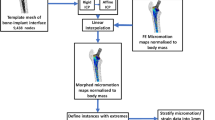
Abstract
Revision knee surgery is complicated by distortion of previous components and removal of additional bone, potentially causing misalignment and inappropriate selection of implants. In this study, we reconstructed the native femoral and tibial surface shapes in simulated total/unicompartmental knee arthroplasty (TKA/UKA) for 20 femurs and 20 tibias using a statistical inference method based on Gaussian Process regression. Compared to the true geometry, the average absolute errors (mean absolute distances) in the prediction of resected femur bones in TKA, medial UKA, and lateral UKA were 1.0 ± 0.3 mm, 1.0 ± 0.3 mm, and 0.8 ± 0.2 mm, respectively, while those in the prediction of tibia resections in the corresponding surgeries were 1.0 ± 0.4 mm, 0.8 ± 0.2 mm, and 0.7 ± 0.2 mm, respectively. Furthermore, it was found that the prediction accuracy depends on the size and gender of the resected bone. For example, the prediction accuracy for UKA cuts was significantly better than that for TKA cuts (p < 0.05). The female and male cuts were often overfit and underfit, respectively. The data indicated that this reconstruction approach can be a viable option for planning of revision surgeries, especially when contralateral anatomy is pathological or cannot be available.









Similar content being viewed by others
References
Albrecht, T., M. Lüthi, T. Gerig, and T. Vetter. Posterior shape models. Med. Image Anal. 17:959–973, 2013.
Asano, T., M. Akagi, K. Tanaka, J. Tamura, and T. Nakamura. In vivo three-dimensional knee kinematics using a biplanar image-matching technique. Clin. Orthop. Relat. Res. 388:157–166, 2001. https://doi.org/10.1097/00003086-200107000-00023.
Audenaert, E. A., J. Van Houcke, D. F. Almeida, L. Paelinck, M. Peiffer, G. Steenackers, and D. Vandermeulen. Cascaded statistical shape model based segmentation of the full lower limb in CT. Comput. Methods Biomech. Biomed. Engin. 22:644–657, 2019.
Baldwin, M. A., J. E. Langenderfer, P. J. Rullkoetter, and P. J. Laz. Development of subject-specific and statistical shape models of the knee using an efficient segmentation and mesh-morphing approach. Comput. Methods Programs Biomed. 97:232–240, 2010.
Berger, R. A., H. E. Rubash, M. J. Seel, W. H. Thompson, and L. S. Crossett. Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylar axis. Clin. Orthop. Relat. Res. 40:47, 1993. https://doi.org/10.1097/00003086-199301000-00008.
Bhandari, M., J. Smith, L. E. Miller, and J. E. Block. Clinical and economic burden of revision knee arthroplasty. Clin. Med. Insights Arthritis Musculoskelet. Disord. 5:CMAMDS0859, 2012.
Biomet Orthopedics. Vanguard XP Total Knee System Design Rationale. 2014.at https://qa-www.zimmerbiomet.com/content/dam/zimmer-biomet/medical-professionals/knee/vanguard-xp-total-knee-system/vanguard-xp-design-rationale.pdf
Caprara, S., F. Carrillo, J. G. Snedeker, M. Farshad, and M. Senteler. Automated pipeline to generate anatomically accurate patient-specific biomechanical models of healthy and pathological FSUs. Front. Bioeng. Biotechnol. 9:1–15, 2021.
Chui, C. S., K. S. Leung, J. Qin, D. Shi, P. Augat, R. M. Y. Wong, S. K. H. Chow, X. Y. Huang, C. Y. Chen, Y. X. Lai, P. S. H. Yung, L. Qin, and W. H. Cheung. Population-based and personalized design of total knee replacement prosthesis for additive manufacturing based on Chinese anthropometric data. Engineering. 7:386–394, 2021.
