the use of specimen-specific joint geometries for clinical studies.
Differently from the studies above, other works used their in silico results to predict the future condition of the joints. For instance, in a computational and experimental study of 15 subjects, Aitken et al. [2], calibrated a discrete element analysis-based method to simulate the hip biomechanics and correctly identify the region in the hip prone to degeneration. They used semi-automatically segmented geometries with a representative loading of hip-dysplastic subjects. Finally, in a computational study by discrete element analyses of 38 subjects, Segal et al., [50] indicated that simulated elevated contact stress may serve for predicting the deterioration of cartilage and bone marrow lesions in the knee. They used manually segmented knee geometries and vertically compressed the knees at a flexed angle with half of the body weight of the subject. However, incorporating detailed subject-specific kinematics, kinetics, and joint geometries for numerous subjects is still challenging [38], even more so if the study aims to provide reproducible methods [17]. In this scenario, we believe that approximating individualized knee geometries with simple methods and predicting individualized loading conditions with machine learning tools can overcome these challenges while providing meaningful results to investigate knee OA. To the best of our knowledge, these analyses have not been carried out before.
Regarding geometry, Mononen et al. 2019 compared the OA predictions of models generated from 21 knee geometries obtained via manual segmentation and by scaling a template model based on knee anatomic measurements. They found that by scaling a single template knee geometry, they could predict the future radiographic condition of the knee better than using a fully subject-specific manually segmented knee geometry. The area under the curve (AUC) values comparing osteoarthritis (OA) groups by Kellgren and Lawrence (KL) grades demonstrated this. Using manually segmented geometries models, the AUCs were 0.91 for KL0 vs. KL3, 0.83 for KL0 vs. KL2, and 0.67 for KL2 vs. KL3. However, when employing the template-based method, the AUCs improved substantially, reaching 1.0 for KL0 vs. KL3, 0.89 for KL0 vs. KL2, and 0.88 for KL2 vs. KL3. However, they used the joint space width to scale the femoral and tibial cartilage thickness of the template instead of measuring the thickness separately for both femoral and tibial cartilage. This assumption raises the question about the impact of considering this femoral-to-tibial cartilage thickness ratio on the predictions since cartilage thickness has a significant effect on articular cartilage stresses [1, 41, 48].
Regarding joint loading, the time needed to specify personalized knee joint reaction forces is the main limitation. The golden standard to obtain these forces involves motion capture data followed by musculoskeletal modeling. To overcome this difficulty, multiple machine learning approaches have estimated the kinematics and kinetics of the joints in the lower limbs during walking [6, 15]. Some of them rely on motion data for predicting knee joint contact forces (JCFs), which may still be a limitation for accessibility. In recent studies [26, 27], we developed shallow neural networks for the estimation of the maximum knee JCF during the stance phase of gait. This approach [27] uses simple demographic and biomechanical information of the subjects to predict the maximum total knee JCF (with Pearson correlation between predictions and musculoskeletal model simulations of r = 0.8) and corresponding peak contact forces for the medial (r = 0.6) and lateral (r = 0.7) compartments, avoiding motion capture.
In this work, we aim to further develop and validate a template-based FE approach to predict the development of knee OA. We hypothesize that individualized knee FE simulations through a template-based modeling approach with neural network-based knee JCFs and personalized femoral and tibial cartilage thickness improve the classification of the future radiographic condition of the knees. To this end, we combined novel methods that overcome the previously mentioned limitations of the template-based method with individualized predictions of peak JCFs.
Materials and Methods
We obtained the radiographic and demographic information of 97 knees from the Osteoarthritis Initiative database (OAI, https://nda.nih.gov/oai/), selected according to the exclusion criteria depicted in Fig. 1, at a healthy baseline and after eight years of follow-up. We grouped the knees according to the Kellgren–Lawrence (KL) grade at the follow-up time. We then created medial and lateral compartment models of the knees by scaling a template FE model using information from the healthy baseline and simulated the stance phase of the gait [35]. At this stage, we varied how we defined (i) the maximum tibiofemoral JCF, (ii) the cartilage thickness scaling, and (iii) the thresholds of maximum principal stress for cartilage degeneration.
Next, we compared the FE model outcomes and the classification performance from the different variations in the pipeline. Finally, we correlated our simulated results and the joint space narrowing (JSN) measured between the healthy baseline and the eighth year of follow-up.
Subject Characteristics
Table 1 presents the baseline characteristics of the subjects and their knees. Out of the 97 subjects, 71% were female, and no differences were observed among the variables for the different KL groups.
Finite Element Models
We implemented a template-based modeling method [36] for the lateral compartment as described previously for the medial compartment (Fig. 2a). Here, to generate individualized compartment models, template FE models of the lateral and medial compartments (single template per compartment), incorporating their corresponding geometries and loading conditions, were scaled based on the information obtained from the subject of interest. The template geometry comprised the femoral and tibial cartilages and considered the other tissues in the knee joint through boundary conditions [35, 36].
