Abstract
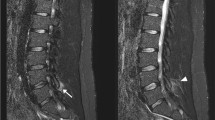
The neurodegenerative disease amyotrophic lateral sclerosis (ALS) results in the death of motor neurons in voluntary muscles. There are no cures for ALS and few available treatments. In studies with small animal models, injection of cellular therapeutics into the anterior horn of the spinal cord has been shown to inhibit the progression of ALS. It was hypothesized that spinal injection could be made faster and less invasive with the aid of a robot. The robotic system presented—SpinoBot—uses MRI guidance to position a needle for percutaneous injection into the spinal cord. With four degrees of freedom (DOF) provided by two translation stages and two rotational axes, SpinoBot proved capable of advanced targeting with a mean error of 1.12 mm and standard deviation of 0.97 mm in bench tests, and a mean error of 2.2 mm and standard deviation of 0.85 mm in swine cadaver tests. SpinoBot has shown less than 3% signal-to-noise ratio reduction in 3T MR imaging quality, demonstrating its compliance to the MRI environment. With the aid of SpinoBot, the length of the percutaneous injection procedure is reduced to less than 60 min with 10 min for each additional insertion. Although SpinoBot is designed for ALS treatment, it could potentially be used for other procedures that require precise access to the spine.







Similar content being viewed by others
References
National Institutes of Health. Amyotrophic Lateral Sclerosis (ALS) Information Page. NIH National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/Disorders/All-Disorders/Amyotrophic-Lateral-Sclerosis-ALS-Information-Page.
Human Physiology Academy. An Overview of the Central Nervous System: The Spinal Cord. Human Physiology Academy, 2014. http://humanphysiology.academy/Neurosciences%202015/Chapter%202/A.2.1%20Spinal%20Cord.html.
Asheuer, M., F. Pflumio, S. Benhamida, A. Dubart-Kupperschmitt, F. Fouquet, Y. Imai, P. Aubourg, and N. Cartier. Human CD34+ cells differentiate into microglia and express recombinant therapeutic protein. Proc. Natl. Acad. Sci. USA 101:3557–3562, 2004.
Bertelsen, A., J. Melo, E. Sánchez, and D. Borro. A review of surgical robots for spinal interventions. Int. J. Med. Robot. 9:407–422, 2013.
Chinzei K., N. Hata, F. A. Jolesz and R. Kikinis. MR compatible surgical assist robot: System integration and preliminary feasibility study. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. New York: Springer, pp. 921–930, 2000.
Chinzei K., R. Kikinis and F. A. Jolesz. MR compatibility of mechatronic devices: design criteria. In: Medical Image Computing and Computer-Assisted Intervention—MICCAI’99. New York: Springer, pp. 1020–1030, 1999.
Cleary, K., A. Melzer, V. Watson, G. Kronreif, and D. Stoianovici. Interventional robotic systems: applications and technology state-of-the-art. Minim. Invas. Ther. Allied Technol. 15:101–113, 2006.
Elhawary, H., Z. T. H. Tse, A. Hamed, M. Rea, B. L. Davies, and M. U. Lamperth. The case for MR-compatible robotics: a review of the state of the art. Int. J. Med. Robot. 4:105–113, 2008.
Elhawary, H., A. Zivanovic, M. Rea, B. L. Davies, C. Besant, I. Young, and M. Lamperth. A modular approach to MRI-compatible robotics. IEEE Eng. Med. Biol. Mag. 27:35–41, 2008.
Fischer, G. S., I. Iordachita, C. Csoma, J. Tokuda, S. P. DiMaio, C. M. Tempany, N. Hata, and G. Fichtinger. MRI-compatible pneumatic robot for transperineal prostate needle placement. IEEE ASME Trans. Mechatron. 13:295–305, 2008.
Gassert, R., E. Burdet, and K. Chinzei. MRI-compatible robotics. IEEE Eng. Med. Biol. Mag. 27:12–14, 2008.
Glass, J. D., N. M. Boulis, K. Johe, S. B. Rutkove, T. Federici, M. Polak, C. Kelly, and E. L. Feldman. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Regen. Med. 30:1144–1151, 2012.
