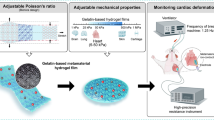
Abstract
Impaired cardiac filling in response to increased passive myocardial stiffness contributes to the pathophysiology of heart failure. By leveraging cardiac MRI data and ventricular pressure measurements, we can estimate in vivo passive myocardial stiffness using personalized inverse finite element models. While it is well-known that this approach is subject to uncertainties, only few studies quantify the accuracy of these stiffness estimates. This lack of validation is, at least in part, due to the absence of ground truth in vivo passive myocardial stiffness values. Here, using 3D printing, we created soft, homogenous, isotropic, hyperelastic heart phantoms of varying geometry and stiffness and simulate diastolic filling by incorporating the phantoms into an MRI-compatible left ventricular inflation system. We estimate phantom stiffness from MRI and pressure data using inverse finite element analyses based on a Neo-Hookean model. We demonstrate that our identified softest and stiffest values of 215.7 and 512.3 kPa agree well with the ground truth of 226.2 and 526.4 kPa. Overall, our estimated stiffnesses revealed a good agreement with the ground truth (\(< 5.8\%\) error) across all models. Our results suggest that MRI-driven computational constitutive modeling can accurately estimate synthetic heart material stiffnesses in the range of 200–500 kPa.








Similar content being viewed by others
References
Aliotta, E., K. Moulin, P. Magrath, and D. B. Ennis. Quantifying precision in cardiac diffusion tensor imaging with second-order motion-compensated convex optimized diffusion encoding. Magn. Reson. Med. 80:1074–1087, 2018.
Arunachalam, S. P., A. Arani, F. Baffour, J. A. Rysavy, P. J. Rossman, K. J. Glaser, D. S. Lake, J. D. Trzasko, A. Manduca, K. P. McGee, R. L. Ehman, and P. A. Araoz. Regional assessment of in vivo myocardial stiffness using 3D magnetic resonance elastography in a porcine model of myocardial infarction. Magn. Reson. Med. 79:361–369, 2018.
Asner, L., M. Hadjicharalambous, R. Chabiniok, D. Peresutti, E. Sammut, J. Wong, G. Carr-White, P. Chowienczyk, J. Lee, A. King, N. Smith, R. Razavi, and D. Nordsletten. Estimation of passive and active properties in the human heart using 3D tagged MRI. Biomech. Model. Mechanobiol. 15:1121–1139, 2016.
Augenstein, K. F., B. R. Cowan, I. J. LeGrice, P. M. F. Nielsen, and A. A. Young. Method and apparatus for soft tissue material parameter estimation using tissue tagged Magnetic Resonance Imaging. J. Biomech. Eng. 127:148–157, 2005.
Avazmohammadi, R., J. S. Soares, D. S. Li, S. S. Raut, R. C. Gorman, and M. S. Sacks. A contemporary look at biomechanical models of myocardium. Annu. Rev. Biomed. Eng. 21:417–442, 2019.
Channer, K. S., W. Culling, P. Wilde, and J. V. Jones. Estimation of left ventricular end-diastolic pressure by pulsed Doppler ultrasound. Lancet 1:1005–1007, 1986.
Cork, T. E., L. E. Perotti, I. A. Verzhbinsky, M. Loecher, and D. B. Ennis. High-resolution ex vivo microstructural MRI after restoring ventricular geometry via 3D printing. Funct. Imaging Model. Heart 11504:177–186, 2019.
Coronel, R., J. de Groot, and J. van Lieshout. Defining heart failure. Cardiovasc. Res. 50:419–422, 2001.
Dual, S. A., J. M. Zimmermann, J. Neuenschwander, N. H. Cohrs, N. Solowjowa, W. J. Stark, M. Meboldt, and M. SchmidDaners. Ultrasonic sensor concept to fit a ventricular assist device cannula evaluated using geometrically accurate heart phantoms. Artif. Organs 43:467–477, 2019.