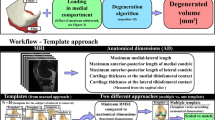
Workflow of the present study. a Generation of knee joint compartment models using simple MRI-based measurements. b Parameters varied in the template-based method: thickness scaling (top) and loading definition (bottom). c Comparison of the predicted degenerated volumes of cartilage tissue and verification against experimental observations. ICD intercondylar distance, A-P anterior–posterior distance, JS joint space
Geometry and Loading
In brief, to generate the geometry, we scaled a single template FE mesh using the ratio between simple MRI-based anatomic measurements from the template knee and the knee to be modeled. After a mesh convergence analysis, the template FE mesh was composed of 18471 linear hexahedral elements in the medial compartment and 17010 elements in the lateral compartment The average edge length of the elements was 0.6 mm, and the aspect ratios were (median [interquartile range]) 1.81 [1.62–2.03] for the medial compartment and 2.04 [1.78–2.37], for the lateral compartment. The anatomic measurements included the femoral intercondylar distance (ICD), maximum femoral anterior–posterior (A-P) distances from the medial and lateral condyles, and the joint space (JS) measured in the same planes as the A-P distances. The A-P and ICD measurements were used to scale the geometry in the X- and Y-directions, respectively (Fig. 2a). The JS measurements were used to scale the tibial cartilage thickness in the Y-direction and radially for the femoral cartilage [36]. Loading was applied to the models by pairing the knee JCF in the axial direction (Y-axis) with the flexion angle (Z-axis) during the stance phase of gait [35]. In this work, to individualize the knee loading, an experimentally determined shape of JCF [24] was scaled to match peak JCFs predicted by three different methods, while the flexion angle trajectory was kept fixed. The Variations overview section describes these methods.
Cartilage Model
The cartilage model followed a biphasic and fibril-reinforced formulation (Table 2) implemented in FEBio 3.7.0 [31] with a depth-dependent arcade-like orientation of collagen fibrils [4, 42]. This formulation allowed us to implement tensile stress thresholds to define the damage onset in the cartilage collagen network. When compressed, this biphasic and fibril-reinforced formulation pressurizes the fluid phase and stretches the stiff collagen fibril network, contributing strongly to the dynamic stiffness of cartilage [12]. The contact between cartilages was defined as sliding elastic [32], which constrains the fluid exudation from the tissue, assuming negligible fluid exchange during the simulated time of approximately one second [13].
Variations Overview
We combined two different approaches to scaling the cartilage thickness and three ways to define the loading of the knee compartments in the template-based method (Fig. 2b; Table 3). In addition, we examined two different equations defining thresholds of maximum principal stress for the initiation of cartilage degeneration.
We used two methods to scale the template cartilage thickness using MRI measurements, as shown in Table 3. By this, we aimed to determine whether using the joint space, keeping the femoral-to-tibial cartilage thickness ratio constant, provides similar predictive results compared to personalizing the femoral-to-tibial cartilage thickness ratio by measuring the femoral and tibial cartilage thickness separately. The former could be possible by X-rays [21], which are more affordable and faster than MRI but unable to directly show cartilage thickness for the femur and tibia separately, and the latter only by MRI.
Regarding the three different approaches we used to define the peak JCFs (Table 3), we wanted to investigate whether using neural network-based peak JCFs and load share between knee compartments would improve the OA predictions compared to simpler assumptions [35, 36]. In the first two cases, we scaled an experimentally determined peak JCF to the subjects using their body weight. In one case, we evenly divided the peak JCF between knee compartments (50%/50%). In the other case, we implemented a load sharing predicted by neural networks (LS—NN). Finally, in the third case, we used load sharing and peak JCFs predicted by neural networks (LS & Peak—NN). See the Neural network predictions section for further details.
With respect to the tissue degeneration mechanism, we assumed that the deterioration in the cartilage tissue begins once the collagen fibrils exceed age-dependent thresholds of tensile stress. We implemented two approaches to evaluate the sensitivity of the response variable to these thresholds. See the Degeneration model section.
Neural Network Predictions
We used feed-forward artificial neural networks (NN) to individualize the peak compartmental knee JCFs [26, 27]. The predictors comprised the height, weight, walking speed, joint frontal alignment, age, and sex of the subjects, and the outputs consisted of the medial (r = 0.61 ± 0.15), lateral (r = 0.67 ± 0.13), and total (r = 0.80 ± 0.10) maximum JCFs during the stance phase of the gait. The load sharing was computed as the ratio of the medial and lateral maximum JCFs to the total predicted maximum knee JCF.