Goolsby, J., M. C. Marty, D. Heletz, J. Chiappelli, G. Tashko, D. Yarnell, P. S. Fishman, S. Dhib-Jalbut, C. T. Bever, Jr, B. Pessac, and D. Trisler. Hematopoietic progenitors express neural genes. Proc. Natl. Acad. Sci. USA 100:14926–14931, 2003.
Memorang, Inc. Gross Anatomy. Memorang, Inc., 2017. https://www.memorangapp.com/flashcards/56760/Gross+Anatomy/.
Hempel, E., H. Fischer, L. Gumb, T. Höhn, H. Krause, U. Voges, H. Breitwieser, B. Gutmann, J. Durke, and M. Bock. An MRI-compatible surgical robot for precise radiological interventions. Comput. Aided. Surg. 8:180–191, 2003.
Ho, M., A. B. McMillan, J. M. Simard, R. Gullapalli, and J. P. Desai. Toward a meso-scale SMA-actuated MRI-compatible neurosurgical robot. IEEE Trans. Robot. 28:213–222, 2012.
Hoehn, M., E. Kustermann, J. Blunk, D. Wiedermann, T. Trapp, S. Wecker, M. Focking, H. Arnold, J. Hescheler, B. K. Fleischmann, W. Schwindt, and C. Buhrle. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc. Natl. Acad. Sci. USA 99:16267–16272, 2002.
International A. ASTM F2554-10. Standard Practice for Measurement of Positional Accuracy of Computer Assisted Surgical Systems, West Conshohocken, PA, 2010.
International Organization for Standardization. ISO 1101:2017 Geometrical product specifications (GPS)—geometrical tolerancing—tolerances of form, orientation, location and run-out, 2017.
Krieger, A., I. I. Iordachita, P. Guion, A. K. Singh, A. Kaushal, C. Ménard, P. A. Pinto, K. Camphausen, G. Fichtinger, and L. L. Whitcomb. An MRI-compatible robotic system with hybrid tracking for MRI-guided prostate intervention. IEEE Trans. Biomed. Eng. 58:3049–3060, 2011.
Krieger, A., S.-E. Song, N. B. Cho, I. I. Iordachita, P. Guion, G. Fichtinger, and L. L. Whitcomb. Development and evaluation of an actuated MRI-compatible robotic system for MRI-guided prostate intervention. IEEE ASME Trans. Mechatron. 18:273–284, 2013.
Krieger, A., R. C. Susil, C. Ménard, J. A. Coleman, G. Fichtinger, E. Atalar, and L. L. Whitcomb. Design of a novel MRI compatible manipulator for image guided prostate interventions. IEEE Trans. Biomed. Eng. 52:306–313, 2005.
Lacey, C., and G. Sutherland. Advancing neurosurgery through translational research. Neurosurgery 72(Suppl 1):176–181, 2013.
Maderer, J., and M. Feeney. Putting the right face on an assistive robot. Biomed. Saf. Stand. 44:113–114, 2014.
Mazzini, L., I. Ferrero, V. Luparello, D. Rustichelli, M. Gunetti, K. Mareschi, L. Testa, A. Stecco, R. Tarletti, M. Miglioretti, E. Fava, N. Nasuelli, C. Cisari, M. Massara, R. Vercelli, G. D. Oggioni, A. Carriero, R. Cantello, F. Monaco, and F. Fagioli. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp. Neurol. 223:229–237, 2010.
Mazzini, L., K. Mareschi, I. Ferrero, M. Miglioretti, A. Stecco, S. Servo, A. Carriero, F. Monaco, and F. Fagioli. Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 14:56–60, 2012.
Melzer, A., B. Gutmann, T. Remmele, R. Wolf, A. Lukoscheck, M. Bock, H. Bardenheuer, and H. Fischer. INNOMOTION for percutaneous image-guided interventions: principles and evaluation of this MR- and CT-compatible robotic system. IEEE Eng. Med. Biol. Mag. 27:66–73, 2008.
Mert, A., L. S. Gan, E. Knosp, G. R. Sutherland, and S. Wolfsberger. Advanced cranial navigation. Neurosurgery 72(Suppl 1):43–53, 2013.
