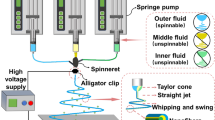
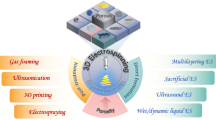
Abstract
The primary goal of bone tissue engineering is to restore and rejuvenate bone defects by using a suitable three-dimensional scaffold, appropriate cells, and growth hormones. Various scaffolding methods are used to fabricate three-dimensional scaffolds, which provide the necessary environment for cell activity and bone formation. Multiple materials may be used to create scaffolds with hierarchical structures that are optimal for cell growth and specialization. This study examines a notion for creating an optimal framework for bone regeneration using a combination of the robocasting method and the electrospinning approach. Research indicates that the integration of these two procedures enhances the benefits of each method and provides a rationale for addressing their shortcomings via this combination. The hybrid approach is anticipated to provide a manufactured scaffold that can effectively replace bone defects while possessing the necessary qualities for bone regeneration.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Campana, V., et al. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. 25(10):2445–2461, 2014.
Liu, X., and P. X. Ma. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 32(3):477–486, 2004.
Meena, L. K., et al. Polymeric microgels for bone tissue engineering applications—A review. Int. J. Polym. Mater. Polym. Biomater. 2019. https://doi.org/10.1080/00914037.2019.1570512.
Martinez, D. A., et al. Prolonged wait time is associated with increased mortality for Chilean waiting list patients with non-prioritized conditions. BMC Public Health. 19(1):233, 2019.
He, F.-L., et al. A novel layer-structured scaffold with large pore sizes suitable for 3D cell culture prepared by near-field electrospinning. Mater. Sci. Eng. C. 86:18–27, 2018.
Farrokhi, M., et al. Artificial intelligence in cancer care: From diagnosis to prevention and beyond. Kindle. 3(1):1–149, 2023.
Ameri, A., et al. Diverse activity of miR-150 in tumor development: Shedding light on the potential mechanisms. Cancer Cell Int. 23(1):261, 2023.
Jammalamadaka, U., and K. Tappa. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 9(1):22, 2018.
Zhang, Y., et al. Association between serum soluble α-klotho and bone mineral density (BMD) in middle-aged and older adults in the United States: A population-based cross-sectional study. Aging Clin. Exp. Res. 35(10):2039–2049, 2023.
Ofudje, E. A., et al. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 29(1):1–8, 2018.
John, A., et al. physico-chemical modification as a versatile strategy for the biocompatibility enhancement of biomaterials. RSC Adv. 5(49):39232–39244, 2015.
Tavakolinejad, Z., Y. Mohammadi Kamalabadi, and A. Salehi. Comparison of the shear bond strength of orthodontic composites containing silver and amorphous tricalcium phosphate nanoparticles: An ex vivo study. J. Dent. (Shiraz). 24(3):285–292, 2023.
Koons, G. L., M. Diba, and A. G. Mikos. Materials design for bone-tissue engineering. Nat. Rev. Mater. 5(8):584–603, 2020.
Jin, H.-H., et al. In vivo evaluation of porous hydroxyapatite/chitosan–alginate composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 51(5):1079–1085, 2012.
Chen, S., et al. lncRNA Xist regulates osteoblast differentiation by sponging miR-19a-3p in aging-induced osteoporosis. Aging Dis. 11(5):1058–1068, 2020.
Beheshtizadeh, N., et al. 3D printing of complicated GelMA-coated alginate/tri-calcium silicate scaffold for accelerated bone regeneration. Int. J. Biol. Macromol. 229:636–653, 2023.
Camilo, C. C., et al. Bone response to porous alumina implants coated with bioactive materials, observed using different characterization techniques. J. Appl. Biomater. Funct. Mater. 15(3):e223–e235, 2017.
Weng, W., et al. Review of zirconia-based biomimetic scaffolds for bone tissue engineering. J. Mater. Sci. 56(14):8309–8333, 2021.
Sari, M., et al. Bioceramic hydroxyapatite-based scaffold with a porous structure using honeycomb as a natural polymeric Porogen for bone tissue engineering. Biomater. Res. 25(1):2, 2021.
Liu, T., et al. Customized design 3D printed PLGA/calcium sulfate scaffold enhances mechanical and biological properties for bone regeneration. Front. Bioeng. Biotechnol. 10:874931, 2022.