Cootes, T., E. Baldock, and J. Graham. An introduction to active shape models. Image Process. Anal. 223–248, 2000.at http://person.hst.aau.dk/lasse/teaching/IACV/doc/asm_overview.pdf
Defrate, L. E., H. Sun, T. J. Gill, H. E. Rubash, and G. Li. In vivo tibiofemoral contact analysis using 3D MRI-based knee models. J. Biomech. 37:1499–1504, 2004.
Feng, Y., T. Y. Tsai, J. S. Li, S. Wang, H. Hu, C. Zhang, H. E. Rubash, and G. Li. Motion of the femoral condyles in flexion and extension during a continuous lunge. J. Orthop. Res. 33:591–597, 2015.
Fliss, B., M. Luethi, P. Fuernstahl, A. M. Christensen, K. Sibold, M. Thali, and L. C. Ebert. CT-based sex estimation on human femora using statistical shape modeling. Am. J. Phys. Anthropol. 169:279–286, 2019.
Iwaki, H., V. Pinskerova, and M. A. R. Freeman. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J. Bone Joint Surg. Br. 82-B:1189–1195, 2000.
Joliffe, I., and B. Morgan. Principal component analysis and exploratory factor analysis. Stat. Methods Med. Res. 1:69–95, 1992.
Lamplot, J. D., A. Bansal, J. T. Nguyen, and R. H. Brophy. Risk of subsequent joint arthroplasty in contralateral or different joint after index shoulder, hip, or knee arthroplasty. J. Bone Jt. Surg. 100:1750–1756, 2018.
Li, P., T. Y. Tsai, J. S. Li, S. Wang, Y. Zhang, Y. M. Kwon, H. E. Rubash, and G. Li. Gender analysis of the anterior femoral condyle geometry of the knee. Knee. 21:529–533, 2014.
Li, P., T. Y. Tsai, J. S. Li, Y. Zhang, Y. M. Kwon, H. E. Rubash, and G. Li. Morphological measurement of the knee: Race and sex effects. Acta Orthop. Belg. 80:260–268, 2014.
Lombardi, A. V., T. H. Mallory, R. A. Waterman, and R. W. Eberle. Intercondylar distal femoral fracture. An unreported complication of posterior-stabilized total knee arthroplasty. J. Arthroplasty. 10:643–50, 1995.
Luthi, M., T. Gerig, C. Jud, and T. Vetter. Gaussian process morphable models. IEEE Trans. Pattern Anal. Mach. Intell. 40:1860–1873, 2018.
Maderbacher, G., A. Keshmiri, F. Zeman, J. Grifka, and C. Baier. Assessing joint line positions by means of the contralateral knee: A new approach for planning knee revision surgery? Knee Surg. Port. Traumatol. Arthrosc. 23:3244–3250, 2015.
McMahon, M., and J. A. Block. The risk of contralateral total knee arthroplasty after knee replacement for osteoarthritis. J. Rheumatol. 30:1822–1824, 2003.
Nolte, D., C. K. Tsang, K. Y. Zhang, Z. Ding, A. E. Kedgley, and A. M. J. Bull. Non-linear scaling of a musculoskeletal model of the lower limb using statistical shape models. J. Biomech. 49:3576–3581, 2016.
Parcells, B. Tka Bone Cuts. In: Hip and Knee Book. 2017.at https://hipandkneebook.com/tja-publication-blog/2017/3/14/tka-bone-cuts
Pinskerova, V., P. Johal, S. Nakagawa, A. Sosna, A. Williams, W. Gedroyc, and M. A. R. Freeman. Does the femur roll-back with flexion? J. Bone Joint Surg. Br. 86:925–931, 2004.
Poltaretskyi, S., J. Chaoui, M. Mayya, C. Hamitouche, M. J. Bercik, P. Boileau, and G. Walch. Prediction of the pre-morbid 3D anatomy of the proximal humerus based on statistical shape modelling. Bone Jt. J. 99B:927–933, 2017.
Rao, C., C. K. Fitzpatrick, P. J. Rullkoetter, L. P. Maletsky, R. H. Kim, and P. J. Laz. A statistical finite element model of the knee accounting for shape and alignment variability. Med. Eng. Phys. 35:1450–1456, 2013.