Degeneration Model
First, we defined the stress thresholds for degeneration (\({T}_{{\sigma }_{\text{f}}}\)) based on experimental observations by Kempson on monotonic tensile tests of human cartilage samples [23, 35]
Second, we used the relationship proposed by Weightman et al. 1978, between the age of the donors, the cyclic tensile stress in human cartilage samples (\({T}_{{\sigma }_{\text{f}}}\)), and the number of cycles to failure (\(N\))
The latter, with three representative numbers of cycles: N = 105 accounting for low cycle fatigue and N = 106 and N = 107 representing 5000–50000 steps a week for eight years [47]. For simplicity, in the Results section, we show outcomes from the models using Eq. 2 with N = 106, and the others are presented in the Supplementary material.
Quantification of Degenerated Tissue
To quantify the simulated degeneration, we summed up the volume of the elements exceeding the age-dependent thresholds for degeneration during the stance phase of gait [35, 36, 41] and divided it by the reference volume shown in Fig. 3. We limited our analysis to the central portion of the joint, following the MOAKS joint partitioning [19], as the FE models were constrained to simulate the contact between cartilages in this region.
Verifications
We not only evaluated how well the template method predicts future KL grades but also looked at how closely the simulated results correlate with other ways of measuring the gradual changes in knee OA. To this end, we compared the predicted volumes of degenerated tissue against the joint space narrowing (JSN), assuming the JSN is an indirect measurement of alterations in knee structures [16, 20].
We defined the JSN as the percentage difference in joint space width (JSW) between the healthy baseline and after 8 years of follow-up, measured in the medial and lateral compartments from load-bearing frontal plane X-rays. We defined the JSW as the vertical distance between the central point of the surface of femoral condyles projected to the tibial plateaus [43]. We implemented an in-house MATLAB (The MathWorks, Natick, MA, USA) tool for the semi-automatic measurement of JSW.
Statistical Analysis
We opted for non-parametric analyses because the data were not normally distributed and did not have homogeneous variance. We compared the simulated degeneration between the different combinations of loading and thickness scaling by Friedman tests, between the medial and lateral compartments by Wilcoxon signed rank test and between KL groups at the eighth year of follow-up by Kruskal–Wallis tests. In cases with multiple comparisons, we applied the Bonferroni correction to adjust the level of significance α = 0.05. To assess how the different combinations of loading, geometry, and thresholds for degeneration influenced the predictive capabilities of the method, we used the area under the curve (AUC) of logistic receiver operating characteristic (ROC) analyses, using the simulated degenerated volumes at the baseline time. This metric is easy to interpret [33], and it has been used before in knee OA predictions to compare the performance across classification algorithms [44]. We used DeLong’s criterion to evaluate the significance of AUCs [10]. We used adjusted-R2 measure and cross-tabulation analysis to evaluate if the compartment with the largest simulated degeneration in the knee correlates with the compartment with the largest JSN in corresponding knees. DeLong’s criterion was implemented in R (v.4.3.1, http://www.r-project.org/) [40] and the other statistical analyses in MATLAB 2022b.
Results
Figure 4 shows that all the varied parameters modified the stress distributions. However, the variations in loading caused a larger impact on tensile stress distributions compared to variations in the thickness scaling method. Using an evenly distributed JCF between compartments caused higher stresses in the lateral compartment. Using predictions of peak JCFs by neural networks increased overall stresses in the knee and shifted the highest values to the medial compartment, compared to using an even distribution of load sharing and peak JCF from the literature. Figure 5 shows the corresponding degenerations caused by the variations in loading in each cartilage and compartment.
a Peak joint contact forces (top, left) and maximum principal stresses in the tibial cartilage for the medial and lateral compartments during the first half of the stance (top, right), and the lateral (bottom, left) and medial (bottom, right) compartments for the models with the fixed and scaled cartilage thickness ratios. b Distribution of maximum principal solid stress in the mid-frontal cross-section of one knee model at the loading response of the stance. The columns represent loading conditions, and the rows represent thickness scaling methods. 50%/50%—generic evenly distributed joint contact force, LS-NN—load sharing predicted by neural networks with maximums from a generic curve, LS & Peak-NN—load sharing and peak forces predicted using neural networks. ***p < 0.001, #p < 0.05.
Average contribution of medial and lateral compartments of tibial and femoral cartilages to the simulated overall degenerated volume using a a 50%/ 50% loading distribution between compartments and peak joint contact forces from the literature, b loading distribution estimated by neural networks (NN) and peak joint contact forces from the literature, and c load sharing and peak joint contact forces estimated by neural networks. Volumes correspond to age-dependent damage thresholds given by Eq. 2 with N = 106 cycles on models using the femoral-to-tibial cartilage thickness ratio of the template model.
Regarding simulated cartilage degeneration for the lateral compartment (Fig. 6a), the generic model with an even distribution of JCF between compartments and the maximum JCF from the literature (50%/50%) yielded the largest degenerated volumes compared to the other models (p < 0.001). In contrast, in the medial compartment (Fig.6b), using peak JCFs from the literature resulted in lower degenerated volumes compared to the model with predicted peak JCFs by neural networks (p < 0.001).