Natalia, D. P., et al. Hydroxyapatite/tricalcium phosphate (HA/beta-TCP) scaffold combined with bone and endothelial cells as a potential candidate for oral and maxillofacial bone regeneration. J. Dent. 121:103960, 2022.
Fu, Q. Chapter 15-Bioactive glass Scaffolds for bone tissue engineering. In: Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses, edited by G. Kaur. Sawston: Woodhead Publishing, 2019, pp. 417–442.
Qu, H., et al. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 9(45):26252–26262, 2019.
Zhao, S., et al. A multiaxial bionic ankle based on series elastic actuation with a parallel spring. IEEE Trans. Ind. Electron. 2023. https://doi.org/10.1109/TIE.2023.3310041.
Wen, Y., et al. 3D printed porous ceramic scaffolds for bone tissue engineering: A review. Biomater. Sci. 5(9):1690–1698, 2017.
Zhang, Y., et al. Variations in deep iliac circumflex artery perforator chimeric flap design for single-stage customized-reconstruction of composite bone and soft-tissue defect. J. Plast. Reconstr. Aesthet. Surg. 87:273–283, 2023.
Kolan, K. C., et al. Near-field electrospinning of a polymer/bioactive glass composite to fabricate 3D biomimetic structures. Int. J. Bioprinting. 5(1):163, 2019.
Ferreira, A. M., et al. Collagen for bone tissue regeneration. Acta Biomater. 8(9):3191–3200, 2012.
Ranganathan, S., K. Balagangadharan, and N. Selvamurugan. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 133:354–364, 2019.
Farokhi, M., et al. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 36(1):68–91, 2018.
Demirtaş, T. T., G. Irmak, and M. Gümüşderelioğlu. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 9(3):035003, 2017.
Hernández-González, A. C., L. Téllez-Jurado, and L. M. Rodríguez-Lorenzo. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 229:115514, 2020.
Coletta, D. J., et al. Bone regeneration mediated by a bioactive and biodegradable extracellular matrix-like hydrogel based on elastin-like recombinamers. Tissue Eng. Part A. 23(23–24):1361–1371, 2017.
Honarpardaz, A., et al. Enhanced chondrogenic differentiation of bone marrow mesenchymal stem cells on gelatin/glycosaminoglycan electrospun nanofibers with different amount of glycosaminoglycan. J. Biomed. Mater. Res. Part A. 107(1):38–48, 2019.
Mondal, S., et al. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 46(3):3443–3455, 2020.
Seraji, A. A., F. Goharpey, and J. KhademzadehYeganeh. Highly crystallized and tough polylactic acid through addition of surface modified cellulose nanocrystals. J. Appl. Polym. Sci. 139(37):e52871, 2022.
Yeo, T., et al. Promoting bone regeneration by 3D-printed poly (glycolic acid)/hydroxyapatite composite scaffolds. J. Ind. Eng. Chem. 94:343–351, 2021.
Davarpanah Jazi, R., et al. Fabrication and characterization of electrospun poly lactic-co-glycolic acid/zeolite nanocomposite scaffolds using bone tissue engineering. J. Bioactive Compat. Polym. 33(1):63–78, 2018.
Hernandez, I., A. Kumar, and B. Joddar. A bioactive hydrogel and 3D printed polycaprolactone system for bone tissue engineering. Gels. 3(3):26, 2017.
Ali, M. G., et al. Dual nanofiber scaffolds composed of polyurethane-gelatin/nylon 6-gelatin for bone tissue engineering. Colloids Surf. A. 597:124817, 2020.
Gopanna, A., et al. Polyethylene and polypropylene matrix composites for biomedical applications. In: Materials for Biomedical Engineering, Amsterdam: Elsevier, 2019, pp. 175–216.
Bhattarai, D. P., et al. A review on properties of natural and synthetic based electrospun fibrous materials for bone tissue engineering. Membranes. 8(3):62, 2018.
La Mantia, F., et al. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 145:79–92, 2017.
Gan, J., et al. Dynamic failure of 3D printed negative-stiffness meta-sandwich structures under repeated impact loadings. Compos. Sci. Technol. 234:109928, 2023.
Xu, J., et al. Dynamic response of chain mail fabrics with variable stiffness. Int. J. Mech. Sci. 264:108840, 2024.