Rao, Z., C. Zhou, Q. Zhang, W. A. Kernkamp, J. Wang, L. Cheng, T. E. Foster, H. S. Bedair, and G. Li. There are isoheight points that measure constant femoral condyle heights along the knee flexion path. Knee Surg. Sport. Traumatol. Arthrosc. 29:600–607, 2021.
Rasmussen, C. E., and C. K. I. Williams. Gaussian Processes for Machine Learning. MIT Press, 2006.at www.GaussianProcess.org/gpml
Ritter, M. A., K. D. Carr, E. M. Keating, and P. M. Faris. Long-term outcomes of contralateral knees after unilateral total knee arthroplasty for osteoarthritis. J. Arthroplasty. 9:347–349, 1994.
Salhi, A., V. Burdin, A. Boutillon, S. Brochard, T. Mutsvangwa, and B. Borotikar. Statistical shape modeling approach to predict missing scapular bone. Ann. Biomed. Eng. 48:367–379, 2020.
Sanders, T. L., H. MaraditKremers, C. D. Schleck, D. R. Larson, and D. J. Berry. Subsequent total joint arthroplasty after primary total knee or hip arthroplasty. J Bone Jt Surg. 99:396–401, 2017.
Sato, T., and T. Mochizuki. Three-dimensional morphology of the distal femur based on surgical epicondylar axis in the normal elderly population. Knee. 30:125–133, 2021.
Scalismo Tutorials Version: 0.90. Tutorial 10: Iterative Closest Points for rigid alignmentat https://scalismo.org/docs/tutorials/tutorial10
Scalismo Tutorials Version: 0.90. Tutorial 11: Model fitting with Iterative Closest Pointsat https://scalismo.org/docs/tutorials/tutorial11/
Smith & Nephew. Smith & Nephew Journey II XR Bi-Cruciate Retaning Knee System Design Rationale. 2017.at https://www.smith-nephew.com/global/assets/pdf/products/surgical/orthopaedics/journeyiixrdesignrationale06791v11017.pdf
Smoger, L. M., C. K. Fitzpatrick, C. W. Clary, A. J. Cyr, L. P. Maletsky, P. J. Rullkoetter, and P. J. Laz. Statistical modeling to characterize relationships between knee anatomy and kinematics. J. Orthop. Res. 33:1620–1630, 2015.
Stegmann, M. B. B., and D. D. D. Gomez. A brief introduction to statistical shape analysis. Informatics Math. … 1–15, 2002.at http://graphics.stanford.edu/courses/cs164-09-spring/Handouts/paper_shape_spaces_imm403.pdf
Thienpont, E. Revision knee surgery techniques. EFORT Open Rev. 1:233–238, 2016.
Varadarajan, K. M., A. A. Freiberg, T. J. Gill, H. E. Rubash, and G. Li. Relationship between three-dimensional geometry of the trochlear groove and in vivo patellar tracking during weight-bearing knee flexion. J. Biomech. Eng. 132:1–7, 2010.
Vlachopoulos, L., C. Dünner, T. Gass, M. Graf, O. Goksel, C. Gerber, G. Székely, and P. Fürnstahl. Computer algorithms for three-dimensional measurement of humeral anatomy: Analysis of 140 paired humeri. J. Shoulder Elb. Surg. 25:e38–e48, 2016.
Vlachopoulos, L., M. Lüthi, F. Carrillo, C. Gerber, G. Székely, and P. Fürnstahl. Restoration of the patient-specific anatomy of the proximal and distal parts of the humerus. J. Bone Jt. Surg. 100:e50, 2018.
Willing, R., and I. Y. Kim. Quantifying the competing relationship between durability and kinematics of total knee replacements using multiobjective design optimization and validated computational models. J. Biomech. 45:141–147, 2012.
Zheng, G., S. Li, and G. Szekely. Statistical shape and deformation analysis - methods. Implement. Appl. 2017. https://doi.org/10.1016/c2015-0-06799-5.