Emig, R., C. M. Zgierski-Johnston, V. Timmermann, A. J. Taberner, M. P. Nash, P. Kohl, and R. Peyronnet. Passive myocardial mechanical properties: meaning, measurement, models. Biophys. Rev. 13:587–610, 2021.
Genet, M., M. Rausch, L. Lee, S. Choy, X. Zhao, G. Kassab, S. Kozerke, J. Guccione, and E. Kuhl. Heterogeneous growth-induced prestrain in the heart. J. Biomech. 48:2080–2089, 2015.
Geuzaine, C., and J.-F. Remacle. GMSH: a 3-D finite element mesh generator with built-in pre- and post-processing facilities. Int. J. Numer. Methods Eng. 79:1309–1331, 2009.
Greenberg, N. L., P. M. Vandervoort, M. S. Firstenberg, M. J. Garcia, and J. D. Thomas. Estimation of diastolic intraventricular pressure gradients by Doppler M-mode echocardiography. Am. J. Physiol. Heart Circ. Physiol. 280:H2507–2515, 2001.
Guccione, J. M., K. D. Costa, and A. D. McCulloch. Finite element stress analysis of left ventricular mechanics in the beating dog heart. J. Biomech. 28:1167–1177, 1995.
Hadjicharalambous, M., C. T. Stoeck, M. Weisskopf, N. Cesarovic, E. Ioannou, V. Vavourakis, and D. A. Nordsletten. Investigating the reference domain influence in personalised models of cardiac mechanics: effect of unloaded geometry on cardiac biomechanics. Biomech. Model Mechanobiol. 20:1579–1597, 2021.
Henning, R. J. Diagnosis and treatment of heart failure with preserved left ventricular ejection fraction. World J. Cardiol. 12:7–25, 2020.
Hopf, R., L. Bernardi, J. Menze, M. Zündel, E. Mazza, and A. E. Ehret. Experimental and theoretical analyses of the age-dependent large-strain behavior of Sylgard 184 (10:1) silicone elastomer. J. Mech. Behav. Biomed. Mater. 60:425–437, 2016.
Hunter, P. J., A. J. Pullan, and B. H. Smaill. Modeling total heart function. Annu. Rev. Biomed. Eng. 5:147–177, 2003.
Jöbsis, P. D., H. Ashikaga, H. Wen, E. C. Rothstein, K. A. Horvath, E. R. McVeigh, and R. S. Balaban. The visceral pericardium: macromolecular structure and contribution to passive mechanical properties of the left ventricle. Am. J. Physiol. Heart Circ. Physiol. 293:H3379–3387, 2007.
Johnston, I. D., D. K. McCluskey, C. K. L. Tan, and M. C. Tracey. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 24:035017, 2014.
Kanzow, C., N. Yamashita, and M. Fukushima. Levenberg–Marquardt methods with strong local convergence properties for solving nonlinear equations with convex constraints. J. Comput. Appl. Math. 172:375–397, 2004.
Kass, D. A., J. G. F. Bronzwaer, and W. J. Paulus. What mechanisms underlie diastolic dysfunction in heart failure? Circ. Res. 94:1533–1542, 2004.
Kolawole, F. O., M. Peirlinck, T. E. Cork, V. Y. Wang, S. A. Dual, M. E. Levenston, E. Kuhl, and D. B. Ennis. A framework for evaluating myocardial stiffness using 3D-printed heart phantoms. In: Functional Imaging and Modeling of the Heart, edited by D. B. Ennis, L. E. Perotti, and V. Y. Wang. Cham: Springer, 2021, pp. 305–314.
Krishnamurthy, A., C. T. Villongco, J. Chuang, L. R. Frank, V. Nigam, E. Belezzuoli, P. Stark, D. E. Krummen, S. Narayan, J. H. Omens, A. D. McCulloch, and R. C. Kerckhoffs. Patient-specific models of cardiac biomechanics. J. Comput. Phys. 244:4–21, 2013.