Papagelopoulos, P. J., H. A. Peterson, M. J. Ebersold, R. P. Emmanuel, S. N. Choudhury, and L. M. Quast. Spinal column deformity and instability after lumbar or thoracolumbar laminectomy for intraspinal tumors in children and young adults. Spine 22:442–451, 1997.
Pappafotis N., W. Bejgerowski, R. Gullapalli, J. M. Simard, S. K. Gupta and J. P. Desai. Towards design and fabrication of a miniature MRI-compatible robot for applications in neurosurgery. In: ASME 2008 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. American Society of Mechanical Engineers, pp. 747–754, 2008.
Riley, J., T. Federici, M. Polak, C. Kelly, J. Glass, B. Raore, J. Taub, V. Kesner, E. L. Feldman, and N. M. Boulis. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery 71:405–416, 2012; ((Discussion 416)).
Ringel, F., D. Ingerl, S. Ott, and B. Meyer. Varioguide: a new frameless image guided stereotactic system—accuracy study and clinical assessment. Neurosurgery 64:ons365–ons373, 2009.
Stoianovici, D., D. Song, D. Petrisor, D. Ursu, D. Mazilu, M. Mutener, M. Schar, and A. Patriciu. “MRI Stealth” robot for prostate interventions. Minim. Invas. Ther. Allied Technol. 16:241–248, 2007.
Sutherland, G. R., I. Latour, and A. D. Greer. Integrating an image-guided robot with intraoperative MRI. IEEE Eng. Med. Biol. Mag. 27:59–65, 2008.
Sutherland, G. R., P. B. McBeth, and D. F. Louw. NeuroArm: an MR compatible robot for microsurgery. Int. Congr. Ser. 1256:504–508, 2003.
Sutherland, G. R., S. Wolfsberger, S. Lama, and K. Zarei-nia. The evolution of neuroArm. Neurosurgery 72(Suppl 1):27–32, 2013.
Taguchi, A., T. Soma, H. Tanaka, T. Kanda, H. Nishimura, H. Yoshikawa, Y. Tsukamoto, H. Iso, Y. Fujimori, D. M. Stern, H. Naritomi, and T. Matsuyama. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 114:330–338, 2004.
Tan, N., W.-C. Lin, P. Khoshnoodi, N. H. Asvadi, J. Yoshida, D. J. Margolis, D. S. Lu, H. Wu, K. H. Sung, and D. Y. Lu. In-bore 3-T MR-guided transrectal targeted prostate biopsy: prostate imaging reporting and data system version 2—based diagnostic performance for detection of prostate cancer. Radiology 283:130–139, 2016.
Tsekos, N. V., E. Yacoub, P. V. Tsekos, and I. G. Koutlas. Design of an MRI-compatible robotic stereotactic device for minimally invasive interventions in the breast. J. Biomech. Eng. 126:458–465, 2004.
Wolter K., G. Decker and W. Willinek. Transperineal MR-guided stereotactic prostate biopsy utilizing a commercially available anorectal biopsy device. In: RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren©. Georg Thieme Verlag KG, pp. 116–120, 2013.
Yasuoka, S., H. A. Peterson, and C. S. MacCarty. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J. Neurosurg. 57:441–445, 1982.
Zhao, Z. M., H. J. Li, H. Y. Liu, S. H. Lu, R. C. Yang, Q. J. Zhang, and Z. C. Han. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transpl. 13:113–122, 2004.
Acknowledgments
This study was supported in part by the National Institutes of Health (NIH) Bench-to-Bedside Award, the NIH Center for Interventional Oncology Grant, the National Science Foundation (NSF) I-Corps Team Grant (1617340), NSF REU site program 1359095, the UGA-AU Inter-Institutional Seed Funding, the American Society for Quality Dr. Richard J. Schlesinger Grant, the PHS Grant UL1TR000454 from the Clinical and Translational Science Award Program, and the NIH National Center for Advancing Translational Sciences.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Xiaoxiang Zheng oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Squires, A., Oshinski, J.N., Boulis, N.M. et al. SpinoBot: An MRI-Guided Needle Positioning System for Spinal Cellular Therapeutics. Ann Biomed Eng 46, 475–487 (2018). https://doi.org/10.1007/s10439-017-1960-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1960-z