Simulated degenerated volume of cartilage for the a lateral and b medial compartments, using Eq. 2 with N = 106. On the left, volumes pooled by force definition, and on the right, volumes pooled by thickness scaling method and Kellgren–Lawrence (KL) grade. Boxes show the first, second, and third quartiles, and whiskers represent the range. 50%/50%—generic evenly distributed joint contact force, LS-NN—load sharing predicted by neural networks with maximums from a generic curve, LS & Peak-NN—load sharing and peak forces predicted using neural networks. ***p < 0.001, #p < 0.05.
Based on the AUC analysis, our method was unable to differentiate between KL 0–1 and KL 2 groups for any of the parameter combinations (Table 4). Overall, implementing personalized thickness scaling for femoral and tibial cartilages did not improve the AUCs (Fig. 7) compared to using the JS and the femoral-to-tibial cartilage fraction from the template. Overall, the model with NN-based compartmental JCFs and the thickness ratio from the template best discriminated the knees using either volume from the lateral, medial, or overall knee (median AUC = 0.70, range [0.50–0.78]), for all the implemented failure thresholds (Eqs. 1 and 2 with N = 105, 106, and 107). See the Supplementary material for further comparisons.
Areas under the curve (AUCs) from comparing the different pairs of KL grades (KL 0–1 vs KL 2 in squares, KL 0–1 vs KL 3–4 in triangles, and KL 2 vs KL 3–4 in circles) for each of the models, considering the degenerated volumes from a the lateral compartment, b the medial compartment, and c the overall knee. Each color represents an age-dependent stress threshold function for degeneration. Dashed lines represent reference AUCs of 0.5 and 0.7 for the stratification by the models to different KL grade groups. Additional comparisons can be found in the Supplementary material.
The model with NN-based JCF predicted 2.6 [2.0–4.2] (median [interquartile range]) times higher simulated degenerated volumes for the medial compartment compared to those for the lateral compartment (p < 0.001) (Fig. 8). In addition, the simulated degeneration in both compartments of severely affected joints (KL 3–4) differed significantly from the healthy (KL 0-1) and mildly (KL 2) affected joints (p < 0.001). In contrast, only the JSN (Fig. 8b) in the medial compartment significantly differed between the KL 0–1 and KL 3–4 groups (p < 0.001). Regarding compartmental JSN differences, it was higher in the medial compartment only in the mildly affected joints (p = 0.025). Linear regression and cross-tabulation analyses suggested that the simulated degenerations do not explain the JSN, with an adj-R2 = 0.104 (p < 0.001) and the model constantly predicting larger damaged volumes in the medial than lateral compartment.
Comparison of the a simulated degenerated volumes, b joint space narrowing, and c linear regression between the maximum joint space narrowing in the knee and the overall joint simulated degenerated volume. These results correspond to using LS & Peaks-NN with scaled thickness ratio and Eq. 2 with N = 106 cycles. *p < 0.05, ***p < 0.001, #p < 0.05.
Discussion
In the present study, we extended the template-based method for the simulation of the knee and the prediction of OA. To this end, we simulated 97 knees, modeling the lateral and medial compartments of the tibiofemoral joint, incorporating neural network-based predictions of peak knee JCFs, evaluating two ways of specifying cartilage thickness, and evaluating four age-dependent thresholds for cartilage degeneration. We found that loading should be considered with special care. Assuming equal contributions from the compartments to withstand the knee JCF resulted in persistently elevated stresses in the lateral compartment and subsequent higher lateral degenerations (Figs. 4, 5). In contrast, models using compartmental load sharing by neural networks resulted in simulated degenerations more consistent with the literature, i.e., OA is more frequent in the medial than in the lateral compartment of the knee [54].
AUC analyses showed that including the lateral compartment in the predictions enhanced the classification performance compared to using the results from the medial compartment only (Table 4; Fig. 7). This could be attributed to the anatomic differences between the medial and lateral compartments and the possibility of using more realistic loadings when both compartments are considered. The geometry of the lateral compartment is less congruent compared to the medial compartment [39], causing the stresses in this compartment to be more sensitive to variations in geometry and loading (Figs. 5, 6). This characteristic caused the lateral femoral and tibial cartilages to similarly contribute to the amount of overstressed tissue, differently to the medial compartment where the femoral cartilage has a larger contribution (Fig. 5).
In the verification, we observed that for the medial compartment, simulated degenerated volumes and JSN differed between KL 0–1 and KL 3–4 groups. However, these parameters were weakly correlated (Fig. 8). One possible reason for this is that JSN is associated with changes in other joint structures, besides cartilage tissue, that we are not including in the models, e.g., meniscal extrusions [16, 20, 43]. Then, it would be valuable to validate our predictions against the loss of cartilage volume measured in the respective knee compartments by MRI [8, 55]. In addition, other modeling-based outcomes could be tested aiming to better conjugate the predictive and explanatory capabilities of the method, like cumulative exposure of cartilage [2, 34]. Preliminary tests with this digital biomarker (not shown) provided similar group-wise differences.