Land, S., V. Gurev, S. Arens, C. M. Augustin, L. Baron, R. Blake, C. Bradley, S. Castro, A. Crozier, M. Favino, T. E. Fastl, T. Fritz, H. Gao, A. Gizzi, B. E. Griffith, D. E. Hurtado, R. Krause, X. Luo, M. P. Nash, S. Pezzuto, G. Plank, S. Rossi, D. Ruprecht, G. Seemann, N. P. Smith, J. Sundnes, J. J. Rice, N. Trayanova, D. Wang, Z. JennyWang, and S. A. Niederer. Verification of cardiac mechanics software: benchmark problems and solutions for testing active and passive material behaviour. Proc. Math. Phys. Eng. Sci. 471:20150641, 2015.
Legland, D., I. Arganda-Carreras, and P. Andrey. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 32:3532–3534, 2016.
Lippi, G., and F. Sanchis-Gomar. Global epidemiology and future trends of heart failure. AME Med. J. 5:15, 2020.
Maas, S. A., B. J. Ellis, G. A. Ateshian, and J. A. Weiss. FEBio: finite elements for biomechanics. J. Biomech. Eng. 134:11005, 2012.
Maggioni, A. P., U. Dahlström, G. Filippatos, O. Chioncel, M. Crespo Leiro, J. Drozdz, F. Fruhwald, L. Gullestad, D. Logeart, G. Fabbri, R. Urso, M. Metra, J. Parissis, H. Persson, P. Ponikowski, M. Rauchhaus, A. A. Voors, O. W. Nielsen, F. Zannad, L. Tavazzi, and Heart Failure Association of the European Society of Cardiology (HFA). EURObservational research programme: regional differences and 1-year follow-up results of the heart failure pilot survey (ESC-HF pilot). Eur. J. Heart. Fail. 15:808–817, 2013.
Meyer, F., and S. Beucher. Morphological segmentation. J. Vis. Commun. Image Represent. 1:21–46, 1990.
Mojsejenko, D., J. R. McGarvey, S. M. Dorsey, J. H. Gorman, J. A. Burdick, J. J. Pilla, R. C. Gorman, and J. F. Wenk. Estimating passive mechanical properties in a myocardial infarction using MRI and finite element simulations. Biomech. Model. Mechanobiol. 14:633–647, 2015.
Namana, V., S. S. Gupta, N. Sabharwal, and G. Hollander. Clinical significance of atrial kick. QJM 111:569–570, 2018.
Nikou, A., S. M. Dorsey, J. R. McGarvey, J. H. Gorman, J. A. Burdick, J. J. Pilla, R. C. Gorman, and J. F. Wenk. Effects of using the unloaded configuration in predicting the in vivo diastolic properties of the heart. Comput. Methods Biomech. Biomed. Eng. 19:1714–1720, 2016.
Omens, J. H., and Y. C. Fung. Residual strain in rat left ventricle. Circ. Res. 66:37–45, 1990.
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. Syst. 9:62–66, 1979.
Palchesko, R. N., L. Zhang, Y. Sun, and A. W. Feinberg. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS ONE 7:e51499, 2012.
Palit, A., S. K. Bhudia, T. N. Arvanitis, G. A. Turley, and M. A. Williams. In vivo estimation of passive biomechanical properties of human myocardium. Med. Biol. Eng. Comput. 56:1615–1631, 2018.
Peirlinck, M., F. S. Costabal, J. Yao, J. M. Guccione, S. Tripathy, Y. Wang, D. Ozturk, P. Segars, T. M. Morrison, S. Levine, and E. Kuhl. Precision medicine in human heart modeling: perspectives, challenges, and opportunities. Biomech. Model. Mechanobiol. 20:803–831, 2021.
Peirlinck, M., M. De Beule, P. Segers, and N. Rebelo. A modular inverse elastostatics approach to resolve the pressure-induced stress state for in vivo imaging based cardiovascular modeling. J. Mech. Behav. Biomed. Mater. 85:124–133, 2018.
Peirlinck, M., F. SahliCostabal, K. L. Sack, J. S. Choy, G. S. Kassab, J. M. Guccione, M. De Beule, P. Segers, and E. Kuhl. Using machine learning to characterize heart failure across the scales. Biomech. Model. Mechanobiol. 18:1987–2001, 2019.