Domingues, R. M., et al. Fabrication of anisotropically aligned nanofibrous scaffolds based on natural/synthetic polymer blends reinforced with cellulose nanocrystals for tendon tissue engineering. Front. Bioeng. Biotechnol. 2016. https://doi.org/10.3389/conf.FBIOE.2016.01.01963.
Jamali, S., Y. Zare, and K. Y. Rhee. Modeling of mechanical behaviors and interphase properties of polymer/nanodiamond composites for biomedical products. J. Mater. Res. Technol. 19:2750–2758, 2022.
Li, H., et al. Abrasion performance and failure mechanism of fiber yarns based on molecular segmental differences. J. Eng. Fibers Fabrics. 19:15589250241228264, 2024.
Du, X., S. Fu, and Y. Zhu. 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview. J. Mater. Chem. B. 6(27):4397–4412, 2018.
Riau, A. K., et al. Functionalization of the polymeric surface with bioceramic nanoparticles via a novel, nonthermal dip coating method. ACS Appl. Mater. Interfaces. 8(51):35565–35577, 2016.
Xu, J., et al. Study of the bending properties of variable stiffness chain mail fabrics. Compos. Struct. 322:117369, 2023.
Kim, G., et al. Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning. Macromol. Rapid Commun. 29(19):1577–1581, 2008.
Huang, B., et al. Engineered dual-scale poly (ε-caprolactone) scaffolds using 3D printing and rotational electrospinning for bone tissue regeneration. Addit. Manuf. 36:101452, 2020.
Naghieh, S., et al. Combinational processing of 3D printing and electrospinning of hierarchical poly(lactic acid)/gelatin-forsterite scaffolds as a biocomposite: Mechanical and biological assessment. Mater. Des. 133:128–135, 2017.
Dong, J., et al. A hybrid platform for three-dimensional printing of bone scaffold by combining thermal-extrusion and electrospinning methods. Microsyst. Technol. 26(6):1847–1861, 2020.
Ghanad, M., et al. Single-step solution combustion synthesis of porous 1393–B3 glass powders and structural characterization via solid-state NMR spectroscopy. Ceram. Int. 49(9, Part A):14689–14701, 2023.
Akrami, N., et al. Microstructural properties and in vitro dissolution of microporous bioactive 13–93B3 glass powders synthesized via solution combustion synthesis. J. Non-Crystall. Solids. 615:122425, 2023.
Park, S. H., et al. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 4(5):1198–1207, 2008.
Kardan-Halvaei, M., et al. Fabrication of 3D-printed hydroxyapatite using freeze-drying method for bone regeneration: RVE and finite element simulation analysis. J. Mater. Res. Technol. 24:8682–8692, 2023.
Moghadam, M. Z., et al. Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J. Mech. Behav. Biomed. Mater. 69:115–127, 2017.
Liu, S., et al. Fabrication and characterization of polylactic acid/polycaprolactone composite macroporous micro-nanofiber scaffolds by phase separation. New J. Chem. 44(40):17382–17390, 2020.
Li, J., et al. 3D printed dual-functional biomaterial with self-assembly micro-nano surface and enriched nano argentum for antibacterial and bone regeneration. Appl. Mater. Today. 17:206–215, 2019.
Sola, A., et al. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C. 96:153–165, 2019.
Huang, Z.-M., et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 63(15):2223–2253, 2003.
Wang, Y., et al. In-situ growth of robust superlubricated nano-skin on electrospun nanofibers for post-operative adhesion prevention. Nat. Commun. 13(1):5056, 2022.
Bürck, J., et al. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 1151:73–80, 2018.
Jarusuwannapoom, T., et al. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 41(3):409–421, 2005.
Geng, X., O.-H. Kwon, and J. Jang. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials. 26(27):5427–5432, 2005.
Henriques, C., et al. A systematic study of solution and processing parameters on nanofiber morphology using a new electrospinning apparatus. J. Nanosci. Nanotechnol. 9(6):3535–3545, 2009.
Babilotte, J., et al. 3D printed polymer–mineral composite biomaterials for bone tissue engineering: fabrication and characterization. J. Biomed. Mater. Res. Part B. 107(8):2579–2595, 2019.
Kondiah, P. P., et al. Recent progress in 3D-printed polymeric scaffolds for bone tissue engineering. In: Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering, Amsterdam: Elsevier, 2020, pp. 59–81.