In this study, the three methods we considered to individualize the peak JCF experienced by the knee compartments during normal walking had significant effects on the simulated degenerations (Fig. 6). However, it is a challenging task to validate these force magnitudes against experimental measurements in uninjured knees because: firstly, the experimentally obtained generic JCF, used in the 50%/50% and LS–NN loading models, corresponded to data from subjects with instrumented knee arthroplasties and, secondly, the neural networks were designed to predict simulated data from musculoskeletal models. Despite these limitations, these three methods induced cartilage stress peaks similar to those reported in experimental studies [14, 18]. Furthermore, these methods provided means to approximate the personalized loading in the knee using simple demographic and biomechanical data comprising age, weight, gender, height, joint alignment, and walking speed [26, 27, 46].
We identified the challenging need for more recent data characterizing the damage mechanisms of articular cartilage under cyclic loading spanning a wide range of subject ages. While the works by Kempson [23] and Weightman et al. [51, 52] studied the tissue from an engineering perspective, characterizing the tensile mode of failure, we believe that studying mixed loadings (e.g., compressive, sliding, shear, and tensile stresses), including biological aspects of the tissue surroundings, e.g., the concentration of inflammatory factors [22, 28], could provide a more comprehensive understanding of damage mechanics with aging in the cartilage constituents.
In this study, we want to highlight three aspects of using physics-based models. Firstly, in addition to the measurable changes in the outcomes from modeling subjects under different risk factors, the results can be interpreted to predict the future condition of the joint, as demonstrated in the present study and others [2, 49]. This predictive capability can aid in better understanding disease progression mechanisms. Secondly, physics-based models can be intuitively modified to quantify the effect of variations of the different parameters involved in the workflow, e.g., uncertainty in tissue material properties [11]. This allows for sensitivity analyses and clarity of the influence of different factors on the outcomes. Finally, these methods could be further developed to model the effects of preventive and conservative treatments, e.g., weight loss and changes in JCF by gait retraining [30], providing valuable insights into the potential benefits of non-surgical interventions and guiding treatment decision-making.
There are several limitations to our study. For instance, we did not explicitly consider the geometry of the menisci but only their load-bearing function, by reducing the cartilage–cartilage JCF in the corresponding compartments [35]. Our method also currently ignores the varying characteristics of the gait load curve with age, which could potentially impact the amount of tissue at risk [5]. For instance, it is known that, in elders, the second peak of JCF during the stance phase of gait is lower, the midstance JCF is higher, and the walking speed is slower than in young people [7]. These alterations may increase cartilage stress exposure, promoting the damage of tissue constituents. In addition, we did not model all the relationships between the local morphology of the joint and its effects on loading [9]. Another limitation consisted of using the same material properties of articular cartilage for all the knees [25]. Yet, it is still challenging to measure and translate these variations into numerous models.
We showed how physics-based methods can be expedited using estimates of joint loading obtained from machine learning tools, avoiding cumbersome motion capture procedures. We accounted for individualized knee geometry by scaling a template, avoiding the time-consuming tasks of image segmentation, processing, and meshing. These methods could aid physicians in quantifying the current risk of patients developing degenerative musculoskeletal conditions, such as knee OA and in envisioning preventive and conservative measures.
In conclusion, our template-based approach demonstrated promising predictive capabilities for OA development in 97 knees. The method is susceptible to variations in loading, geometry, and threshold for simulating degeneration. Based on our results, the interplay of loading and the criterion for degeneration showed a greater impact on improving the predictions compared to the procedures used to scale cartilage thickness. In addition, the analysis of the lateral compartment should be considered in further developments of our method since it improved the subject stratification by the models into different KL grade groups. Regarding clinical relevance, our method cannot distinguish between future healthy (KL 0–1) and moderate knee OA (KL 2) subjects (AUCs, p > 0.05) using information from a radiographically healthy baseline. However, mechanical modeling allows us to identify patients developing severe knee OA (KL 3–4) in the next eight years.
References
Adam, C., F. Eckstein, S. Milz, E. Schulte, C. Becker, and R. Putz. The distribution of cartilage thickness in the knee-joints of old-aged individuals measurement by A-mode ultrasound. Clin. Biomech. 13:1–10, 1998.
Aitken, H. D., R. W. Westermann, N. I. Bartschat, A. M. Meyer, M. J. Brouillette, N. A. Glass, J. C. Clohisy, M. C. Willey, and J. E. Goetz. Chronically elevated contact stress exposure correlates with intra-articular cartilage degeneration in patients with concurrent acetabular dysplasia and femoroacetabular impingement. J. Orthop. Res. 40:2632–2645, 2022.
Allen, K. D., and Y. M. Golightly. Epidemiology of osteoarthritis: state of the evidence. Curr. Opin. Rheumatol. 27:276–283, 2015.