Peirlinck, M., K. L. Sack, P. De Backer, P. Morais, P. Segers, T. Franz, and M. De Beule. Kinematic boundary conditions substantially impact in silico ventricular function. Int. J. Numer. Methods Biomed. Eng. 35:e3151, 2019.
Pfaller, M. R., J. M. Hörmann, M. Weigl, A. Nagler, R. Chabiniok, C. Bertoglio, and W. A. Wall. The importance of the pericardium for cardiac biomechanics: from physiology to computational modeling. Biomech. Model. Mechanobiol. 18:503–529, 2019.
Rodríguez-Cantano, R., J. Sundnes, and M. E. Rognes. Uncertainty in cardiac myofiber orientation and stiffnesses dominate the variability of left ventricle deformation response. Int. J. Numer. Methods Biomed. Eng. 35:e3178, 2019.
Røe, Å. T., J. M. Aronsen, K. Skårdal, N. Hamdani, W. A. Linke, H. E. Danielsen, O. M. Sejersted, I. Sjaastad, and W. E. Louch. Increased passive stiffness promotes diastolic dysfunction despite improved Ca$^{2+}$ handling during left ventricular concentric hypertrophy. Cardiovasc. Res. 113:1161–1172, 2017.
Sakata, Y., T. Ohtani, Y. Takeda, K. Yamamoto, and T. Mano. Left ventricular stiffening as therapeutic target for heart failure with preserved ejection fraction. Circ. J. 77:886–892, 2013.
Savarese, G., and L. H. Lund. Global public health burden of heart failure. Card. Fail. Rev. 3:7–11, 2017.
Sidebotham, D., and I. J. Le Grice. Chapter 1—physiology and pathophysiology. In: Cardiothoracic Critical Care, edited by D. Sidebotham, A. Mckee, M. Gillham, and J. H. Levy, Philadelphia: Butterworth-Heinemann, 2007, pp. 3–27.
Stehlin, E. F., D. McCormick, S. C. Malpas, B. P. Pontré, P. A. Heppner, and D. M. Budgett. MRI interactions of a fully implantable pressure monitoring device. J. Magn. Reson. Imaging 42:1441–1449, 2015.
Stimm, J., D. A. Nordsletten, J. Jilberto, R. Miller, E. Berberoğlu, S. Kozerke, and C. T. Stoeck. Personalization of biomechanical simulations of the left ventricle by in vivo cardiac DTI data: impact of fiber interpolation methods. Front. Physiol. 13:1042537, 2022.
Suinesiaputra, A., B. R. Cowan, A. O. Al-Agamy, M. A. Elattar, N. Ayache, A. S. Fahmy, A. M. Khalifa, P. Medrano-Gracia, M.-P. Jolly, A. H. Kadish, D. C. Lee, J. Margeta, S. K. Warfield, and A. A. Young. A collaborative resource to build consensus for automated left ventricular segmentation of cardiac MR images. Med. Image Anal. 18:50–62, 2014.
Trankle, C., J. M. Canada, L. Buckley, S. Carbone, D. Dixon, R. Arena, B. Van Tassell, and A. Abbate. Impaired myocardial relaxation with exercise determines peak aerobic exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail. 4:351–355, 2017.
Virani, S. S., A. Alonso, H. J. Aparicio, E. J. Benjamin, M. S. Bittencourt, C. W. Callaway, A. P. Carson, A. M. Chamberlain, S. Cheng, F. N. Delling, M. S. V. Elkind, K. R. Evenson, J. F. Ferguson, D. K. Gupta, S. S. Khan, B. M. Kissela, K. L. Knutson, C. D. Lee, T. T. Lewis, J. Liu, M. S. Loop, P. L. Lutsey, J. Ma, J. Mackey, S. S. Martin, D. B. Matchar, M. E. Mussolino, S. D. Navaneethan, A. M. Perak, G. A. Roth, Z. Samad, G. M. Satou, E. B. Schroeder, S. H. Shah, C. M. Shay, A. Stokes, L. B. VanWagner, N.-Y. Wang, C. W. Tsao, and American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation 143:e254–e743, 2021.