Yu, G. Z., et al. Biomimetic rotated lamellar plywood motifs by additive manufacturing of metal alloy scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 3(4):648–657, 2017.
Lim, H.-K., et al. 3D-printed ceramic bone scaffolds with variable pore architectures. Int. J. Mol. Sci. 21(18):6942, 2020.
Bruyas, A., et al. Systematic characterization of 3D-printed PCL/β-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 33(14):1948–1959, 2018.
Le Guéhennec, L., et al. In vitro and in vivo biocompatibility of calcium-phosphate scaffolds three-dimensional printed by stereolithography for bone regeneration. J. Biomed. Mater. Res. Part A. 108(3):412–425, 2020.
Winarso, R., et al. Application of fused deposition modeling (FDM) on bone scaffold manufacturing process: A review. Heliyon. 8:e11701, 2022.
Hameed, P., et al. Biomorphic porous Ti6Al4V gyroid scaffolds for bone implant applications fabricated by selective laser melting. Progr. Addit. Manuf. 6:455–469, 2021.
Chi, C.-Y., et al. Preparation and in-vitro evaluation of Fe2O3-doped DP-bioglass in combination with 3D-printing and selective laser sintering process (3DP-SLS) for alveolar bone augmentation. Ceram. Int. 47(9):12725–12734, 2021.
Kuah, K. X., et al. Analysis of the corrosion performance of binder jet additive manufactured magnesium alloys for biomedical applications. J. Magn. Alloys. 10(5):1296–1310, 2022.
Turnbull, G., et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioactive Mater. 3(3):278–314, 2018.
Yakout, M., et al. The selection of process parameters in additive manufacturing for aerospace alloys. Int. J. Adv. Manuf. Technol. 92(5):2081–2098, 2017.
Moore, T. A. Evaluating the augmentation of army resupply with additive manufacturing in a deployed environment. Defense AR J. 26(4):424–425, 2018.
Leal, R., et al. Additive manufacturing tooling for the automotive industry. Int. J. Adv. Manuf. Technol. 92(5):1671–1676, 2017.
Park, J. H., H.-K. Jung, and J. R. Lee. Development and evaluation of fall impact protection pads using additive manufacturing. Materials. 12(20):3440, 2019.
Vanderploeg, A., S.-E. Lee, and M. Mamp. The application of 3D printing technology in the fashion industry. Int. J. Fashion Des. Technol. Educ. 10(2):170–179, 2017.
Du Plessis, A., et al. Numerical comparison of lattice unit cell designs for medical implants by additive manufacturing. Virtual Phys. Prototyp. 13(4):266–281, 2018.
Yi, L., et al. Application and progress of bioactive scaffolds in bone tissue engineering. Chin. J. Tissue Eng. Res. 23(6):963, 2019.
Zhou, X., et al. Recent advances in additive manufacturing technology for bone tissue engineering scaffolds. Int. J. Adv. Manuf. Technol. 108:1–16, 2020.
Bose, S., S. Vahabzadeh, and A. Bandyopadhyay. Bone tissue engineering using 3D printing. Mater. Today. 16(12):496–504, 2013.
Yu, J., et al. Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers. 12(12):2958, 2020.
Karamian, E., et al. Fabrication of hydroxyapatite-baghdadite nanocomposite scaffolds coated by PCL/Bioglass with polyurethane polymeric sponge technique. Nanomed. J. 4(3):177–183, 2017.
Malekshahi, Y., et al. Effects of prantschimgin and grandivitin from Ferulago macrocarpa on VEGF, MMP9, MMP2 and research of binding modes using computational methods. Int. Pharm. Acta. 1(1):92–93, 2018.
Yan, F., et al. A multi-scale controlled tissue engineering scaffold prepared by 3D printing and NFES technology. AIP Adv.4(3):031321, 2014.
Seraji, A. A., and A. A. Bajgholi. Dual role of nanoclay in the improvement of the in-situ nanofibrillar morphology in polypropylene/polybutylene terephthalate nanocomposites. J. Ind. Text. 52:15280837221133570, 2022.
Nadaf, A., et al. Recent update on electrospinning and electrospun nanofibers: current trends and their applications. RSC Adv. 12(37):23808–23828, 2022.