Ateshian, G. A., V. Rajan, N. O. Chahine, C. E. Canal, and C. T. Hung. Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J. Biomech. Eng. 131:612–615, 2009.
Boyer, K. A., K. L. Hayes, B. R. Umberger, P. G. Adamczyk, J. F. Bean, J. S. Brach, B. C. Clark, D. J. Clark, L. Ferrucci, J. Finley, J. R. Franz, Y. M. Golightly, T. Hortobágyi, S. Hunter, M. Narici, B. Nicklas, T. Roberts, G. Sawicki, E. Simonsick, and J. A. Kent. Age-related changes in gait biomechanics and their impact on the metabolic cost of walking: Report from a National Institute on Aging workshop. Exp. Gerontol. 173, 2023.
Burton, W. S., C. A. Myers, and P. J. Rullkoetter. Machine learning for rapid estimation of lower extremity muscle and joint loading during activities of daily living. J. Biomech.123:110439, 2021.
Chen, C. P. C., M. J. L. Chen, Y.-C. Pei, H. L. Lew, P.-Y. Wong, and S. F. T. Tang. Sagittal plane loading response during gait in different age groups and in people with knee osteoarthritis. Am. J. Phys. Med. Rehabil. 82:307–312, 2003.
Cicuttini, F. M., G. Jones, A. Forbes, and A. E. Wluka. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann. Rheum. Dis. 63:1124–1127, 2004.
Clouthier, A. L., C. R. Smith, M. F. Vignos, D. G. Thelen, K. J. Deluzio, and M. J. Rainbow. The effect of articular geometry features identified using statistical shape modelling on knee biomechanics. Med. Eng. Phys. 66:47–55, 2019.
DeLong, E. R., D. M. DeLong, and D. L. Clarke-Pearson. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44:837, 1988.
Dhaher, Y. Y., T. Kwon, and M. Barry. The effect of connective tissue material uncertainties on knee joint mechanics under isolated loading conditions. J. Biomech. 43:3118–3125, 2010.
Ebrahimi, M., A. Turkiewicz, M. A. J. Finnilä, S. Saarakkala, M. Englund, R. K. Korhonen, and P. Tanska. Associations of human femoral condyle cartilage structure and composition with viscoelastic and constituent-specific material properties at different stages of osteoarthritis. J. Biomech.145:111390, 2022.
Eckstein, F., M. Tieschky, S. Faber, K. H. Englmeier, and M. Reiser. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anat. Embryol. (Berl). 200:419–424, 1999.
Gilbert, S., T. Chen, I. D. Hutchinson, D. Choi, C. Voigt, R. F. Warren, and S. A. Maher. Dynamic contact mechanics on the tibial plateau of the human knee during activities of daily living. J. Biomech. 47:2006–2012, 2014.
Halilaj, E., A. Rajagopal, M. Fiterau, J. L. Hicks, T. J. Hastie, and S. L. Delp. Machine learning in human movement biomechanics: best practices, common pitfalls, and new opportunities. J. Biomech. 81:1–11, 2018.
Hall, J., L. L. Laslett, J. Martel-Pelletier, J. P. Pelletier, F. Abram, C. H. Ding, F. M. Cicuttini, and G. Jones. Change in knee structure and change in tibiofemoral joint space width: a five year longitudinal population-based study. BMC Musculoskelet. Disord. 17:1–11, 2016.
Halloran, J. P., N. Abdollahi Nohouji, M. A. Hafez, T. F. Besier, S. K. Chokhandre, S. Elmasry, D. R. Hume, C. W. Imhauser, N. B. Rooks, M. T. Y. Schneider, A. Schwartz, K. B. Shelburne, W. Zaylor, and A. Erdemir. Assessment of reporting practices and reproducibility potential of a cohort of published studies in computational knee biomechanics. J. Orthop. Res. 2022. https://doi.org/10.1002/jor.25358
Huang, A., M. L. Hull, and S. M. Howell. The level of compressive load affects conclusions from statistical analyses to determine whether a lateral meniscal autograft restores tibial contact pressure to normal: a study in human cadaveric knees. J. Orthop. Res. 21:459–464, 2003.
Hunter, D. J., A. Guermazi, G. H. Lo, A. J. Grainger, P. G. Conaghan, R. M. Boudreau, and F. W. Roemer. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 19:990–1002, 2011.
Khan, H. I., L. Chou, D. Aitken, A. McBride, C. Ding, L. Blizzard, J. P. Pelletier, J. Martel-Pelletier, F. Cicuttini, and G. Jones. Correlation between changes in global knee structures assessed by magnetic resonance imaging and radiographic osteoarthritis changes over ten years in a midlife cohort. Arthritis Care Res. 68:958–964, 2016.