Zhu, Z., and G. Li. Construction of 3D human distal femoral surface models using a 3D statistical deformable model. J. Biomech. 44:2362–2368, 2011.
Acknowledgments
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix A: Formulations of Gaussian Process and Gaussian Process Regression
Gaussian Process
Mathematically, a Gaussian Process (GP) is used to model a vector-valued random variable, such as the 3D deformation field (\(u\)) of a shape group, which is a function of the spatial coordinates (\(x\in {\mathbb{R}}^{3}\))1:
where the subscripts (1, 2, 3) of \(u\) represent its three components for the 3D case. \(\mu (x)\in {\mathbb{R}}^{3}\) is the mean deformation, and \(k(x,x{^{\prime}})\in {\mathbb{R}}^{3\times 3}\) is the covariance function, which is also termed as the kernel.
In terms of the discrete deformation field (as the reference surface mesh, \({\Gamma }_{R}\), in nature consisting of a set of points, is the feasible region, i.e., \(x\in {\Gamma }_{R}\)), the kernel of the GP can be converted to a covariance matrix (\(\Sigma\)), when reducing the GP to a multivariate normal distribution that models scalar-valued random variables. By singular value decomposition of \(\Sigma\) (i.e., principal component analysis), eigenvalues (\({\lambda }_{i}\)) and eigenvectors (\({\varphi }_{i}\)) can be obtained, and the deformation field can be expressed as a linear combination of \(\sqrt{{\lambda }_{i}}\) and \({\varphi }_{i}(x)\)15,44:
where \({\alpha }_{i} \sim N(\mathrm{0,1})\) are random variables which follow the standard normal distribution, and \(n\) is the number of principal components. It can be noted that Eq. A2 is the formulation of the well-known SSM.15,44 Therefore, the SSM is a special application of the GP model (Eq. A1), when the discrete deformation field was considered. In this study, the SSMs were also created with an intent to visualize shape variations, where either femur or tibia SSM included all principal components (\(n=51\), equal to the sample size minus 1).
Gaussian Process Regression
Gaussian process regression (GPR) is a Bayesian inference method designed to make predictions incorporating prior knowledge (kernels) and to provide uncertainty measures over predictions.29 Technically, GPR computes a posterior GP model, \({u}_{p} \sim GP({\mu }_{p}, {k}_{p})\), which is the conditional distribution of the prior GP model (Eq. A1), given partial observations (\(\tilde{u }\)). Considering the uncertainty of observations, we have assumed that:
where \({y}_{i}\in {\mathbb{R}}^{3}\), \(i=1,\cdots ,m\) are the coordinates of observations. \(\epsilon \sim N(0, {\sigma }^{2}{I}_{3m\times 3m})\) is the random noise, which follows a normal distribution; where \({\sigma }^{2}\) is the variance controlling the degree of uncertainty, and \({I}_{3m\times 3m}\) represents the identity matrix with the dimension of \(3m\times 3m\).
As a merit, the posterior GP model has a closed-form (explicit) solution, and the posterior mean (\({\mu }_{p}\)) and kernel (\({k}_{p}\)) can be formulated as:
where \(Y={[{y}_{1}^{1} {y}_{1}^{2} {y}_{1}^{3} \cdots {y}_{m}^{1} {y}_{m}^{2} {y}_{m}^{3}]}^{T}\). In particular, the mean of the posterior GP model, \({\mu }_{p}\), is the solution to GPR, as it has the maximal probability of the conditional distribution. Please refer to more mathematical details of GPR in the application of shape completion, which have been documented previously.1
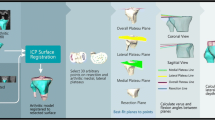
Appendix B: Rigid and Non-rigid Iterative Closest Point Algorithms
See Figs.
B1 and
B2.
Rights and permissions
About this article
Cite this article
Zhou, C., Cha, T., Peng, Y. et al. 3D Geometric Shape Reconstruction for Revision TKA and UKA Knees Using Gaussian Process Regression. Ann Biomed Eng 49, 3685–3697 (2021). https://doi.org/10.1007/s10439-021-02871-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02871-3