Patel, M., H. J. Hong, and W.-G. Koh. Micropatterned fibrous scaffolds for biomedical application. J. Ind. Eng. Chem. 80:729–738, 2019.
Fuh, Y.-K., et al. The control of cell orientation using biodegradable alginate fibers fabricated by near-field electrospinning. Mater. Sci. Eng. C. 62:879–887, 2016.
He, X.-X., et al. Near-field electrospinning: Progress and applications. J. Phys. Chem. C. 121(16):8663–8678, 2017.
Luo, G., et al. High aspect-ratio 3D microstructures via near-field electrospinning for energy storage applications. In 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS). 2016. IEEE.
Chang, C., K. Limkrailassiri, and L. Lin. Continuous near-field electrospinning for large area deposition of orderly nanofiber patterns. Appl. Phys. Lett. 93(12):123111, 2008.
Krysiak, Z. J., et al. Hierarchical composite meshes of electrospun PS microfibers with PA6 nanofibers for regenerative medicine. Materials. 13(8):1974, 2020.
Ren, K., et al. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C. 78:324–332, 2017.
Shitole, A. A., et al. Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration. J. Mater. Sci. 30(5):1–17, 2019.
Li, Y., C. Liao, and S. C. Tjong. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials. 9(7):952, 2019.
Singh, Y. P., et al. Optimization of electrospinning process & parameters for producing defect-free chitosan/polyethylene oxide nanofibers for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 31(6):781–803, 2020.
Shadamarshan, R. P., et al. Fabrication of PCL/PVP electrospun fibers loaded with trans-anethole for bone regeneration in vitro. Colloids Surf. B. 171:698–706, 2018.
Hou, J., et al. Biomimetic growth of hydroxyapatite on electrospun CA/PVP core–shell nanofiber membranes. Polymers. 10(9):1032, 2018.
Zhu, L., D. Luo, and Y. Liu. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 12(1):6, 2020.
Reznikov, N., R. Shahar, and S. Weiner. Bone hierarchical structure in three dimensions. Acta Biomater. 10(9):3815–3826, 2014.
Zhu, L., D. Luo, and Y. Liu. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 12(1):1–15, 2020.
Yu, Y., et al. Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nat. Commun. 13(1):4241, 2022.
Li, T., et al. 3D Printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Adv. Sci. 6(19):1901146, 2019.
Dalton, P. D., et al. Electrospinning and additive manufacturing: converging technologies. Biomater. Sci. 1(2):171–185, 2013.
Kim, M. S., and G. Kim. Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 114:213–221, 2014.
Song, Z. H., et al. Effects of PEMFs on Osx, Ocn, TRAP, and CTSK gene expression in postmenopausal osteoporosis model mice. Int. J. Clin. Exp. Pathol. 11(3):1784–1790, 2018.
Yavari, Z., et al. Stoichiometry influence of oxide support on the catalytic efficiency of nano-palladium towards CH3OH electrooxidation. Chem. Papers. 75(6):2317–2329, 2021.
Wang, Y., et al. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 171:118–132, 2018.
Wang, C., et al. 3D printing of bone tissue engineering scaffolds. Bioactive Mater. 5(1):82–91, 2020.
Zhang, J., et al. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 10(3):1035–1049, 2014.
Wang, Q., et al. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials. 10(6):1244, 2020.
Salehi, N., and A. Salehi. Contact lichenoid reaction in the oral cavity: A comprehensive focus on amalgam restoration. World J. Adv. Res. Rev. 18:134–144, 2023.
Codrea, C. I., et al. Advances in osteoporotic bone tissue engineering. J. Clin. Med. 10(2):253, 2021.
Herath, B., et al. Mechanical and geometrical study of 3D printed Voronoi scaffold design for large bone defects. Mater. Des. 212:110224, 2021.
Xu, Y., et al. Unraveling of advances in 3D-printed polymer-based bone Scaffolds. Polymers. 14:566, 2022. https://doi.org/10.3390/polym14030566.
Zieliński, P. S., et al. 3D printing of bio-instructive materials: Toward directing the cell. Bioactive Mater. 19:292–327, 2023.
Loh, Q. L., and C. Choong. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B. 19(6):485–502, 2013.
Kai, D., et al. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology. 23(9):095705, 2012.