Jahangir, S., A. Mohammadi, M. E. Mononen, J. Hirvasniemi, J.-S. Suomalainen, S. Saarakkala, R. K. Korhonen, and P. Tanska. Rapid X-ray-based 3-D finite element modeling of medial knee joint cartilage biomechanics during walking. Ann. Biomed. Eng. 50:666–679, 2022.
Kar, S., D. W. Smith, B. S. Gardiner, Y. Li, Y. Wang, and A. J. Grodzinsky. Modeling IL-1 induced degradation of articular cartilage. Arch. Biochem. Biophys. 594:37–53, 2016.
Kempson, G. E. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann. Rheum. Dis. 41:508–511, 1982.
Kutzner, I., A. Bender, J. Dymke, G. Duda, P. von Roth, and G. Bergmann. Mediolateral force distribution at the knee joint shifts across activities and is driven by tibiofemoral alignment. Bone Jt. J. 99:779–787, 2017.
Lampen, N., H. Su, D. D. Chan, and P. Yan. Finite element modeling with subject-specific mechanical properties to assess knee osteoarthritis initiation and progression. J. Orthop. Res. 41:72–83, 2023.
Lavikainen, J., L. Stenroth, T. Alkjær, P. A. Karjalainen, R. K. Korhonen, and M. E. Mononen. Prediction of knee joint compartmental loading maxima utilizing simple subject characteristics and neural networks. Ann. Biomed. Eng. 51:2479–2489, 2023.
Lavikainen, J., L. Stenroth, T. Alkjær, R. Korhonen, M. Henriksen, and M. Mononen. Prediction of knee joint loading from subject characteristics using machine learning. ORS Annu. Meet. 2022:1, 2022.
Li, Y., Y. Wang, S. Chubinskaya, B. Schoeberl, E. Florine, P. Kopesky, and A. J. Grodzinsky. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthr. Cartil. 23:266–274, 2015.
Litwic, A., M. H. Edwards, E. M. Dennison, and C. Cooper. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 105:185–199, 2013.
Liukkonen, M. K., M. E. Mononen, P. Vartiainen, P. Kaukinen, T. Bragge, J.-S. Suomalainen, M. K. H. Malo, S. Venesmaa, P. Käkelä, J. Pihlajamäki, P. A. Karjalainen, J. P. Arokoski, and R. K. Korhonen. Evaluation of the effect of bariatric surgery-induced weight loss on knee gait and cartilage degeneration. J. Biomech. Eng. 140:041008, 2018.
Maas, S. A., B. J. Ellis, G. A. Ateshian, and J. A. Weiss. FEBio: finite elements for biomechanics. J. Biomech. Eng. 134:1–10, 2012.
Maas, S., D. Rawlins, J. Weiss, and G. Athesian. FEBio theory manual version 2.9. , 2019.at https://help.febio.org/FEBio/FEBio_tm_2_9/
Mandrekar, J. N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 5:1315–1316, 2010.
Miller, R. H., and R. L. Krupenevich. Medial knee cartilage is unlikely to withstand a lifetime of running without positive adaptation: a theoretical biomechanical model of failure phenomena. PeerJ. 8:e9676, 2020.
Mononen, M. E., M. K. Liukkonen, and R. K. Korhonen. Utilizing atlas-based modeling to predict knee joint cartilage degeneration: data from the osteoarthritis initiative. Ann. Biomed. Eng. 47:813–825, 2019.
Mononen, M. E., A. Paz, M. K. Liukkonen, and M. J. Turunen. Atlas-based finite element analyses with simpler constitutive models predict personalized progression of knee osteoarthritis: data from the osteoarthritis initiative. Sci. Rep. 13:1–12, 2023.
Mootanah, R., C. W. Imhauser, F. Reisse, D. Carpanen, R. W. Walker, M. F. Koff, M. W. Lenhoff, S. R. Rozbruch, A. T. Fragomen, Z. Dewan, Y. M. Kirane, K. Cheah, J. K. Dowell, H. J. Hillstrom, C. W. Imhauser, F. Reisse, D. Carpanen, R. W. Walker, and M. F. Koff. Development and validation of a computational model of the knee joint for the evaluation of surgical treatments for osteoarthritis. Comput. Methods Biomech. Biomed. Engin. 17:1502–1517, 2014.
Mukherjee, S., M. Nazemi, I. Jonkers, and L. Geris. Use of computational modeling to study joint degeneration: a review. Front. Bioeng. Biotechnol. 8:1–20, 2020.
Nuño, N., and A. M. Ahmed. Sagittal profile of the femoral condyles and its application to femorotibial contact analysis. J. Biomech. Eng. 123:18–26, 2001.
Parodi, S., D. Verda, F. Bagnasco, and M. Muselli. The clinical meaning of the area under a receiver operating characteristic curve for the evaluation of the performance of disease markers. Epidemiol. Health.44:e2022088, 2022.
Paz, A., J. J. García, R. K. Korhonen, and M. E. Mononen. Towards a transferable modeling method of the knee to distinguish between future healthy joints from osteoarthritic joints: data from the osteoarthritis initiative. Ann. Biomed. Eng. 51:2192–2203, 2023.