Walker, J. C., M. B. Ratcliffe, P. Zhang, A. W. Wallace, E. W. Hsu, D. A. Saloner, and J. M. Guccione. Magnetic resonance imaging-based finite element stress analysis after linear repair of left ventricular aneurysm. J. Thorac. Cardiovasc. Surg. 135:1094–1102, 1102.e1–2, 2008.
Wang, B., and S. Krause. Properties of dimethylsiloxane microphases in phase-separated dimethylsiloxane block copolymers. Macromolecules 20:2201–2208, 1987.
Wang, V. Y., H. I. Lam, D. B. Ennis, B. R. Cowan, A. A. Young, and M. P. Nash. Modelling passive diastolic mechanics with quantitative MRI of cardiac structure and function. Med. Image Anal. 13:773–784, 2009.
Wang, V. Y., P. M. F. Nielsen, and M. P. Nash. Image-based predictive modeling of heart mechanics. Annu. Rev. Biomed. Eng. 17:351–383, 2015.
Wang, Z. J., V. Y. Wang, C. P. Bradley, M. P. Nash, A. A. Young, and J. J. Cao. Left ventricular diastolic myocardial stiffness and end-diastolic myofibre stress in human heart failure using personalised biomechanical analysis. J. Cardiovasc. Transl. Res. 11:346–356, 2018.
Watanabe, S., J. Shite, H. Takaoka, T. Shinke, Y. Tanino, H. Otake, D. Matsumoto, D. Ogasawara, T. Sawada, K.-I. Hirata, and M. Yokoyama. Predictive importance of left ventricular myocardial stiffness for the prognosis of patients with congestive heart failure. J. Cardiol. 58:245–252, 2011.
Xi, J., P. Lamata, S. Niederer, S. Land, W. Shi, X. Zhuang, S. Ourselin, S. G. Duckett, A. K. Shetty, C. A. Rinaldi, D. Rueckert, R. Razavi, and N. P. Smith. The estimation of patient-specific cardiac diastolic functions from clinical measurements. Med. Image Anal. 17:133–146, 2013.
Zhang, W., D. S. Li, T. Bui-Thanh, and M. S. Sacks. Simulation of the 3D hyperelastic behavior of ventricular myocardium using a finite-element based neural-network approach. Comput. Methods. Appl. Mech. Eng. 394:114871, 2022.
Zhu, Y., X. Luo, H. Gao, C. McComb, and C. Berry. A numerical study of a heart phantom model. Int. J. Comput. Math. 91:1535–1551, 2014.
Zile, M. R., C. F. Baicu, and W. H. Gaasch. Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 350:1953–1959, 2004.
Zile, M. R., C. F. Baicu, J. S. Ikonomidis, R. E. Stroud, P. J. Nietert, A. D. Bradshaw, R. Slater, B. M. Palmer, P. Van Buren, M. Meyer, M. M. Redfield, D. A. Bull, H. L. Granzier, and M. M. LeWinter. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131:1247–1259, 2015.
Zile, M. R., W. H. Gaasch, J. D. Carroll, M. D. Feldman, G. P. Aurigemma, G. L. Schaer, J. K. Ghali, and P. R. Liebson. Heart failure with a normal ejection fraction. Circulation 104:779–782, 2001.
Zimmermann, J., M. Loecher, F. O. Kolawole, K. Bäumler, K. Gifford, S. A. Dual, M. Levenston, A. L. Marsden, and D. B. Ennis. On the impact of vessel wall stiffness on quantitative flow dynamics in a synthetic model of the thoracic aorta. Sci. Rep. 11:6703, 2021.
Acknowledgments
F.K. receives research support from the Stanford Bio-X Stanford Interdisciplinary Graduate Fellowship. This project was funded, in part, by NIH R01 HL131823 to D.B.E.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolawole, F.O., Peirlinck, M., Cork, T.E. et al. Validating MRI-Derived Myocardial Stiffness Estimates Using In Vitro Synthetic Heart Models. Ann Biomed Eng 51, 1574–1587 (2023). https://doi.org/10.1007/s10439-023-03164-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03164-7