Yoon, Y., et al. 3D bioprinted complex constructs reinforced by hybrid multilayers of electrospun nanofiber sheets. Biofabrication. 11(2):025015, 2019.
Liu, J., et al. Injectable, tough and adhesive zwitterionic hydrogels for 3D-printed wearable strain sensors. Chem. Eng. J. 475:146340, 2023.
Yu, S., et al. Anisotropic microstructure dependent mechanical behavior of 3D-printed basalt fiber-reinforced thermoplastic composites. Composites B. 224:109184, 2021.
Kurokawa, N., S. Kimura, and A. Hotta. Mechanical properties of poly (butylene succinate) composites with aligned cellulose-acetate nanofibers. J. Appl. Polym. Sci. 135(24):45429, 2018.
Wang, Y., et al. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 11(8):1371–1394, 2023.
Amorim, P., et al. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting. 22:e00129, 2021.
Zhang, Y., et al. Applications of electrospun scaffolds with enlarged pores in tissue engineering. Biomater. Sci. 10(6):1423–1447, 2022.
Pattanashetti, N. A., et al. Development of novel 3D scaffolds using BioExtruder by varying the content of hydroxyapatite and silica in PCL matrix for bone tissue engineering. J. Polym. Res. 27(4):1–13, 2020.
Dhandayuthapani, B., et al. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011:1–19, 2011.
Lin, K.-F., et al. Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl. Mater. Interfaces. 8(11):6905–6916, 2016.
Vaezi, M., et al. Extrusion-based 3D printing technologies for 3D scaffold engineering. In: Functional 3D Tissue Engineering Scaffolds, Amsterdam: Elsevier, 2018, pp. 235–254.
Lee, S. C., et al. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 120(19):10834–10886, 2020.
Fu, Q., E. Saiz, and A. P. Tomsia. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 7(10):3547–3554, 2011.
Qu, H. Additive manufacturing for bone tissue engineering scaffolds. Mater. Today Commun. 24:101024, 2020.
Khedri, M., et al. Artificial intelligence deep exploration of influential parameters on physicochemical properties of curcumin-loaded electrospun nanofibers. Adv. NanoBiomed Res. 2(6):2100143, 2022.
Collins, M. N., et al. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 31(21):2010609, 2021.
Phogat, K., S. B. Ghosh, and S. Bandyopadhyay-Ghosh. Recent advances on injectable nanocomposite hydrogels towards bone tissue rehabilitation. J. Appl. Polym. Sci. 140(4):e53362, 2023.
Chen, Y., et al. Associations of bone mineral density with lean mass, fat mass, and dietary patterns in postmenopausal Chinese women: A 2-year prospective study. PLoS ONE. 10(9):e0137097, 2015.
Bagi, M., et al. Advances in technical assessment of spiral inertial microfluidic devices toward bioparticle separation and profiling: A critical review. BioChip J. 18:45–67, 2024.
Kong, B., et al. Tailoring micro/nano-fibers for biomedical applications. Bioactive Mater. 19:328–347, 2023.
Keshvardoostchokami, M., et al. Electrospun nanofibers of natural and synthetic polymers as artificial extracellular matrix for tissue engineering. Nanomaterials. 11(1):21, 2020.
Seraji, A. A., M. Aghvami-Panah, and F. Shams-Ghahfarokhi. Evaluation of ultimate engineering properties of polytetrafluoroethylene/carbon-aerogel/glass fiber porous composite. Colloids Surf. A.647:128975, 2022.
Seraji, A. A., et al. Microstructural design and mechanical performance of epoxy/carbon nanotube fiber composite. J. Compos. Mater. 56(23):3591–3602, 2022.
Zhao, S., et al. A review of magnesium corrosion in bio-applications: Mechanism, classification, modeling, in-vitro, and in-vivo experimental testing, and tailoring Mg corrosion rate. J. Mater. Sci. 58(30):12158–12181, 2023.
Acknowledgements
Not applicable.
Funding
This study has not been funded by any institute.
Author information
Authors and Affiliations
Contributions
All authors contributed to investigation, conceptualization, analysis, and were involved in the writing process.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Not applicable.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Y., Lv, H., Huang, Q. et al. The Synergetic Effect of 3D Printing and Electrospinning Techniques in the Fabrication of Bone Scaffolds. Ann Biomed Eng (2024). https://doi.org/10.1007/s10439-024-03500-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10439-024-03500-5