Paz, A., G. A. Orozco, P. Tanska, J. J. García, R. K. Korhonen, and M. E. Mononen. A novel knee joint model in FEBio with inhomogeneous fibril-reinforced biphasic cartilage simulating tissue mechanical responses during gait: data from the osteoarthritis initiative. Comput. Methods Biomech. Biomed. Eng. 26:1353–1367, 2023.
Pradsgaard, D. Ø., A. Hørlyck, A. H. Spannow, C. Heuck, and T. Herlin. A comparison of radiographic joint space width measurements versus ultrasonographic assessment of cartilage thickness in children with juvenile idiopathic arthritis. J. Rheumatol. 46:301–308, 2019.
Ramazanian, T., S. Fu, S. Sohn, M. J. Taunton, and H. Maradit. Prediction models for knee osteoarthritis: review of current models and future directions. Arch. Bone Jt. Surg. 11:1–10, 2023.
Van Rossom, S., M. Wesseling, C. R. Smith, D. G. Thelen, B. Vanwanseele, V. A. Dieter, and I. Jonkers. The influence of knee joint geometry and alignment on the tibiofemoral load distribution: a computational study. Knee. 26:813–823, 2019.
Saito, Y., S. Nakamura, A. Tanaka, R. Watanabe, H. Narimatsu, and U. I. Chung. Evaluation of the validity and reliability of the 10-meter walk test using a smartphone application among Japanese older adults. Front. Sport. Act. Living. 4:904924, 2022.
Schantz, P., K. S. E. Olsson, J. S. Eriksson, and H. Rosdahl. Perspectives on exercise intensity, volume, step characteristics and health outcomes in walking for transport. Front. Public Heal. 10:911863, 2022.
Schneider, M. T. Y., N. Rooks, and T. Besier. Cartilage thickness and bone shape variations as a function of sex, height, body mass, and age in young adult knees. Sci. Rep. 12:1–10, 2022.
Segal, N. A., D. D. Anderson, K. S. Iyer, J. Baker, J. C. Torner, J. A. Lynch, D. T. Felson, C. E. Lewis, and T. D. Brown. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J. Orthop. Res. 27:1562–1568, 2009.
Segal, N. A., A. M. Kern, D. D. Anderson, J. Niu, J. Lynch, A. Guermazi, J. C. Torner, T. D. Brown, and M. Nevitt. Elevated tibiofemoral articular contact stress predicts risk for bone marrow lesions and cartilage damage at 30 months. Osteoarthr. Cartil. 20:1120–1126, 2012.
Weightman, B. Tensile fatigue of human articular cartilage. J. Biomech. 9:193–200, 1976.
Weightman, B., D. J. Chappell, and E. A. Jenkins. A second study of tensile fatigue properties of human articular cartilage. Ann. Rheum. Dis. 37:58–63, 1978.
Wesseling, M., S. Van Rossom, I. Jonkers, and C. R. Henak. Subject-specific geometry affects acetabular contact pressure during gait more than subject-specific loading patterns. Comput. Methods Biomech. Biomed. Eng. 22:1323–1333, 2019.
Wise, B. L., J. Niu, M. Yang, N. E. Lane, W. Harvey, D. T. Felson, J. Hietpas, M. Nevitt, L. Sharma, J. Torner, C. E. Lewis, and Y. Zhang. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res. 64:847–852, 2012.
Wluka, A. E., A. Forbes, Y. Wang, F. Hanna, G. Jones, and F. M. Cicuttini. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res. Ther. 8:1–9, 2006.
Yao, J., J. Crockett, M. D. Souza, G. A. Day, R. K. Wilcox, and A. C. Jones. Effect of meniscus modelling assumptions in a static tibiofemoral finite element model: importance of geometry over material. Biomech. Model. Mechanobiol. 2024. https://doi.org/10.1007/s10237-024-01822-w.
Acknowledgements
The authors wish to thank the patients and staff of all the hospitals who have contributed to the Osteoarthritis Initiative (OAI). The OAI is a public–private partnership comprised five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Funding
Open access funding provided by University of Eastern Finland (including Kuopio University Hospital). Funding was provided by The Sigrid Jusélius Foundation (grant numbers 240098, 240130), Finnish Cultural Foundation, Research Council of Finland (Grant Numbers 352666, 328920, 324994), Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (grant 5041814) and Doctoral Programme in Science, Forestry, and Technology of the University of Eastern Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mikael J. Turunen, Rami K. Korhonen, and Mika E. Mononen own shares in Aikoa Technologies Oy. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paz, A., Lavikainen, J., Turunen, M.J. et al. Knee-Loading Predictions with Neural Networks Improve Finite Element Modeling Classifications of Knee Osteoarthritis: Data from the Osteoarthritis Initiative. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03549-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03549-2